Abstract
Background
Psoriasis is a chronic inflammatory skin disease. The Wnt/β-catenin signaling pathway is essential for the regulation of adult stem cells, homeostasis, and tissue regeneration; however, the relationship between this pathway and interleukin (IL)-36γ in the pathogenesis of psoriasis remains unclear.
Methods
In this study, psoriasiform model mice were established using imiquimod (IMQ) induction. Hematoxylin and eosin (H&E) staining was used to evaluate pathological morphologies, while immunohistochemistry was used to verify the expression patterns of β-catenin and the inflammatory factors IL-6, IL-17 A, and interferon (IFN)-γ.
Results
IL-36γ treatment increased psoriasis area and severity index scores, and enhanced proliferation of keratinocytes in IMQ-induced psoriatic mice. The effects of IL-36γ on the severity of psoriasiform lesions and epidermal hyperplasia were partly inhibited by IWR-1, which is an inhibitor of the Wnt/β-catenin signaling pathway. Furthermore, the levels of proinflammatory cytokines and molecules involved in the Wnt/β-catenin signaling pathway in psoriatic mouse skin, including IL-6, IL-17 A, IFN-γ, β-catenin, and Dickkopf-1 (DKK1), were upregulated by treatment with IL-36γ. Consistently, the effects of IL-36γ on the inflammatory response and the Wnt/β-catenin signaling pathway were alleviated by IWR-1.
Conclusions
Taken together, our findings suggested that inhibition of the Wnt/β-catenin signaling pathway may be useful in the alleviation of IL-36γ-induced psoriasis-like lesions.
Keywords: IWR-1, Psoriasis, IL-36ʏ, Wnt/β-catenin signaling pathway
Introduction
The IL-36 family belongs to the IL-1 superfamily of cytokines, which are considered to be critical regulators of inflammation [1]. This family mainly comprises four members: IL-36α, IL-36β, IL-36γ, and IL-36Ra [2, 3]. IL-36Ra negatively impacts the IL-36 signaling pathway by competitively binding to IL-36 receptor (IL-36R) [4]. IL-36 family of cytokines are expressed in skin, gut, lung, renal, and cervical tissues. The IL-36 family of cytokines are expressed at low levels in the skin under physiological conditions, but are upregulated in keratinocytes, epithelial cells, and inflammatory monocytes/macrophages during inflammation [2]. Several stimuli can promote the expression of IL-36 cytokines, including lipopolysaccharide, viral infection, and tissue injury [5]. Blockage of IL-36R can inhibit inflammatory disorders in the skin, kidneys, and lungs [6].
Psoriasis is an immune-associated inflammatory disease characterized by scaly erythematous plaques, which mainly result from increased proliferation of keratinocytes [7]. Previous studies have demonstrated that IL-36 cytokines enhance the skin inflammation of psoriasis by promoting pro-inflammatory responses in keratinocytes, leukocyte recruitment, and angiogenesis [8]. In the IMQ-induced psoriasiform mouse model, the loss of function of IL-36Ra enhances skin lesions, whereas deficiency of IL-36R ameliorates psoriasiform dermatitis [9, 10]. The serum levels of IL-36γ have been found to be higher in psoriasis vulgaris patients than in control groups. Additionally, post-treatment serum levels of IL-36γ are downregulated compared with pre-treatment levels [11]. Previous studies showed upregulated expression of IL-36γ in the lesions of psoriasis patients [12]. Our previous study found that the clinical symptoms and histopathological characteristics of psoriasiform lesions induced by IMQ treatment in IL-36γ-deficient mice can be significantly exacerbated by IL-36γ. These findings suggest that IL-36γ plays an important role in the pathogenesis of psoriasis. However, the exact mechanism remains unclear.
Inhibition of Wnt/β-catenin signaling reportedly alleviates the severity of psoriatic inflammation in the IMQ-induced psoriasis model [13]. The pathological features of psoriasis are epidermal hyperplasia, dilated blood vessels in the dermis, and infiltration of inflammatory cells into the dermis [14]. In this study, we aimed to investigate the potential roles of IL-36γ and Wnt/β-catenin signaling in the regulation of keratinocyte proliferation.
Methods
Animals and reagents
Balb/c mice (age: 6–8 weeks) obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) were used in this study. All mice were maintained in a specific pathogen-free facility. Recombinant IL-36γ was purchased from R&D Systems (Minneapolis, MN, USA). IWR-1 was purchased from Sigma (St. Louis, MO, USA). An area of about 2 cm × 3 cm on the back of each mouse was shaven and treated with a daily topical dose of IMQ cream (Aldara cream, 3 M Pharmaceuticals, UK) or Vaseline for 5 consecutive days. The mice were randomly divided into the following five groups (n = 4 per group): Vaseline, IMQ model, IMQ model treated with IWR-1 (10 mg/Kg on days 1, 3, 5), IMQ model treated with recombinant IL-36γ (2 µg/mouse on days 1, 3, 5), and IMQ model treated with IWR-1 and IL-36γ (IWR-1 subcutaneously injected 2 h prior to IL-36γ exposure).
Psoriasis area and severity index (PASI) assessment
Erythema, scaling, and thickening were scored independently from 0 to 4 using the following PASI-based scale: 0, none; 1, slight; 2, moderate; 3, severe; 4, very severe. The total score (0–12) was used to evaluate the severity of psoriatic inflammation.
Histological analysis
Mice from each group were sacrificed via cervical dislocation. Samples of back skin were fixed in 4% paraformaldehyde solution for 48 h, dehydrated, and embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin (H&E) for histological analysis. Histopathological epidermal thickness was evaluated using NDP.view software (Hamamatsu Photonics, Hamamatsu, Japan).
For immunohistochemistry analyses, sections were incubated with primary antibodies against IL-6 (Abcam, Cambridge, MA, USA), IL-17A (Abcam), and IFN-γ (Abcam), β-catenin (Abcam), and DKK1(Abcam), followed by secondary biotinylated monoclonal antibodies, and processed using staining kits (Proteintech, Rosemont, IL, USA). A semiquantitative scoring system (H-score method; range: 0–300) based on the percentage of cells observed under microscopy at different staining intensities was applied to quantify the immunohistochemical staining intensity. H-score analysis was performed by two experienced pathologists in a double-blinded fashion.
Statistical analysis
Data are presented as means ± standard deviation. SPSS 21.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) were used for statistical analysis. Statistical analysis was performed using the Student’s t-test or the Mann–Whitney test. A P value < 0.05 was considered to indicate statistical significance.
Results
IWR-1 ameliorates IL-36γ-mediated exacerbation of psoriatic skin lesions in an IMQ-induced mouse model
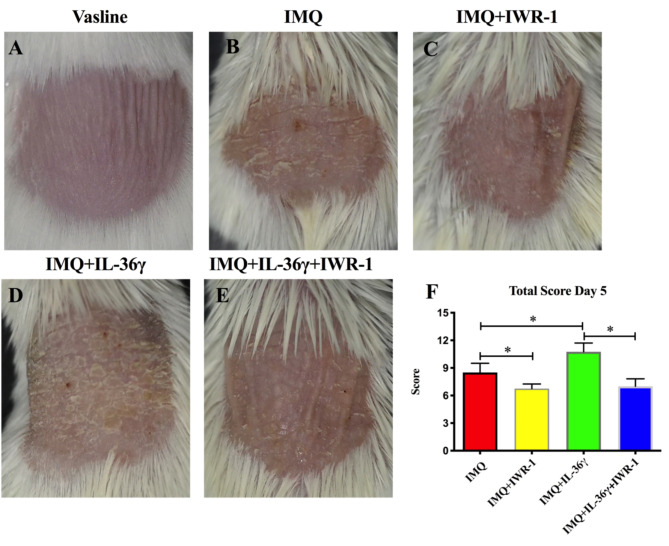
IMQ-induced psoriasis-like mice were used to evaluate the effects of IL-36γ on psoriasis-like skin inflammation. Vaseline (control) or Aldara (IMQ) cream was smeared on the shaved back skin of wild-type mice for 5 consecutive days, with or without subcutaneous injection of IL-36γ and IWR-1. As shown in Fig. 1B, compared with the Vaseline group (Fig. 1A), the IMQ-treated group developed psoriasis-like symptoms. The effects of IMQ were inhibited by subcutaneous injection of IWR-1 (Fig. 1C). While the subcutaneous administration of IL-36γ aggravated the characteristics of psoriasis, including erythema, thickening, and scaling (Fig. 1D), these effects were inhibited by subcutaneous injection of IWR-1 (Fig. 1E). In general, the IL-36γ-treated groups showed the highest PASI scores, which were decreased by administration of IWR-1(Fig. 1F).
Fig. 1.
Aggravated symptoms of psoriasis in imiquimod (IMQ)-induced mice treated with interleukin (IL)-36γ. Groups of mice were treated with Vaseline (A), IMQ (B), IMQ and IWR-1 (IMQ + IWR-1; C), IMQ and IL-36γ (IMQ + IL-36γ; D), or IMQ, IWR-1, and IL-36γ (IMQ + IWR-1 + IL-36γ; E). (F) Total psoriasis area and severity index (PASI) scores, including individual scales (0–4) for erythema, scaling, and thickness in each group. *P < 0.05
Ameliorative effects of IWR-1 administration on pathological changes in IL-36γ-induced psoriasiform skin lesions
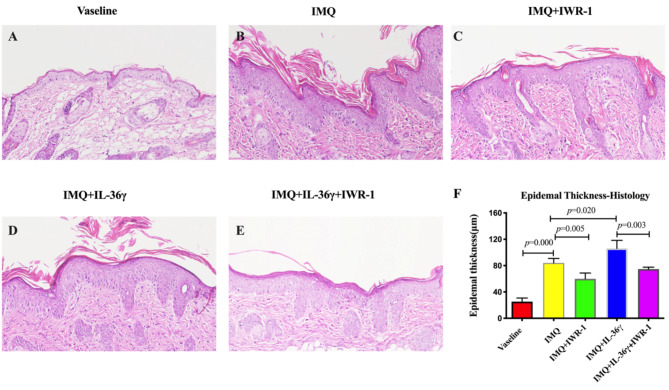
To compare the pathological changes in lesions among the groups, H&E-stained sections were analyzed. Compared with the Vaseline group, the IMQ-treated group exhibited increased epidermal thickening (Fig. 2A, B), with keratinocyte thickness measurements of 20.30 (± 5.45) µm and 84.08 (± 6.79) µm in the two groups, respectively. The effect of IMQ on hyperplasia was significantly diminished in the IMQ + IWR-1 group (Fig. 2C), which exhibited keratinocyte thickness of 59.88 (± 8.91) µm, in contrast to 106.10 (± 12.29) µm in the IMQ + IL-36γ group (Fig. 2D) and 74.78 (± 2.84) µm in the IMQ + IL-36γ + IWR-1 group (Fig. 2E). In short, IL-36γ treatment significantly increased epidermal thickness in IMQ-treated mice, but could be reduced by IWR-1 treatment (Fig. 2F).
Fig. 2.
Increased epidermal thickness in imiquimod (IMQ)-induced model mice treated with interleukin (IL)-36γ. Representative hematoxylin and eosin-stained sections (magnification: 200×) from the Vaseline (A), IMQ (B), IMQ and IWR-1 (IMQ + IWR-1; C), IMQ and IL-36γ (IMQ + IL-36γ; D), and IMQ, IWR-1, and IL-36γ (IMQ + IWR-1 + IL-36γ; E) groups. (F) Calculated epidermal thickness. Data expressed as means ± standard deviation; * P < 0.05
IWR-1 reverses IL-36γ-mediated upregulation of inflammatory factors in psoriatic lesions of IMQ-induced mice
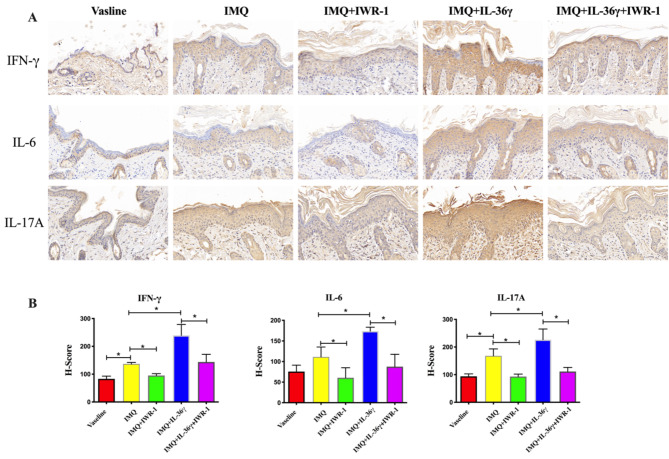
We further investigated the role of IL-36γ in the inflammatory reaction in IMQ-induced psoriasiform dermatitis. Compared with the Vaseline group, the expression of IL-17 A and IFN-γ were significantly increased in IMQ group, but there is no difference between Vaseline group and IMQ group in the expression of IL-6. Subcutaneous injection of IL-36γ at day 1, 3, 5, concomitantly to topical IMQ application, increase the expression of IL-6, IL-17 A and IFN-γ in epidermal layer. As shown in Fig. 3, reduced expression of IL-6, IL-17 A and IFN-γ was also observed after IWR-1 treatment.
Fig. 3.
Effect of interleukin (IL)-36γ on inflammatory cytokines in psoriatic-like skin tissues. (A, B) Immunohistochemical staining of IL-6, IL-17A, and interferon (IFN)-γ in imiquimod (IMQ)-induced skin lesions (magnification: 200×; A) and H-scores in each experimental group (B); *P < 0.05
IL-36γ exposure leads to increased expression of β-catenin and DKK1 in IMQ-induced mice
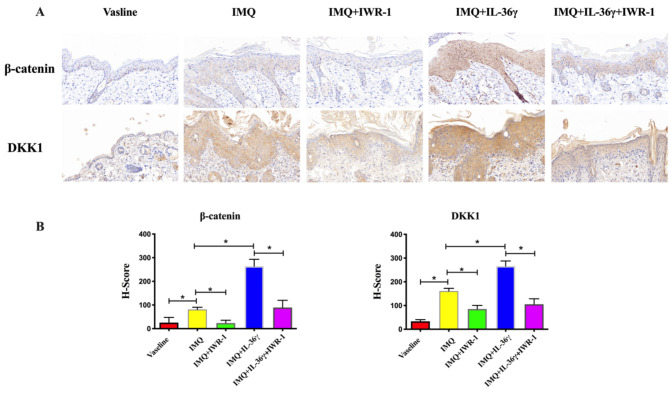
To explore the potential role of Wnt/β-catenin signaling in the inflammatory process, we examined the protein expression levels of β-catenin and DKK1 following exposure to IL-36γ and/or IWR-1. As shown in Fig. 4, compared with the Vaseline group, expression of β-catenin and DKK1 increased significantly in the IMQ-treated group. Further significant increases in β-catenin and DKK1 expression in IL-36γ-exposed IMQ model mice were reversed by treatment with IWR-1.
Fig. 4.
IWR-1-mediated attenuation of the interleukin (IL)-36γ exposure-induced elevations in epidermal β-catenin and DKK1 in imiquimod (IMQ)-induced model mice. Immunohistochemistry staining to determine the protein expressions of β-catenin and DKK1 in IMQ or Vaseline-treated back skin (magnification 200×) (A) and H scores in each experimental group (B). Data expressed as means ± standard deviation; * P < 0.05
Discussion
The keratinocyte is the predominant cell type of human skin. It is well established that the abnormal proliferation and differentiation of keratinocytes plays an important role in the pathogenesis of psoriasis [15]. In this study, we found that IMQ-induced psoriasis-like skin inflammation was significantly exacerbated by IL-36γ treatment. Furthermore, IL-36γ-induced exacerbation of skin lesions was attenuated by administration of an inhibitor of Wnt/β-catenin signaling. Histopathologically, IMQ-treated mice exhibited increased epidermal thickness that was worsened by treatment with IL-36γ, while suppressed by the inhibition of Wnt/β-catenin signaling. Our study highlights the potential of IL-36γ as a pro-psoriatic factor and suggests that the effects of IL-36γ on this mouse model of psoriasis might be ameliorated by inhibitors of Wnt/β-catenin signaling.
Psoriasis is a chronic skin disease that presents as uncontrolled keratinocyte proliferation and pathological differentiation. Biomedical and immunological disturbances are important in the pathogenesis of psoriasis [16]. The IL-36 family, which belongs to IL-1 superfamily, was discovered in 2000. Previous studies showed that IL-36 plays a significant role in innate and adaptive immune responses, transmitting signals through the MAPK and NF-κB signaling pathways [17]. Furthermore, in both the acute and chronic phases of psoriasis, the activities of IL-36 pathways are increased [18]. Some psoriasis patients exhibit the Koebner phenomenon, which refers to the occurrence of new lesions in normal skin following skin injury [19]. Researchers previously discovered that plasmacytoid dendritic cells can infiltrate the skin rapidly after skin injury, and that IFN-α derived from these cells may play a crucial role in triggering psoriasis [20]. IL-36 cytokines enhance the expression of systemic IFN-I [19, 21]. Therefore, the IL-36 family may play a role in the Koebner phenomenon in psoriasis. Previous studies showed that the increased expression of IL-36 cytokines in psoriasis-like model mice was reduced by vitamin D supplementation or corticosteroid treatment [22, 23]. The microvessels in psoriatic lesions are elongated, widened, and tortuous, and hypervascularization of psoriatic lesions has been positively correlated with disease severity [24, 25]. Furthermore, IL-36γ has been shown to activate angiogenesis [24]. In this study, we observed that the severity of psoriasis and expression levels of IL-6, IL-17A, and IFN-γ were significantly increased by IL-36γ treatment, with IWR-1 attenuating the promotional effects of IL-36γ. These findings suggested that IL-36γ may promote the proliferation and inflammatory response of keratinocytes. However, the role of Wnt/β-catenin signaling in this process deserves further investigation.
The Wnt family of proteins, which are divided into canonical and non-canonical signaling pathways, play important roles in cell regeneration, growth, division, migration, and polarity [26]. DKK1 is a well-characterized inhibitor and, together with β-catenin, is a key molecule in the canonical Wnt pathway. Previous studies of β-catenin yielded conflicting results, with reports of both increased and decreased expression of β-catenin in biopsy specimens from psoriasis lesions [27–29]. In our study, we observed significantly increased expression of β-catenin and DKK1 in psoriatic lesions compared with that in control skin samples, indicating possible activation of the canonical pathway in psoriasis model mice. Furthermore, treatment with IL-36γ further increased the expression of β-catenin and DKK1. We surmised that DKK1, as an inhibitor of the Wnt/β-catenin canonical signaling pathway, was upregulated secondarily to the elevation of β-catenin. Increased expression of both β-catenin and DKK1 was related to the severity of psoriasis in our psoriasis mice. Nevertheless, the relationship between IL-36γ and Wnt/β-catenin signaling requires further study.
In conclusion, our study further established the importance of IL-36γ and reinforced the involvement of the Wnt/β-catenin signaling pathway in the disease pathogenesis of psoriasis. Further studies are needed to elucidate the exact role of the Wnt/β-catenin signaling pathway and the molecular mechanism of the crosstalk between IL-36γ and the Wnt/β-catenin signaling pathway in psoriasis.
Acknowledgements
Not applicable.
Author contributions
All the authors contributed to this manuscript. WW, XZ and HJ designed the experiments; WW performed the experiments; WW and YG analyzed the data; WW wrote the paper. HJ reviewed the manuscript. All authors read and approved the final manuscript.
Funding
We received grants from the following: National Key R&D Program of China (2022YFC3601800), National High Level Hospital Clinical Research Funding (2022-PUMCH-A-251), Beijing Natural Science Foundation (7242109) and National Key Clinical Specialty Project of China.
Data availability
The data used in the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
This study protocols were approved by Animal Ethics committee of Peking Union Medical College Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Han Y, Huard A, Mora J, da Silva P, Brune B, Weigert A. IL-36 family cytokines in protective versus destructive inflammation. Cell Signal. 2020;75:109773. [DOI] [PubMed] [Google Scholar]
- 2.Ngo VL, Kuczma M, Maxim E, Denning TL. IL-36 cytokines and gut immunity. Immunology. 2021;163(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Yu X, Wu C, Jin H. IL-36gamma inhibits differentiation and induces inflammation of keratinocyte via wnt signaling pathway in psoriasis. Int J Med Sci. 2017;14(10):1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe S, Iwata Y, Fukushima H, Saito K, Tanaka Y, Hasegawa Y, Akiyama M, Sugiura K. Neutrophil extracellular traps are induced in a psoriasis model of interleukin-36 receptor antagonist-deficient mice. Sci Rep. 2020;10(1):20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster AM, Baliwag J, Chen CS, Guzman AM, Stoll SW, Gudjonsson JE, Ward NL, Johnston A. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J Immunol. 2014;192(12):6053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neurath MF. IL-36 in chronic inflammation and cancer. Cytokine Growth Factor Rev. 2020;55:70–9. [DOI] [PubMed] [Google Scholar]
- 7.Nussbaum L, Chen YL, Ogg GS. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br J Dermatol. 2021;184(1):14–24. [DOI] [PubMed] [Google Scholar]
- 8.Iznardo H, Puig L. Exploring the role of IL-36 cytokines as a New Target in Psoriatic Disease. Int J Mol Sci 2021, 22(9). [DOI] [PMC free article] [PubMed]
- 9.Tortola L, Rosenwald E, Abel B, Blumberg H, Schafer M, Coyle AJ, Renauld JC, Werner S, Kisielow J, Kopf M. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest. 2012;122(11):3965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahil SK, Catapano M, Di Meglio P, Dand N, Ahlfors H, Carr IM, Smith CH, Trembath RC, Peakman M, Wright J et al. An analysis of IL-36 signature genes and individuals with IL1RL2 knockout mutations validates IL-36 as a psoriasis therapeutic target. Sci Transl Med. 2017;9(411). [DOI] [PubMed]
- 11.Chen J, Du J, Han Y, Wei Z. Correlation analysis between IL-35, IL-36gamma, CCL27 and psoriasis vulgaris. J Dermatolog Treat. 2021;32(6):621–4. [DOI] [PubMed] [Google Scholar]
- 12.D’Erme AM, Wilsmann-Theis D, Wagenpfeil J, Holzel M, Ferring-Schmitt S, Sternberg S, Wittmann M, Peters B, Bosio A, Bieber T, et al. IL-36gamma (IL-1F9) is a biomarker for psoriasis skin lesions. J Invest Dermatol. 2015;135(4):1025–32. [DOI] [PubMed] [Google Scholar]
- 13.Xue Y, Liu Y, Bian X, Zhang Y, Li Y, Zhang Q, Yin M. Mir-205-5p inhibits psoriasis-associated proliferation and angiogenesis: Wnt/beta-catenin and mitogen-activated protein kinase signaling pathway are involved. J Dermatol. 2020;47(8):882–92. [DOI] [PubMed] [Google Scholar]
- 14.Tokuyama M, Mabuchi T. New Treatment addressing the pathogenesis of Psoriasis. Int J Mol Sci. 2020;21(20). [DOI] [PMC free article] [PubMed]
- 15.Ni X, Lai Y. Keratinocyte: a trigger or an executor of psoriasis? J Leukoc Biol. 2020;108(2):485–91. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Song K, Hiebert P, Werner S, Kim TG, Kim YS. Tussilagonone ameliorates psoriatic features in keratinocytes and Imiquimod-Induced Psoriasis-Like lesions in mice via NRF2 activation. J Invest Dermatol. 2020;140(6):1223–e12321224. [DOI] [PubMed] [Google Scholar]
- 17.Byrne J, Baker K, Houston A, Brint E. IL-36 cytokines in inflammatory and malignant diseases: not the new kid on the block anymore. Cell Mol Life Sci. 2021;78(17–18):6215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugiura K, Fujita H, Komine M, Yamanaka K, Akiyama M. The role of interleukin-36 in health and disease states. J Eur Acad Dermatol Venereol 2024. [DOI] [PubMed]
- 19.Ji YZ, Liu SR. Koebner phenomenon leading to the formation of new psoriatic lesions: evidences and mechanisms. Biosci Rep. 2019;39(12). [DOI] [PMC free article] [PubMed]
- 20.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202(1):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catapano M, Vergnano M, Romano M, Mahil SK, Choon SE, Burden AD, Young HS, Carr IM, Lachmann HJ, Lombardi G, et al. IL-36 promotes systemic IFN-I responses in severe forms of Psoriasis. J Invest Dermatol. 2020;140(4):816–e826813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.German B, Wei R, Hener P, Martins C, Ye T, Gottwick C, Yang J, Seneschal J, Boniface K, Li M. Disrupting the IL-36 and IL-23/IL-17 loop underlies the efficacy of calcipotriol and corticosteroid therapy for psoriasis. JCI Insight. 2019;4(2). [DOI] [PMC free article] [PubMed]
- 23.Towne JE, Sims JE. IL-36 in psoriasis. Curr Opin Pharmacol. 2012;12(4):486–90. [DOI] [PubMed] [Google Scholar]
- 24.Bridgewood C, Fearnley GW, Berekmeri A, Laws P, Macleod T, Ponnambalam S, Stacey M, Graham A, Wittmann M. IL-36gamma is a strong inducer of IL-23 in psoriatic cells and activates angiogenesis. Front Immunol. 2018;9:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosina P, Giovannini A, Gisondi P, Girolomoni G. Microcirculatory modifications of psoriatic lesions during topical therapy. Skin Res Technol. 2009;15(2):135–8. [DOI] [PubMed] [Google Scholar]
- 26.Ismaeel A, Alhashimi F, Almossali Z, Alshaikh S, Selvam S, Janahi D. Immunohistochemical expression of vitamin D receptor and wnt signaling pathway molecules in psoriasis. Acta Dermatovenerol Alp Pannonica Adriat. 2023;32(4):129–33. [PubMed] [Google Scholar]
- 27.Farag AGA, Shoaib MA, Samaka RM, Abdou AG, Mandour MM, Ibrahim RAL. Progranulin and beta-catenin in psoriasis: an immunohistochemical study. J Cosmet Dermatol. 2019;18(6):2019–26. [DOI] [PubMed] [Google Scholar]
- 28.Gudjonsson JE, Johnston A, Stoll SW, Riblett MB, Xing X, Kochkodan JJ, Ding J, Nair RP, Aphale A, Voorhees JJ, et al. Evidence for altered wnt signaling in psoriatic skin. J Invest Dermatol. 2010;130(7):1849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hampton PJ, Ross OK, Reynolds NJ. Increased nuclear beta-catenin in suprabasal involved psoriatic epidermis. Br J Dermatol. 2007;157(6):1168–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the current study are available from the corresponding author on reasonable request.