Abstract
Background:
Immune tolerance induction (ITI) is the gold standard for inhibitor eradication to restore the clinical efficacy of factor replacement therapy in haemophilia. However, as ITI often requires frequent administration over extended periods, it can be considered burdensome for patients and healthcare resources. Therefore, there is a need to optimise ITI treatment, particularly in patients who failed previous ITI attempts.
Objectives:
The ReITIrate study aimed to prospectively evaluate rescue ITI with efmoroctocog alfa, an extended half-life recombinant FVIII Fc fusion protein (herein rFVIIIFc), within a limited 60-week timeframe in patients with severe haemophilia A and inhibitors who failed previous ITI attempts.
Design:
ReITIrate was a phase IV, open-label, single-arm, interventional, multicentre study.
Methods:
Primary endpoint was ITI success (negative titre, <0.6 BU/mL; incremental recovery >66%; elimination half-life ⩾7 hours) within 60 weeks. Exploratory immunophenotype analyses were performed to characterise anti-drug antibodies (ADA) and cellular immune responses.
Results:
Nine of 16 enrolled subjects completed the ITI period during ReITIrate, of which one subject attained all 3 ITI success criteria after 46 weeks with no relapse. Two subjects achieved partial success (one subject met 2/3 success criteria; one met all criteria, but not simultaneously, with inhibitor recurrence). One additional subject (ITI failure) achieved negative inhibitor titre. Across these four subjects, median (range) time to negative titre was 19 (11–60) weeks. No new safety concerns were identified. IgG4 was the major contributor to the ADA IgG response. Subjects with partial/complete ITI success had fewer IgG subclasses involved than those who failed/withdrew. Immunophenotyping indicated an increase in regulatory T-cells (CD4+CD25+CD127low), supporting the ability to perform sensitive blood sampling to identify immune tolerance markers.
Conclusion:
This study demonstrates that ITI with rFVIIIFc given within a limited timeframe has potential benefit in a difficult-to-treat inhibitor haemophilia population who failed previous ITI attempts.
Trial registration:
Keywords: factor VIII, haemophilia A, immune tolerance, recombinant factor VIII Fc fusion protein, recombinant fusion proteins
Introduction
Inhibitors are the most severe treatment-related complication of exogenous factor VIII (FVIII) in haemophilia A.1,2 Approximately one-third of previously untreated patients with severe haemophilia A develop neutralising antibodies (NAb; or inhibitors) against FVIII, rendering FVIII replacement ineffective for prevention and treatment of bleeds and maintenance of surgical haemostasis.1–3
Immune tolerance induction (ITI) is the gold standard to eradicate inhibitors and restore the clinical efficacy of FVIII replacement therapy by tolerising the immune system to FVIII. 1 Despite the availability of non-factor therapies with emicizumab becoming a preferred first-line approach to protect against bleeds and as an alternative to ITI in certain patient groups, inhibitor eradication by ITI remains the preferred management strategy for patients with haemophilia A who develop inhibitors.2,4–7 However, there is currently no clear consensus on an optimal ITI regimen. 8 ITI therapy with standard half-life (SHL) FVIII products is considered burdensome for patients and healthcare resources as it usually requires frequent administration of high-dose FVIII over extended periods to achieve tolerance and optimise haemostatic control.3,9–12 Many patients need central venous access devices (CVADs) for frequent infusions.12,13
Moreover, ITI therapy is not always successful, with complete ITI success rates between 38% and 83% for patients receiving first-time ITI.14–19 ITI outcomes may be influenced by therapeutic regimens and patient-related prognostic factors, which could guide treatment decisions and optimise ITI.19–21 A pre-ITI inhibitor titre <10 BU/mL, peak historical inhibitor titre ⩽200 BU/mL, and ITI initiated <5 years after inhibitor diagnosis have been identified as predictors of ITI success.11,21 The International ITI (I-ITI) study indicated that patients with good prognosis had similar ITI success rates with both high-dose and low-dose FVIII regimens, although low-dose subjects bled more often and required longer treatment periods to achieve success. 14 Patients who have failed ITI can be exposed to subsequent ITI attempts, or ‘rescue ITI’ therapy. Although there are limited data on the success rate and time to tolerance of rescue ITI treatments, a successful outcome is generally less likely than the first ITI attempt.17–19,22 Therefore, there is a need to optimise ITI treatment in patients who failed previous ITI attempts, especially with the availability of non-factor therapy. 2
Preclinical data suggests efmoroctocog alfa, an extended half-life (EHL) recombinant FVIII Fc fusion protein (herein referred to as rFVIIIFc), has immunomodulatory properties and may induce tolerance to FVIII; certain epitopes in the Fc domain have been shown to activate regulatory T-cells (Tregs) and interact with monocyte-derived macrophages expressing Fc receptors to induce regulatory macrophage polarisation.23–25 However, additional immunological analyses are required to more fully characterise the immune mechanisms underlying ITI with rFVIIIFc.
Retrospective clinical ITI data and case reports indicate earlier tolerance can be achieved using rFVIIIFc compared with SHL products.9,10,22,26–28 However, data are limited and use varying treatment protocols and definitions of success.9,10,22,26–28 Therefore, it would be valuable to assess the effectiveness of ITI with rFVIIIFc within a limited timeframe using a standardised ITI protocol, especially in patients with a poor prognosis of ITI success.
Given the expected differences in first-time ITI and rescue ITI treatment success rates, two phase IV studies were designed to prospectively evaluate ITI with rFVIIIFc. VerITI-8 (NCT03093480) evaluated ITI outcomes with rFVIIIFc in patients with severe haemophilia A and inhibitors undergoing their first ITI treatment over 48 weeks. 29 Here, we report results from ReITIrate (NCT03103542), the first prospective study to evaluate the outcomes of rescue ITI with rFVIIIFc over a limited, predefined 60-week timeframe using a standardised rFVIIIFc protocol.
Methods
Study design and participants
ReITIrate was a phase IV, open-label, single-arm, interventional study conducted in nine countries across Europe and North America.
The primary objective was to describe the outcome of ITI treatment performed within a limited, predefined 60-week timeframe with rFVIIIFc in patients who failed previous attempts of tolerisation (including immunosuppressant use in previous ITI attempts). Secondary and exploratory objectives are listed in the Supplementary Methods.
Enrolled subjects were male, of any age, with severe haemophilia A and high-titre inhibitors (historical peak: ⩾5 BU/mL according to medical records), with ⩾0.6 BU/mL inhibitor titre at screening. Eligible subjects had undergone ⩾1 previous failed ITI treatment with any FVIII product (minimum dose: 50 IU/kg three times weekly) and a minimum ITI treatment period of 33 months or <33 months if there was no downward trend of ⩾20% in inhibitor titre in any 6-month period after the initial 3 months of ITI.
Concurrent systematic treatment with immunosuppressants within 12 weeks prior to screening was not permitted. Emicizumab was not yet approved and launched when this study was planned. As there was a lack of experience with the safety of emicizumab during ITI treatment, patients concurrently or previously treated with emicizumab were excluded from this study. Full exclusion criteria are reported in the Supplementary Methods.
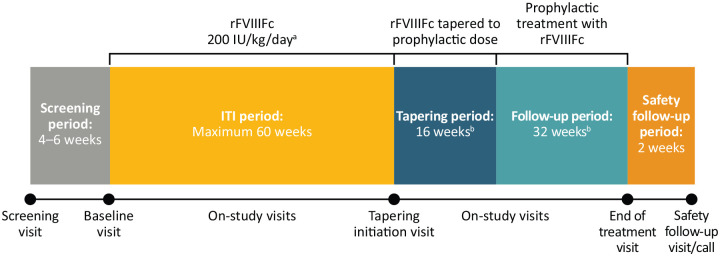
ReITIrate consisted of a 4- to 6-week screening period, followed by an ITI treatment period, during which subjects initially received rFVIIIFc 200 IU/kg daily (subsequently adjusted based on FVIII:C levels and according to investigator judgement to avoid FVIII plasma activity >200 IU/dL or <1 IU/dL) until successful tolerisation or for a maximum of 60 weeks (Figure 1). rFVIIIFc was administered as a once-daily injection or divided into two injections per day at the investigator’s discretion.
Figure 1.
ReITIrate study design.
aAfter the initial rFVIIIFc dose of 200 IU/kg/day, dosing was subsequently adjusted based on FVIII:C levels and according to investigator judgement to avoid FVIII plasma activity >200 or <1 IU/dL. bOnly subjects who achieved ITI success within 60 weeks entered the tapering and follow-up periods. Concomitant use of immunosuppressants was not permitted during the study. If the tapering period was prolonged, the follow-up period was shortened accordingly (minimum of 24 weeks).
FVIII, factor VIII; ITI, immune tolerance induction; rFVIIIFc, recombinant FVIII Fc fusion protein.
Subjects who achieved ITI success within the treatment period were eligible to enter the 16-week tapering period (where rFVIIIFc dose was gradually reduced to prophylactic levels; dose and regimen adjusted by the investigator in accordance with local practice) and subsequent 32-week follow-up period. During follow-up, subjects continued prophylactic treatment with the dose and frequency prescribed by the investigator according to clinical response. A final safety follow-up was conducted 7–14 days after the end of treatment (EOT) visit.
During the ITI period, bleeds were treated according to investigator judgement and local practice; concomitant treatment with bypassing agents (BPAs) was permitted. During tapering/follow-up, subjects were monitored for relapse, defined as a positive inhibitor ⩾0.6 BU/mL (Nijmegen-modified Bethesda assay) and an incremental recovery (IR) ⩽66% of expected IR, both on two consecutive assessments performed within 2–4 weeks, with or without clinical signs/symptoms. Subjects with confirmed relapse proceeded directly to the EOT visit.
Endpoints
The primary endpoint was ITI success within 60 weeks, which was assessed continuously. ITI success was defined as simultaneously achieving all the following criteria: negative inhibitor titre (<0.6 BU/mL) at two consecutive visits, calculated FVIII IR > 66% of the expected IR at two consecutive visits and FVIII elimination half-life (t½) ⩾7 hours.11,30 Partial success was defined as achieving negative inhibitor titre and one pharmacokinetic parameter of ITI success (IR > 66% or elimination t½ ⩾7 hours). Treatment failure was defined as fulfilling one of the following criteria: no downward trend of ⩾20% in inhibitor titre in any 6-month period after the initial 3 months of ITI treatment 30 ; presence of a sustained positive inhibitor (⩾0.6 BU/mL) after 60 weeks of ITI; or negative inhibitor titre without achieving either IR >66% of expected IR or elimination t½ ⩾7 hours after 60 weeks of ITI. As per the protocol, outcomes could also be deemed ‘not determinable’ due to withdrawal during the ITI period.
Secondary endpoints included time to tolerisation (i.e. ITI success), relapse rate following successful ITI, number of bleeds during ITI and follow-up (also reported as annualised bleeding rate (ABR)), adverse events (AEs), number of hospitalisations and days of missed school, rFVIIIFc consumption, and dose and frequency adherence (calculated as the number of administered doses/number of prescribed doses and the number of days in which a dose was taken/number of days in which a dose was prescribed, respectively).
Exploratory endpoints included the presence of FVIII-specific anti-drug antibodies (ADA; both neutralising and non-neutralising) and cellular immune response characterisation (details reported in the Supplementary Methods).
Data collection and analysis
Pre-dose blood samples were collected at all visits during the study for the determination of FVIII inhibitors using local procedures. Blood samples were collected for FVIII activity analysis to assess IR where t½ was not assessed, starting at the visit after the confirmed negative inhibitor titre. Blood samples for assessment of t½ were collected starting at the visit after confirmed IR >66% of the expected IR. Assessments were repeated at each consecutive visit until the t½ was ⩾7 hours and at the EOT visit. At least a 24-hour washout was required prior to the t½ pre-dose sample collection, without additional rFVIIIFc doses, during the pharmacokinetic sampling period.
Blood samples for assessment of inhibitor titre and rFVIIIFc activity were analysed using the Nijmegen-modified Bethesda assay at both local and central laboratories (which were accredited or participated in an external quality assurance scheme). Local laboratory analyses were used for clinical decision making and evaluation of ITI success criteria. Calculations were conducted by the investigator or designee. For assessment of exploratory endpoints, blood samples, including peripheral blood mononuclear cells and plasma, were analysed at a central laboratory and a central research laboratory.
Further details on data collection and analyses are provided in the Supplementary Methods.
Statistical analysis
Due to the limited number of subjects with severe haemophilia A and persistent inhibitors who could fulfil the strict inclusion criteria, no formal sample size calculation was possible.
All subjects who received ⩾1 rFVIIIFc dose were included in the ITI full analysis set. Outcomes were summarised using descriptive statistics; no inferential statistics were performed. Safety data and consumption were presented descriptively across the study and by each period. Time to ITI success was analysed using Kaplan-Meier estimates. Individual age and inhibitor titres data are presented as ranges to help protect subject identification.
Results
Subject characteristics
Of 18 screened subjects, 16 were enrolled in ReITIrate between November 2017 and December 2018. Enrolled subjects had multiple risk factors for poor ITI outcome and a median (range) total previous ITI duration of 51.7 (13–155) months (Tables 1 and 2). One subject was treated with rFVIIIFc during the 12 months prior to baseline. No subjects used rFVIIIFc as their last treatment prior to first inhibitor development. All subjects with a known causative FVIII gene (F8) genotype (not available for n = 2) had high-risk mutations (i.e. inversions, large deletion, frameshift and nonsense mutations). In addition to risk factors listed in Tables 1 and 2, nine subjects had >5 years between diagnosis of inhibitor and start of ITI in ReITIrate (data not shown). Five subjects had previously received immunomodulation.
Table 1.
Baseline demographics and disease characteristics of ReITIrate subjects.
| Characteristic | Enrolled subjects a (N = 16) |
|---|---|
| Age at enrolment (years), median (range) | 7.5 (3–46) b |
| Weight at screening (kg), mean (SD) | 35.5 (20.3) |
| Race/Ethnicity, n (%) | |
| White | 15 (93.8) |
| Black or African American | 1 (6.3) |
| Family history of inhibitors, n (%) | |
| Yes | 7 (43.8) |
| No | 5 (31.3) |
| Unknown | 4 (25.0) |
| F8 genotype, n (%) | |
| Intron 22 inversion | 9 (56.3) |
| Frameshift | 2 (12.5) |
| Intron 1 inversion | 1 (6.3) |
| Large deletion | 1 (6.3) |
| Nonsense | 1 (6.3) |
| Not available | 2 (12.5) |
| Historical peak inhibitor titre (BU/mL), median (range c ) | 127.4 (10–3,000) |
| Inhibitor titre at screening (BU/mL), median (range) d | 11 (1–635) |
| Number of previous ITI attempts,e,f median (range) | 1 (1–3) |
| 1 treatment, n (%) | 10 (62.5) |
| 2 treatments, n (%) | 4 (25.0) |
| 3 treatments, n (%) | 2 (12.5) |
| Total duration of previous ITI (months), median (range) | 51.7 (13–155) |
| Previous ITI with high dose, g n (%) | 16 (100) |
| Time elapsed since previous ITI (months), h median (range) | 1.5 (0.0–59.4) |
| Previous immunomodulation used, i n (%) | 5 (31) |
Percentages may not sum to 100 due to rounding.
Subjects were enrolled between November 2017 and December 2018.
Only one subject was ⩾18 years of age.
Rounded to the nearest 10.
Rounded to the nearest integer.
Ten of the previous ITI attempts (n = 10 subjects) were with concomitant use of prophylactic bypassing agents.
FVIII products used in previous ITI included recombinant FVIII (16 treatments), plasma-derived FVIII with (11 treatments) or without (1 treatment) von Willebrand factor.
High retrospective ITI dose was defined as ⩾100 IU/kg once a day.
Derived from the end date of the last ITI attempt until the start date of ITI in the ReITIrate study.
With sirolimus, rituximab, vincristine and prednisolone, in combination or alone.
BU, Bethesda unit; F8, factor VIII gene; FVIII, factor VIII; ITI, immune tolerance induction; SD, standard deviation.
Table 2.
Demographics and baseline disease/previous treatment characteristics of individual subjects enrolled in ReITIrate.
| Subject a | Family history of inhibitors | Age category at enrolment (years) | Historic peak inhibitor titre (BU/mL) | Number of previous ITI attempts | Products used for previous ITI (recombinant, plasma or both b ) | Previous high-dose ITI c | Total duration of previous ITI (months) | Time elapsed since last ITI attempt (months)d,e | Previous immuno-modulation used | Inhibitor titre at screening (BU/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | ⩾12 | 10–<200 | 1 | Recombinant | Yes | 155 | 0.0 | No | 10–<200 |
| 2 | No | 6 to <12 | 500 to <1,000 | 1 | Both | Yes | 74 | 0.0 | No | 10 to <200 |
| 3 | Unknown | ⩾12 | 500 to <1,000 | 3 | Both | Yes | 25 | 6.8 | Yes | 10 to <200 |
| 4 | Yes | 6 to <12 | 1,000 to <10,000 | 1 | Recombinant | Yes | 21 | 24.5 | No | 500 to <1,000 |
| 5 | Unknown | 6 to <12 | 1,000 to <10,000 | 2 | Plasma | Yes | 84 | 27.6 | No | 10 to <200 |
| 6 | Yes | <6 | 10 to <200 | 1 | Recombinant | Yes | 43 | 0.0 | No | 10 to <200 |
| 7 | Yes | ⩾12 | 10 to <200 | 1 | Plasma | Yes | 63 | 0.0 | Yes | 10 to <200 |
| 8 | No | 6 to <12 | 1,000 to <10,000 | 2 | Both | Yes | 62 | 2.1 | Yes | 0.6 to <5 |
| 9 | Yes | <6 | 10 to <200 | 1 | Recombinant | Yes | 57 | 0.1 | No | 0.6 to <5 |
| 10 | Unknown | 6 to <12 | 500 to <1,000 | 1 | Both | Yes | 68 | 3.4 | No | 10 to <200 |
| 11 | Yes | <6 | 10 to <200 | 2 | Both | Yes | 45 | 0.0 | No | 0.6 to <5 |
| 12 | No | ⩾12 | 10 to <200 | 1 | Recombinant | Yes | 13 | 1.0 | No | 10 to <200 |
| 13 | No | <6 | 10 to <200 | 1 | Both | Yes | 15 | 0.9 | No | 10 to <200 |
| 14 | No | <6 | 10 to <200 | 3 | Recombinant | Yes | 28 | 11.6 | Yes | 5 to <10 |
| 15 | Yes | ⩾12 | 200 to <500 | 2 | Both | Yes | 97 | 59.4 | No | 5 to <10 |
| 16 | Yes | 6 to <12 | 10 to <200 | 1 | Plasma | Yes | 46 | 17.9 | Yes | 0.6 to <5 |
Age and inhibitor titres were presented as ranges to help protect subject identification.
Numbers were randomly assigned and not related to subject identification numbers in the study.
For subjects marked as ‘both’, both recombinant and plasma-derived products were used during one ITI attempt.
High retrospective ITI dose was defined as ⩾100 IU/kg once a day.
Derived from the end date of the last ITI attempt until the start date of ITI in the ReITIrate study.
For all four subjects who had one previous ITI attempt and 0 months elapsed since their last ITI attempt, ITI was ongoing at enrolment for an extended duration (43–155 months) so were consequently classified as ITI failures.
BU, Bethesda unit; ITI, immune tolerance induction.
ITI success, time to tolerisation and occurrence of relapse
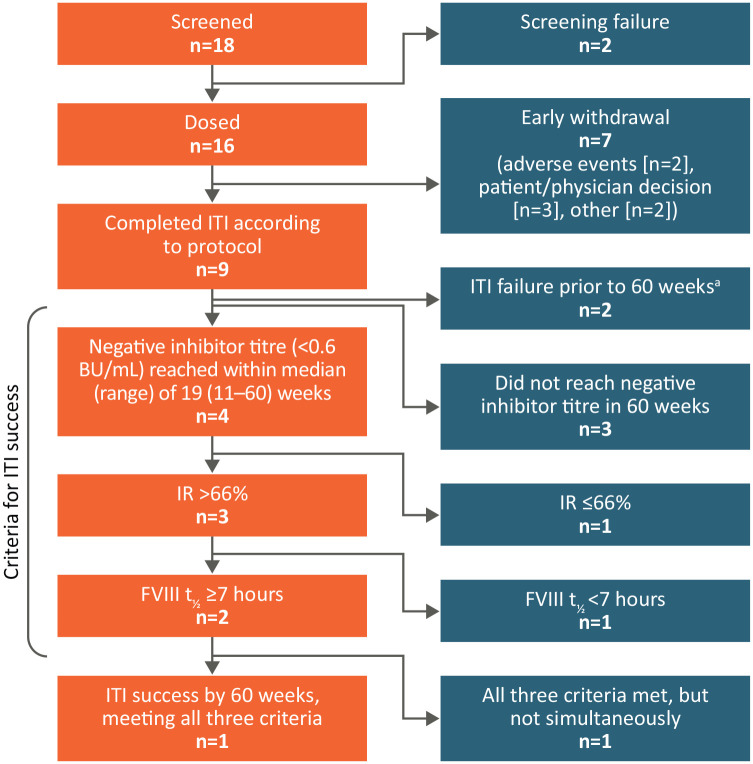
Within the 60-week timeframe, 3/16 (18.8%) enrolled subjects achieved complete or partial ITI success, while six (37.5%) failed to reach ITI success and seven (43.8%) had undetermined criteria according to study definition (Figure 2; Supplementary Table S1). Nine subjects completed the ITI period according to protocol and four of these (44%) achieved negative inhibitor titres. Two subjects entered the tapering period (one subject entered without achieving ITI success due to site error and subsequently stopped the study) and one subject entered the follow-up period.
Figure 2.
ITI outcomes for subjects enrolled in ReITIrate.
aNo downward trend of ⩾20% in inhibitor titre in 6 months of ITI.
BU, Bethesda units; FVIII, factor VIII; IR, incremental recovery; ITI, immune tolerance induction; t½, terminal half-life.
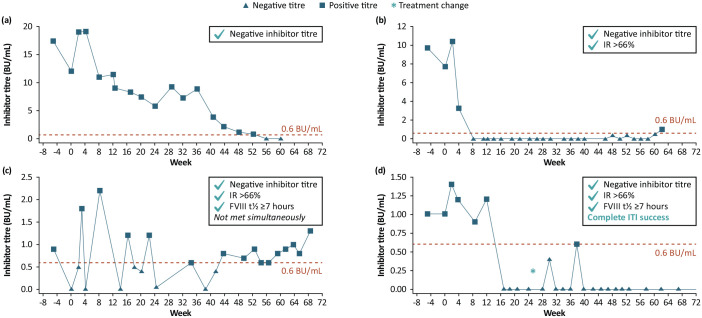
Complete ITI success was achieved by one subject (6.3%) after 46 weeks. This subject did not experience relapse during the 48-week tapering and follow-up periods. Partial success was achieved by two subjects (12.5%) after 30 weeks and 56 weeks. Of these subjects, one met the criteria for IR but not terminal t½ and the other met all ITI success criteria but not simultaneously and had inhibitor recurrence towards the end of the ITI period (Figure 3).
Figure 3.
Inhibitor titres in the four subjects who achieved negative inhibitor titres (<0.6 BU/mL) within 60 weeks of ITI with rFVIIIFc and achievement of ITI success criteria: (a) Subject that obtained a negative inhibitor titre at week 60; (b, c) Subjects deemed as partial success; (d) Subject who simultaneously achieved complete ITI success.
Inhibitor titre scale on the y-axis differs between subjects. Inhibitor titres were assessed at local laboratories. Top right boxes indicate the tolerance criteria achieved according to study protocol definitions. *One subject had a treatment change (dose increase due to low FVIII activity level) at week 24.4.
BU, Bethesda unit; FVIII, factor VIII; IR, incremental recovery; ITI, immune tolerance induction; rFVIIIFc, recombinant factor VIII Fc fusion protein; t½, terminal half-life.
Negative inhibitor titres were achieved by four subjects who completed the ITI period (Figure 3; failure (n = 1; Figure 3(a)), partial success (n = 2; Figure 3(b) and (c)) and complete ITI success (n = 1; Figure 3(d)) within a median (range) of 19 (11–60) weeks. Of these, three subjects reached an IR > 66% of the expected IR (at weeks 30, 30.1 and 56, respectively) and two subjects reported terminal t½ ⩾7 hours (at weeks 46 and 59).
Of the six subjects who failed ITI, two did not have a downward trend of ⩾20% in inhibitor titres in 6 months of ITI, three had sustained positive inhibitor after 60 weeks of ITI and one had negative inhibitor titre without achieving either IR > 66% of the expected IR or terminal t½ ⩾7 hours after 60 weeks of ITI.
Success criteria were not determinable for seven subjects due to early withdrawal for reasons including: AEs (n = 2; described under Safety), physician decision (n = 2), subject decision (n = 1) and other (n = 2).
Dosing, consumption and duration of rFVIIIFc exposure
Median (range) average daily rFVIIIFc dose was 193.3 (98.6–206.3) IU/kg for the ITI period (n = 16), 82.6 (74.9–90.3) IU/kg for the tapering period (n = 2) and 61.5 IU/kg for the follow-up period (n = 1).
Across the 16 enrolled subjects, median (range) annualised rFVIIIFc consumption during the ITI period was 70,520 (48,650–75,337) IU/kg/year. In the subject who achieved complete ITI success and proceeded to the tapering and follow-up periods, annual rFVIIIFc consumption was 27,236 IU/kg/year and 20,927 IU/kg/year during each period, respectively.
Subjects were exposed to rFVIIIFc for a median (range) of 51.6 (17.4–99.0) weeks during the study; median (range) exposure during the ITI, tapering and follow-up periods was 49.5 (17.4–64.3), 12.6 (7.3–18.0) and 31.7 weeks, respectively.
Data on rFVIIIFc adherence, concomitant medication, number of hospitalisations and days missed at school/work are provided in the Supplementary Data.
Bleeds
A median (range) of 5 (0–26) bleeding episodes per subject (n = 16 subjects) were reported during the 12 months prior to baseline. Of these, 14 subjects had traumatic bleeds (n = 74 events) and 11 had spontaneous bleeds (n = 68 events). Refer to the Supplementary Data for concomitant BPA dosing information.
Median (range) ABR was 4.7 (0–45.7) during the ITI period (Supplementary Table S2). In the subject who achieved complete ITI success, ABR during the 48-week follow-up period was 5.1 bleeds/year (all bleeds were traumatic). During the ITI treatment period, 146 bleeding episodes (58 traumatic, 86 spontaneous and 2 missing information) occurred in 12 subjects. Of these, 72 events in 11 subjects were localised to joints (24 traumatic and 48 spontaneous).
Safety
Overall, 188 treatment-emergent AEs (TEAEs; 18 serious and 170 non-serious) were reported across all 16 subjects during the study. The most common TEAEs (⩾15%) are included in Supplementary Table S3.
Of the 18 serious TEAEs (n = 7 subjects), 14 were observed during the ITI period (1 severe, 12 moderate and 1 mild) and 4 during the tapering period (all moderate). Two serious TEAEs, brachiocephalic vein thrombosis and superior vena cava thrombosis, occurred in the same subject during the ITI period (with concomitant BPAs) and were considered related to ITI treatment by the investigator. The subject’s blood culture was positive for staphylococcal infection (medical history of note included serious AE (SAE) of device-related sepsis and insertion of a ventricular assist device). The subject’s central line was removed and BPA stopped. rFVIIIFc treatment continued as planned and the subject recovered. There was one SAE of thrombosis during the tapering period which was considered not related to rFVIIIFc by the investigator. One SAE of CVAD-related thrombosis was reported during the ITI period but was determined not related to rFVIIIFc; this subject had an inhibitor titre of 11.35 BU/mL prior to the event and received BPAs. The aetiology of reported thromboembolic events was concluded to be multifactorial; confounding factors include infection, presence of a CVAD and concomitant use of BPAs.
Three AEs (infection Hickman catheter, bleeds in right thigh and right ankle) in two subjects led to discontinuation (one severe and two moderate); all were reported during the ITI period and considered unrelated to ITI treatment. No deaths or new safety concerns were identified.
Exploratory analysis
ADA screening
A total of 272 plasma samples were analysed for anti-rFVIIIFc antibodies; 258 (95%) were confirmed positive and further characterised.
NAb/ADA titres
Despite differences in methodology, consistent trends were observed between individual NAb titres (Nijmegen-modified Bethesda assay; Supplementary Figure S1), anti-rFVIIIFc titres (electrochemiluminescence) and anti-FVIII IgG titres (enzyme-linked immunosorbent assay; Supplementary Figure S2). Four subjects achieved negative inhibitor titres (<0.6 BU/mL, local laboratory) in the study. Furthermore, some subjects showed a tendency towards decreased ADA levels without reaching negative inhibitor titres (Supplementary Figures S1 and S2).
FVIII/Fc specificity
The 258 ADA-positive samples were not specific for IgG1 (containing the Fc domain of rFVIIIFc). When tested towards FVIII (octocog alfa), 257 ADA-positive samples were positive; 1 sample was negative for octocog alfa although confirmed positive for rFVIIIFc.
FVIII-specific IgA and IgM
After week 2 of the treatment period, two subjects were positive for FVIII-specific IgA (Supplementary Table S4); both subjects had high ADA levels. IgA optical density values for these subjects decreased over time. Both subjects terminated the study early.
IgM responses were obtained at a single timepoint for several subjects but only three subjects had responses at >1 timepoint. One subject with partial ITI success had an IgM response in the outcome assessment period and during the tapering period.
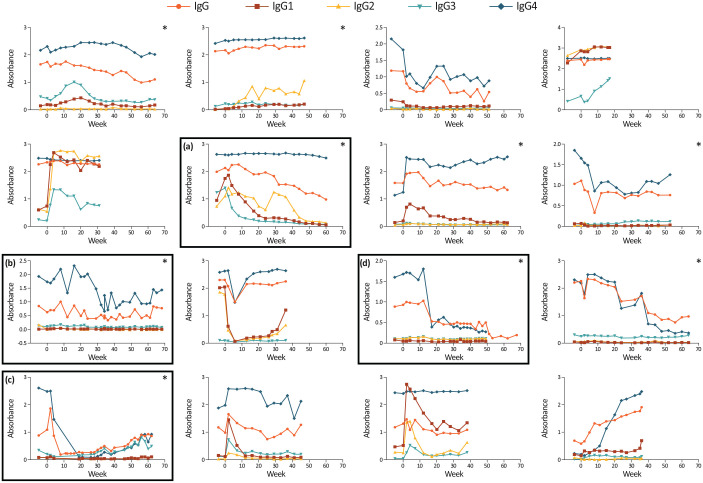
FVIII-specific IgG subclasses
The IgG4 subclass was involved in the immune response for all subjects and was the major contributor to the overall IgG response (Figure 4). For most subjects, the IgG3 subclass also had a positive titre at some timepoints. IgG4 was the only subclass involved in the immune response for the subject with complete ITI success, while IgG3 was also involved for the two subjects with partial success. Subjects that completed ITI had negative or low IgG1 titres.
Figure 4.
The titre of FVIII-specific IgG1–IgG4 during the ReITIrate study period for individual subjects (OD): (a) Subject that obtained a negative inhibitor titre at week 60; (b, c) Subjects deemed as partial success according to protocol definitions; (d) Subject who achieved complete ITI success.
The y-axis scale differs between subjects. *Subjects that completed ITI and the ReITIrate study.
FVIII, factor VIII; Ig, immunoglobulin; ITI, immune tolerance induction; OD, optical density.
Distribution of anti-FVIII antibody binding domains
The FVIII light chain was more frequently recognised by anti-FVIII antibodies than the heavy chain (Supplementary Figure S3). The FVIII domains mainly recognised by antibodies from patient plasma were C2 (in >85% subjects) and A2 (in >50% subjects).
Determination of anti-FVIII affinity
The binding affinities of the IgG antibodies to FVIII were relatively high (Supplementary Figure S4). Two of three subjects that achieved a negative inhibitor titre and were included in the affinity dataset had the lowest antibody affinities to FVIII; the third subject also had affinities in the lower range.
Characterisation of the cellular immune status
Flow cytometry immunophenotyping demonstrated an overall increase in the CD4+CD25+CD127low Tregs subpopulation (Supplementary Figure S5). For the eight subjects with both baseline and EOT measurements available, the mean (SD) proportion of CD4+CD25+CD127low Tregs of total CD4+ T cells increased from 3.2% (0.9) at baseline to 6.1% (1.8) at EOT. T cell activation was evaluated by CD69 expression, but no clear patterns were observed (Supplementary Figure S6). Further data are reported in the Supplementary Data.
Discussion
To the best of our knowledge, ReITIrate is the first and only prospective study to evaluate rescue ITI treatment with an EHL FVIII product in people with severe haemophilia A who have failed previous attempts at tolerisation, including use of immunosuppressants, and within a limited 60-week timeframe.
Of the nine subjects enrolled in ReITIrate who completed the pre-determined ITI treatment period, one achieved complete ITI success with rFVIIIFc after 46 weeks, two reached partial success and six were deemed ITI failures. One of the two subjects deemed as partial success (according to study criteria) achieved all three success criteria but not simultaneously and had inhibitor recurrence towards the end of the ITI period. Four subjects achieved negative inhibitor titres at a median of 19 weeks. The achievement of a negative inhibitor titre may provide clinical benefit with the potential to respond to FVIII treatment and prophylaxis with higher FVIII doses, even if the other two study success criteria were not met. 14
Since only one subject achieved complete ITI success, as defined by the strict study criteria, general conclusions on the secondary objectives (time to tolerisation, relapse and bleeding rate after successful ITI treatment) could not be drawn. Nevertheless, this subject did not experience any relapse within the follow-up period. It should be noted that although this subject completed the formal ITI period per the protocol, the relatively high median dose of 61.5 IU/kg/day administered at follow-up could be considered as ongoing ITI treatment rather than conventional prophylaxis for non-inhibitor patients.
Average rates for missing days from school/work and for hospitalisations during the ITI and follow-up periods were low, indicating a limited impact of ITI treatment on school/work attendance. Despite intense ITI treatment, subjects had high treatment adherence.
rFVIIIFc was well tolerated with no new safety concerns. Eighteen serious TEAEs were reported across seven subjects during the study, including three serious vascular thrombotic events with confirmed multifactorial aetiology. Several risk factors may have contributed to these thromboembolic AEs, such as indwelling CVADs, infections or concomitant use of BPAs.
The exploratory objective was to further understand the mechanism of ITI by investigating antibody response and immune cell status. Antibody specificity was specific for FVIII but not IgG1, suggesting the immune response was directed to the FVIII molecule and not the Fc domain of rFVIIIFc. The high affinity to FVIII observed was expected given that enrolled subjects had an established immune response due to previous challenge with FVIII. The IgG4 subclass was involved in the immune response for all subjects and was the major contributor to the overall IgG response, consistent with previous studies, while IgG1 levels were relatively low in most subjects.31,32 A less broad immune response was seen in subjects with partial/complete ITI success, with only the IgG3 and IgG4 subclasses involved. Most subjects had no IgA response and/or >1 IgM response. Indeed, anti-FVIII IgA and IgM have been identified as potential predictors of poor treatment outcomes in acquired haemophilia A.33,34
Despite no changes in the proportions of CD4+ or CD8+ T cells during ReITIrate, immunophenotype analysis indicated a trend towards an overall increase in CD4+CD25+CD127low Tregs, although further studies would be required to confirm these findings. This result is in agreement with previous non-clinical data associating rFVIIIFc tolerance with attenuation of the immune response to FVIII through a higher proportion of Tregs, a lower percentage of pro-inflammatory T cells and upregulation of tolerogenic cytokines. 23 This is the first study to investigate the T cell and B cell responses in ITI. The ability to perform this degree of immunophenotyping indicates it is possible in relatively young children, despite challenges involved with blood sampling, transport and storage of blood samples and assays involved. However, it should be taken into account that all ReITIrate subjects had undergone at least one previous ITI treatment, which may have influenced the immunological response.
A key strength of ReITIrate was the use of strict criteria to define previous ITI failure, ensuring subjects had undergone and failed a previous intense ITI treatment, and three highly stringent criteria to define ITI success in ReITIrate. It is therefore difficult to compare results with previous studies that may have included subjects that failed shorter or less intense previous ITI attempts or used less stringent success criteria.17,18 Further, comparisons with retrospective studies may not provide a fair benchmark as these analyses may risk selection of more successful cases that completed ITI treatment, excluding subjects who terminated treatment prematurely.
An additional strength was the ReITIrate trial design, including the initial high dose of rFVIIIFc for ITI (200 IU/kg/day), which aligned with the most current and experienced ITI practice and treatment guidelines.14,21,35,36 A detailed immunological follow-up was also conducted to help elucidate the mechanisms underlying rescue ITI outcomes with rFVIIIFc.
Prior to this study, real-world data indicated that tolerance may be achieved more rapidly when ITI is performed with rFVIIIFc compared with SHL products, thus providing possibilities to reduce treatment burden.9,10,22 This study was therefore limited to a short, predefined 60-week timeframe instead of the recommended duration of 33 months (~143 weeks) for establishing failure, which was defined by consensus groups and adopted by clinical studies such as IITI.11,14 It cannot be ruled out that an ITI treatment period longer than 60 weeks might have allowed for more subjects to achieve tolerance, as supported by the inhibitor trends observed in this study, though this was beyond the scope of the study.
A study limitation was the low number of subjects, both overall and completing the ITI treatment period, which hampered the evaluation of results. Patients who failed previous ITI attempts may be less willing to complete another full ITI treatment period and therefore may have contributed to the high early withdrawal rate, particularly in light of newer prophylactic treatment options for inhibitor patients. The use of emicizumab was evolving during the study period; although it was not specifically reported by the investigators, some subjects who withdrew early may have switched to emicizumab. Since patients who had concurrent or prior treatment with emicizumab were excluded from this study, there remains a possibility of selection bias.
The low study success rate may be explained by the stringent endpoints, such as the limited timeframe, as well as the strict inclusion criteria which identified patients who were considered difficult to tolerise with a high-risk profile for ITI failure (including previous ITI failures, peak historical inhibitor titre >200 BU/mL, pre-ITI inhibitor titre >10 BU/mL and >5 years between inhibitor diagnosis and start of ITI).11,17,19,21
The assessments of pharmacokinetic parameters used to define success in this study and inhibitor testing were primarily conducted according to local laboratory protocols and the investigator’s discretion. This could introduce inherent variability in procedures and measurements across different sites. Although this approach reflects real-world clinical practice, it may affect the consistency and comparability of the results.
As a landmark study, there are currently no comparable data from studies that prospectively assess the same patient population as ReITIrate. The RESIST study in patients with previous ITI failure was terminated early with no results published to date. 37 It is difficult to compare ReITIrate results with other studies as they are also limited by low patient numbers and differ in patient population risk profiles, definitions of previous ITI treatment failure, ITI treatment intensity and duration and criteria for ITI success.17–19 Furthermore, while this single-arm study offers valuable insights into the potential benefits of rFVIIIFc for ITI therapy, the lack of control may restrict the generalisability of the findings.
Conclusions
In summary, from this difficult-to-treat population, 4 of 16 enrolled subjects achieved negative inhibitor titres and 1 subject achieved complete ITI success, as defined by the strict study criteria, within the limited 60-week timeframe. Further, although the experimental data had relatively little impact on the overall clinical findings, the study supports the ability to perform sensitive blood sampling for some of the immunophenotypic analyses. Final data from ReITIrate indicate a potential benefit of rFVIIIFc for ITI therapy, over a short ITI duration, in patients with previous ITI failure with no new safety concerns identified.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207241300809 for Rescue immune tolerance induction with a recombinant factor Fc-fused VIII: prospective ReITIrate study of clinical, humoral and cellular immune responses by Christoph Königs, Shannon L. Meeks, Beatrice Nolan, Anja Schmidt, Malin Löfqvist, Jennifer Dumont, Lisa Leickt, Sushrusha Nayak and Stefan Lethagen in Therapeutic Advances in Hematology
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study. The authors acknowledge Louise Edvardsson, Kathleen York and Daniela Bruni, from Sobi, for publication coordination. The authors also acknowledge Riddhi Naik, MSci, and Abbie Rogers, BSc, from Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction. This medical writing and editorial assistance was funded by Sobi and Sanofi. Some data reported in this manuscript have previously been presented at the 11th Annual Congress of the European Association for Haemophilia and Allied Disorders (EAHAD), Madrid, Spain, 6th–9th February 2018, the 10th BIC International Conference, Genoa, Italy, 6th–8th September 2019 and the International Society on Thrombosis and Haemostasis (ISTH) 2021 Congress, Virtual, 17th–21st July 2021. All authors meet the ICMJE criteria for authorship, having contributed significantly to the study’s design, data collection, analysis, manuscript drafting or reviewing, and approved the final version for publication.
Footnotes
ORCID iDs: Shannon L. Meeks  https://orcid.org/0000-0002-3683-8644
https://orcid.org/0000-0002-3683-8644
Beatrice Nolan  https://orcid.org/0000-0003-0145-4736
https://orcid.org/0000-0003-0145-4736
Stefan Lethagan  https://orcid.org/0000-0002-8436-1780
https://orcid.org/0000-0002-8436-1780
Supplementary material: Supplementary material for this article is available online.
Contributor Information
Christoph Königs, Department of Pediatrics and Adolescent Medicine, Clinical and Molecular Hemostasis, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany.
Shannon L. Meeks, Aflac Cancer and Blood Disorders Center, Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, GA, USA
Beatrice Nolan, Children’s Health Ireland at Crumlin, Dublin, Ireland.
Anja Schmidt, Department of Pediatrics and Adolescent Medicine, Clinical and Molecular Hemostasis, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany.
Malin Löfqvist, Sobi, Stockholm, Sweden.
Jennifer Dumont, Sanofi, Cambridge, MA, USA.
Lisa Leickt, Sobi, Stockholm, Sweden.
Sushrusha Nayak, Sobi, Stockholm, Sweden.
Stefan Lethagen, Sobi, Swedish Orphan Biovitrum AB, SE-112 76, Sweden.
Declarations
Ethics approval and consent to participate: The ReITIrate (NCT03103542) study protocol was approved by institutional review boards and/or ethics committees at participating institutions (Saint-Luc – UCL Hospital-Faculty Ethics Committee (Belgium): 2017/22MAY/287; Hamilton Integrated Research Ethics Board (HiREB; Canada) Project Number: 3700; UBC C&W Research Ethics Board (Canada) Number: H17-02414; CPP Ouest IV (France) Project Number: 14/18_1; Ethics Committee of the Faculty of Medicine at Goethe University (Germany) Project Number: 182/17 F; Medical Research Ethics Committee, Our Lady’s Children’s Hospital Crumlin (Ireland) REC Reference: GEN/563/17; National Medical Ethics Committee of the Republic of Slovenia (Slovenia) Reference: 0120-23/2018/8; Regional Ethics Review Board in Gothenburg (Sweden) Reference: 5.1-2018-17094; North-West – Liverpool East Research Ethics Committee (UK) Reference: 17/NW/0511; Emory University Institutional Review Board (USA) Reference: IRB00098261). Subjects/their guardians provided written informed consent prior to participation; if appropriate, adolescent/paediatric subjects also provided assent. ReITIrate was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and ethical principles that comply with the Declaration of Helsinki.
Consent for publication: Subjects/their guardians provided consent for publication; if appropriate, adolescent/paediatric subjects also provided assent.
Author contributions: Christoph Königs: Conceptualisation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Shannon L. Meeks: Visualisation; Writing – original draft; Writing – review & editing.
Beatrice Nolan: Conceptualisation; Writing – original draft; Writing – review & editing.
Anja Schmidt: Investigation; Methodology; Writing – original draft; Writing – review & editing.
Malin Löfqvist: Conceptualisation; Visualisation; Writing – original draft; Writing – review & editing.
Jennifer Dumont: Conceptualisation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Lisa Leickt: Conceptualisation; Visualisation; Writing – original draft; Writing – review & editing.
Sushrusha Nayak: Conceptualisation; Visualisation; Writing – original draft; Writing – review & editing.
Stefan Lethagen: Conceptualisation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Sobi and Sanofi. This article was based on data from the ReITIrate study (ClinicalTrials.gov identifier: NCT03103542). Sobi and Sanofi reviewed and provided feedback on the manuscript. Support for third-party writing assistance for this article, provided by Riddhi Naik, MSc, and Abbie Rogers, BSc, Costello Medical, UK, was funded by Sobi and Sanofi in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Competing interests: CK: Speaker/advisory boards for Bayer, Biotest, CSL Behring, Novo Nordisk, Roche/Chugai, Sanofi/Sobi and Takeda; institutional research support from Bayer, Biotest, CSL Behring, EU H2020 ITN, Intersero, Novo Nordisk, Pfizer, Roche/Chugai, Sanofi/Sobi and Takeda. SLM: Consulting fees from Bayer, BioMarin, CSL Behring, Genentech, Novo Nordisk, Pfizer, Sangamo, Sanofi, Spark and Takeda; research support from Genentech and Octapharma. BN: Speaker for Roche and Sobi; advisory boards for Biogen and Sobi; principal investigator for studies sponsored by Alnylam, Bayer, CSL Behring, Roche, Sanofi and Sobi. AS: Recipient of the Bayer Hemophilia Awards program. LL and SN: Employees of Sobi at the time of study. JD: Employee of Sanofi. ML and SL: Employees and shareholders of Sobi.
Availability of data and materials: Sobi is committed to responsible and ethical sharing of data on participant level and summary data for medicines and indications approved by EMA and/or FDA while protecting individual participant integrity and compliance with applicable legislation. Data access will be granted in response to qualified research requests. All requests are evaluated by a cross-functional panel of experts within Sobi and a decision on sharing will be based on the scientific merit and feasibility of the research proposal, maintenance of personal integrity and commitment to publication of the results. To request access to study data, a data-sharing request form (available on www.sobi.com) should be sent to medical.info@sobi.com. Further information on Sobi’s data sharing policy and process for requesting access can be found at: https://www.sobi.com/en/policies.
References
- 1. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood 2014; 124: 3365–3372. [DOI] [PubMed] [Google Scholar]
- 2. Ljung R, Auerswald G, Benson G, et al. Inhibitors in haemophilia A and B: management of bleeds, inhibitor eradication, and strategies for difficult-to-treat patients. Eur J Haematol 2019; 102: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Königs C, Ozelo MC, Dunn A, et al. First study of extended half-life rFVIIIFc in previously untreated patients with hemophilia A: PUPs A-LONG final results. Blood 2022; 139: 3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carcao M, Escuriola-Ettingshausen C, Santagostino E, et al. The changing face of immune tolerance induction in haemophilia A with the advent of emicizumab. Haemophilia 2019; 25: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holstein K, Albisetti M, Bidlingmaier C, et al. Practical guidance of the GTH Haemophilia Board on the use of emicizumab in patients with haemophilia A. Hamostaseologie 2020; 40: 561–571. [DOI] [PubMed] [Google Scholar]
- 6. Escuriola-Ettingshausen C, Auerswald G, Königs C, et al. Optimizing the management of patients with haemophilia A and inhibitors in the era of emicizumab: recommendations from a German expert panel. Haemophilia 2021; 27: e305–e313. [DOI] [PubMed] [Google Scholar]
- 7. Holstein K, Le Quellec S, Klamroth R, et al. Immune tolerance induction in the era of emicizumab – still the first choice for patients with haemophilia A and inhibitors? Haemophilia 2022; 28: 215–222. [DOI] [PubMed] [Google Scholar]
- 8. Coppola A, Di Minno MN, Santagostino E. Optimizing management of immune tolerance induction in patients with severe haemophilia A and inhibitors: towards evidence-based approaches. Br J Haematol 2010; 150: 515–528. [DOI] [PubMed] [Google Scholar]
- 9. Malec LM, Journeycake J, Ragni MV. Extended half-life factor VIII for immune tolerance induction in haemophilia. Haemophilia 2016; 22: 552–554. [DOI] [PubMed] [Google Scholar]
- 10. Carcao M, Shapiro A, Staber JM, et al. Recombinant factor VIII Fc fusion protein for immune tolerance induction in patients with severe haemophilia A with inhibitors: a retrospective analysis. Haemophilia 2018; 24: 245–252. [DOI] [PubMed] [Google Scholar]
- 11. DiMichele D, Hoots W, Pipe S, et al. International workshop on immune tolerance induction: consensus recommendations. Haemophilia 2007; 13: 1–22. [DOI] [PubMed] [Google Scholar]
- 12. Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence 2017; 11: 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carcao M. Changing paradigm of prophylaxis with longer acting factor concentrates. Haemophilia 2014; 20: 99–105. [DOI] [PubMed] [Google Scholar]
- 14. Hay CRM, DiMichele DM; International Immune Tolerance Study. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood 2012; 119: 1335–1344. [DOI] [PubMed] [Google Scholar]
- 15. Coppola A, Margaglione M, Santagostino E, et al. Factor VIII gene (F8) mutations as predictors of outcome in immune tolerance induction of hemophilia A patients with high-responding inhibitors. J Thromb Haemost 2009; 7: 1809–1815. [DOI] [PubMed] [Google Scholar]
- 16. DiMichele D. The North American Immune Tolerance Registry: contributions to the thirty-year experience with immune tolerance therapy. Haemophilia 2009; 15: 320–328. [DOI] [PubMed] [Google Scholar]
- 17. Kreuz W, Escuriola Ettingshausen C, Vdovin V, et al. First prospective report on immune tolerance in poor risk haemophilia A inhibitor patients with a single factor VIII/von Willebrand factor concentrate in an observational immune tolerance induction study. Haemophilia 2015; 22: 87–95. [DOI] [PubMed] [Google Scholar]
- 18. Kurth M, Puetz J, Kouides P, et al. The use of a single von Willebrand factor-containing, plasma-derived FVIII product in hemophilia A immune tolerance induction: the US experience. J Thromb Haemost 2011; 9: 2229–2234. [DOI] [PubMed] [Google Scholar]
- 19. Oldenburg J, Jimenez-Yuste V, Peiro-Jordan R, et al. Primary and rescue immune tolerance induction in children and adults: a multicentre international study with a VWF-containing plasma-derived FVIII concentrate. Haemophilia 2014; 20: 83–91. [DOI] [PubMed] [Google Scholar]
- 20. Mancuso ME, Cannavò A. Immune tolerance induction in hemophilia. Clin Investig 2015; 5: 321–335. [Google Scholar]
- 21. Valentino LA, Kempton CL, Kruse-Jarres R, et al. US Guidelines for immune tolerance induction in patients with haemophilia A and inhibitors. Haemophilia 2015; 21: 559–567. [DOI] [PubMed] [Google Scholar]
- 22. Carcao M, Shapiro A, Hwang N, et al. Real-world data of immune tolerance induction using recombinant factor VIII Fc fusion protein in patients with severe haemophilia A with inhibitors at high risk for immune tolerance induction failure: a follow-up retrospective analysis. Haemophilia 2021; 27: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krishnamoorthy S, Liu T, Drager D, et al. Recombinant factor VIII Fc (rFVIIIFc) fusion protein reduces immunogenicity and induces tolerance in hemophilia A mice. Cell Immunol 2016; 301: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Groot AS, Moise L, McMurry JA, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide ‘Tregitopes’. Blood 2008; 112: 3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kis-Toth K, Rajani GM, Simpson A, et al. Recombinant factor VIII Fc fusion protein drives regulatory macrophage polarization. Blood Adv 2018; 2: 2904–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malec L, Ragni M, Journeycake J, et al. Immune tolerance induction using rFVIIIFc (Eloctate). Blood 2015; 126: 3531. [Google Scholar]
- 27. Ragni MV, Malec LM, Journeycake JM. Durability of ITI utilizing rFVIIIFc. Blood 2016; 128: 3793. [Google Scholar]
- 28. Groomes CL, Gianferante DM, Crouch GD, et al. Reduction of factor VIII inhibitor titers during immune tolerance induction with recombinant factor VIII-Fc fusion protein. Pediatr Blood Cancer 2016; 63: 922–924. [DOI] [PubMed] [Google Scholar]
- 29. Malec LD, Van Damme A, Chan AK, et al. Recombinant factor VIII Fc fusion protein for first-time immune tolerance induction: final results of the verITI-8 study. Blood 2023; 141: 1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Collins PW, Chalmers E, Hart DP, et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: (4th edition). Br J Haematol 2013; 160: 153–170. [DOI] [PubMed] [Google Scholar]
- 31. Montalvao SA, Tucunduva AC, Siqueira LH, et al. A longitudinal evaluation of anti-FVIII antibodies demonstrated IgG4 subclass is mainly correlated with high-titre inhibitor in haemophilia A patients. Haemophilia 2015; 21: 686–692. [DOI] [PubMed] [Google Scholar]
- 32. Van Helden PMW, van den Berg HM, Gouw SC, et al. IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A. Br J Haematol 2008; 142: 644–652. [DOI] [PubMed] [Google Scholar]
- 33. Tiede A, Hofbauer CJ, Werwitzke S, et al. Anti-factor VIII IgA as a potential marker of poor prognosis in acquired hemophilia A: results from the GTH-AH 01/2010 study. Blood 2016; 127: 2289–2297. [DOI] [PubMed] [Google Scholar]
- 34. Bonnefoy A, Merlen C, Dubé E, et al. Predictive significance of anti-FVIII immunoglobulin patterns on bleeding phenotype and outcomes in acquired hemophilia A: results from the Quebec Reference Center for Inhibitors. J Thromb Haemost 2021; 19: 2947–2956. [DOI] [PubMed] [Google Scholar]
- 35. Brackmann HH, Gormsen J. Massive factor-VIII infusion in haemophiliac with factor-FVIII inhibitors, high responder. Lancet 1977; 310: 933. [DOI] [PubMed] [Google Scholar]
- 36. Mariani G, Kroner B; Immune Tolerance Study Group (ITSG). Immune tolerance in hemophilia with factor VIII inhibitors: predictors of success. Haematologica 2001; 86: 1186–1193. [PubMed] [Google Scholar]
- 37. Gringeri A. VWF/FVIII concentrates in high-risk immunotolerance: the RESIST study. Haemophilia 2007; 13: 73–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207241300809 for Rescue immune tolerance induction with a recombinant factor Fc-fused VIII: prospective ReITIrate study of clinical, humoral and cellular immune responses by Christoph Königs, Shannon L. Meeks, Beatrice Nolan, Anja Schmidt, Malin Löfqvist, Jennifer Dumont, Lisa Leickt, Sushrusha Nayak and Stefan Lethagen in Therapeutic Advances in Hematology