Abstract
Background
The causal relationships between sexually transmitted infections, depression, and self-harm remain unclear.
Methods
We executed various Mendelian Randomization (MR) analyses. At the same time, a cross-sectional analysis from NHANES was used for verification and an enrichment analysis was also utilized to explore the potential common gene functions.
Results
We found that STIs may have a potential causal effect on depression (P = 0.002) and self-harm (P = 0.003). Conversely, self-harm has been identified as a risk factor for the acquisition of STIs (P = 0.006), while there is no evidence to support an effect of depression on STIs. Furthermore, mediation MR indicated that monocyte absolute count played a mediating role in the association between STIs and depression, accounting for 7.7%. And then, the weighted regression analysis of the cross-sectional analysis demonstrated a significant association between one of the common STIs, HPV, and depression. Gene enrichment analysis suggested that the PI3K-Akt signalling pathway and the infectious virus signalling pathway may represent a common underlying pathogenesis.
Conclusion
STIs may increase the risk of depression and self-harm, while self-harm might also represent a risk factor for STIs, which could provide insights and a foundation for the control of STIs and mental health monitoring in clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10218-1.
Keywords: Depression, Self-harm, Sexually transmitted infections, Mendelian randomization, NHANES
Introduction
Sexually transmitted infections (STIs, or sexually transmitted diseases (STDs)) have traditionally been associated with the specific range of diseases, including syphilis, HPV, HIV, gonorrhoea and so on [1]. And even, all diseases that are primarily transmitted through sexual contact are included in a broad sense [2, 3].
STIs constitute a variety of challenges to public health in the clinical arena, due to prolonged duration, high recurrence rate, and widespread prevalence [4, 5]. Especially, we really have observed that visits to the clinic by patients of STDs are frequently accompanied by psycho-emotional problems of varying degrees. And multiple studies reported also support this. A small sample cohort from the UK and Greece studied the life quality of individuals affected by HIV/AIDS, genital warts, or genital herpes, and found that depressive symptoms increased among patients in both STIs cohorts [6]. And some researchers have reported that men diagnosed with AIDS or those who are HIV-positive are at a higher risk of self-harm compared with those who are undiagnosed [7, 8]. Moreover, a cross-sectional survey of 103 young individuals aged 15 to 25 from the mental health clinics revealed that young people with mental health disorders have a strong demand for sexual health services [9]. However, some observational studies were limited by reverse causation, residual confounding, or small sample size [10, 11]. And at present, the relationship between STIs and psychological disorders still lack of definitive genetic causal research.
Mendelian Randomization (MR) methods are being increasingly used to explore the causes of complex diseases [12]. These methods use genetic variation to explore causal relationships. It usually uses single nucleotide polymorphisms (SNPs) as an instrumental variable (IV) [13]. This can reduce the traditional causal and mixed deviation in observational studies, which provide more reliable basis for establishing a causal relationship [14, 15].
In this study, we aimed to use MR methods to evaluate the genetic factors of STIs and depression, self-harm, and to gain insight into their potential biological associations, which provide perspective and theoretical basis for their clinical prevention.
Method
Study design
At first, we conducted bidirectional MR analyses to investigate the causal relationships between STIs, depression and self-harm. Additionally, we performed Multivariable MR (MVMR) analyses to minimize bias, as well as two-step MR analyses to explore potential mediating factors [16, 17]. Furthermore, we utilized data from the National Health and Nutrition Examination Survey (NHANES) to conduct a cross-sectional analysis on the association between classic STIs and depression to further validat their relationship. The data of this cross-sectional survey are sourced from the National Center for Health Statistics (NCHS) and can be accessed at https://www.cdc.gov/nchs/index.htm. MR analysis was performed by using summary-level GWAS data and NHANES individual-level data. The study flow is illustrated in Figure S1. This research employed publicly available online data and did not involve ethical review.
Sources of MR data
The dataset for depression (ebi-a-GCST90018833) were obtained from the UK Biobank; the SNPs of self-harm (finn-b-VWXY20_SUICI_OTHER_INTENTI_SELF_H) and STDs (FINN-B-AB1_SEXUAL_TRANSMISSION) were sourced from the FinnGen biobank. Detailed information about the data can be found in Table S1.
Selection of IVs
Only SNPs that met the genome-wide significance threshold (P < 5e-6 to 5e-8) were deemed potential instrumental variables (IVs). The SNPs with P < 5e-8 were used as IVs in causal effect of Self-Harm on STIs and reverse MR analysis (causal effect of STIs on Depression, Self-Harm). The reason we used P < 5e-6 is that there are not enough SNPs to execute analysis in P < 5e-8, which is supported in some of the published literature [18, 19]. Independent SNPs were chosen based on linkage disequilibrium (LD) criteria (kb = 10,000, r² < 0.001). At the same time, the strength of the IVs was assessed using F-statistics (F > 10), ensuring a robust correlation between the identified IVs and the exposure factors. Afterward, palindromic SNPs were excluded, and the exposure-outcome datasets were harmonized to avoid any distortion in strand orientation or allele coding.
MR analysis
Bidirectional MR analysis was conducted to assess potential causal relationships between STIs and depression, as well as self-harm. The primary method used for this evaluation was the inverse variance-weighted (IVW) approach, as it yields reliable causal estimates in the absence of directional pleiotropy. In addition, MR-Egger and Weighted Mode methods were used as supplementary analyses. To investigate the genetic links among exposure variables and their effects on outcomes, an MVMR analysis based on the IVW method was performed. The mediation effect of immune cells was examined through a two-step MR approach, utilizing data on 731 immune cells obtained from the IEU Open GWAS dataset, covering IDs GCST90001391 to GCST90002121 based on a sample of 3757 Europeans [20]. Among the 731 immune cells, 118 represented absolute cell counts, 389 reflected the median fluorescence intensity, 32 were morphological parameters, and 192 were relative cell counts. The mediation effect was calculated as Beta = Beta (ZY) * Beta (XZ) (let Beta (ZY) represent the estimated causal effect of exposure immune cells on outcome depression, Beta (XZ) represent the estimated causal effect of exposure STIs on outcome immune cells). The proportion of this mediation effect within the total effect was expressed as R = Beta / Beta (XY) * 100%, with the direct effect defined as Direct Effect = Beta (XY) – Beta (let Beta (XY) represent the estimated causal effect of exposure STIs on outcome depression) (Figure S1 A). To evaluate potential heterogeneity or horizontal pleiotropy, we employed MR-Egger, Cochran’s Q statistic and MR-PRESSO. Additionally, funnel plots and leave-one-out analyses were included as part of the sensitivity analysis.
NHANES database
The NHANES is a nationally representative research that includes interviews, physical examinations, and laboratory tests, releasing data biennially since 1999. In this study, we utilized NHANES data from 1999 to 2004, incorporating depression survey responses and HPV (types 6, 11, 16, and 18) infection data. The inclusion criteria was all participants of NHANES data from 1999 to 2004. And exclusion criteria as follows: (1) individuals with missing HPV tests; (2) individuals with missing depression score.
The Composite International Diagnostic Interview (CIDI) is a fully structured diagnostic interview used to assess psychiatric disorders following criteria defined in the fourth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders. The diagnoses of depression are designated as part of NHANES data-processing and the dichotomous diagnosis variables were in the NHANES downloaded datasets. A value of “1” indicates a positive diagnosis, and a value of “5” indicates a negative diagnosis [21]. Competitive Luminex assay was used for HPV 6, 11, 16, 18 in NHANES laboratory data, and value description is positive and negative. Based on prior research and clinical expertise, the following variables were included in the present study: age, gender, race/ethnicity, educational attainment, and street drug use were selected. All covariate data were derived from demographic and questionnaire responses.
Gene enrichment analysis
To identify potential targets related to sexually transmitted diseases, depression, and self-harm, we retrieved data from the GeneCards database using keywords (“depression,” “suicide,” and “HPV”) [22]. The intersection of these targets was obtained using Venny 2.1.0. Further analysis of potential targets was conducted using the WEB-based Gene Set Analysis Toolkit for disease enrichment, alongside R’s cluster profile package for GO and KEGG pathway enrichment.
Statistical analysis
A dataset of 6 years (1999–2004) from NHANES was utilized. We used the weighted analysis as recommended by the NCHS Analysis Guide to maintain national representation [23]. Categorical variables were listed as frequencies, and continuous variables that followed a normal distribution were presented as mean ± standard error. Both univariate and multivariate regression analyses were carried out to evaluate the relationship between HPV and depression. All statistical analyses were conducted using R software 4.2 and EmpowerStats [24]. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. A p-value of less than 0.05 was deemed statistically significant.
Results
Causal effect of depression and self-harm on STIs
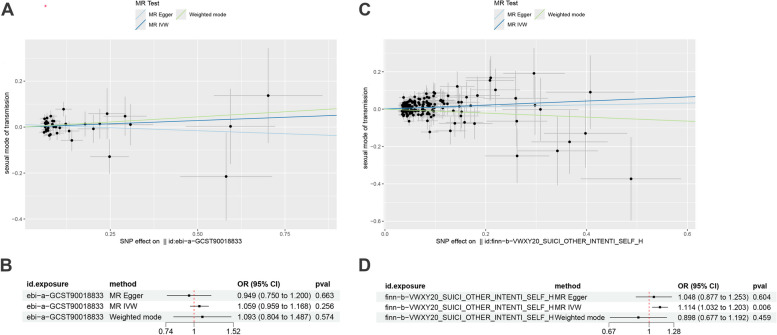
Two-sample MR analysis indicates that the causal effect of depression on the risk of STIs was not statistically significant (IVW: OR = 1.059, 95% CI: 0.959–1.168, P = 0.256) (Fig. 1A, B). However, the primary IVW analysis (OR = 1.114, 95% CI: 1.032–1.203, P = 0.006) indicates a potential causal effect of self-harm on STIs (Fig. 1C, D). To minimize bias, we conducted a MVMR analysis, which still showed a significant association between self-harm and increased risk of STIs (OR = 1.119, 95% CI: 1.022–1.224, P = 0.016), while depression showed no correlation with STIs (Table 1). Detailed information about the IVs of Depression and Self-Harm can be found in Supporting Information Table S2−3. There is some evidence that the effect detected by IVW may be in part explained by horizontal pleiotropy since supposedly pleiotropy-robust methods such as MR-mode had causal effect estimates of opposite signs. And sensitivity analysis did not reveal significant heterogeneity or horizontal pleiotropy in Table S4. Detailed information about the IVs used in the MR analysis can be found in Supporting Information Tables S2–S3. Funnel plots and leave-one-out results are available in Figure S2.
Fig. 1.
Scatter plots and forest plot for causal effect of Depression (A, B), Self-Harm (C, D) on STIs
Table 1.
The result of multivariable mendelian randomization
| Exposure | Outcome | Pvalue | OR 95%CI |
|---|---|---|---|
| Depression | Sexually transmitted infection | 0.222 | 1.030(0.983–1.080) |
| Suicide or other Intentional self-harm | Sexually transmitted infection | 0.016 | 1.119(1.022–1.224) |
OR odds ratio, CI confidence interval
Causal effect of STIs on depression, self-harm
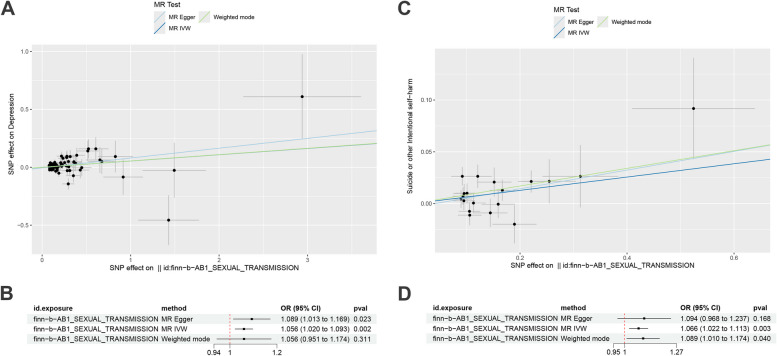
Reverse MR analysis suggests that STIs potentially have a causal effect on depression (MR-Egger: OR = 1.089, 95% CI: 1.013–1.169, P = 0.023; IVW: OR = 1.056, 95% CI: 1.020–1.093, P = 0.002; although the Weighted mode did not show a significant P-value, the trend was similar) (Fig. 2A, B). STIs also potentially affect self-harm (IVW: OR = 1.066, 95% CI: 1.022–1.113, P = 0.002; Weighted mode: OR = 1.089, 95% CI: 1.010–1.174, P = 0.040; while MR-Egger did not yield significant P-values, the trend remained consistent) (Fig. 2C, D). Detailed information about the IVs involved in the reverse MR can be found in Supporting Information Table S5. Sensitivity analyses did not reveal significant heterogeneity or horizontal pleiotropy (Table S4); funnel plots and leave-one-out results are available in Figure S2.
Fig. 2.
Scatter plots and forest plot for causal effect of STIs on Depression (A, B), Self-Harm (C, D)
Monocytes could mediate the association between STIs and depression
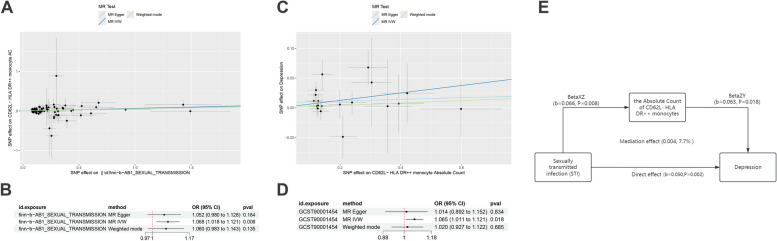
A two-step mediation MR analysis was conducted. First, MR analysis of STIs and 731 immune cell types was performed, revealing that STIs only significantly promote the Absolute Count of CD62L- HLA DR + + monocytes (OR = 1.068, 95% CI: 1.018–1.121, P = 0.008) (Fig. 3A, B). The subsequent MR analysis also indicated that the Absolute Count of CD62L- HLA DR + + monocytes had a significant positive effect on depression (Fig. 3C, D). MR results of depression with other immune cells in Figure S3. The Q statistic measuring pleiotropy was greater than 0.05 in both models, indicating no horizontal pleiotropy in these analyses (Table S4). So, the Absolute Count of CD62L- HLA DR + + monocytes mediate the association between STDs and depression, with a mediation effect of 0.004 (7.7% of the total effect) and a direct effect of 0.050 (Fig. 3E).
Fig. 3.
Mediation mendelian randomization analysis. Scatter plots (A) and forest plot (B) for causal effect of STIs on CD62L- HLA DR + + monocytes Absolute Count; Scatter plots (C) and forest plot (D) for causal effect of CD62L- HLA DR + + monocytes Absolute Count on depression. (E), schematic diagram of mediation analysis
Cross-sectional analysis
After inclusion and exclusion criteria, this study comprised 648 NHANES participants. Table S6 reports the baseline characteristics of participants under different depression conditions, showing significant differences in HPV infection rates between depressed and non-depressed subjects (P < 0.05). Table 2 displays results from weighted logistic regression analysis. The weighted logistic regression results indicate that in model I (unadjusted model), most HPV types (6, 16, 18) are positively correlated with depression risk (P < 0.05). The results remain similar in model II, adjusted for age, gender, race; while model III, further adjusted for education and street drug, only showed correlation in HPV 06 (P < 0.05). In Table 3, Subgroup analysis revealed that the increased risk of depression associated with HPV infection is more statistically significant in younger group (age < 30). The age was considered the interactive factors that affect the relationship between sectional HPV (6, 16) and depression by the interaction analysis.
Table 2.
Weighted regression analysis of depression risk in NHANES
| HPV-positive | Model I | Model II | Model III |
|---|---|---|---|
| OR (95%CI) P-value | |||
| HPV 06 | 3.51 (1.83, 6.71) 0.0020 | 3.18 (1.56, 6.49) 0.0131 | 2.98 (1.48, 5.98) 0.0279 |
| HPV 11 | 1.35 (0.38, 4.79) 0.6491 | 1.09 (0.33, 3.60) 0.8852 | 1.06 (0.33, 3.40) 0.9318 |
| HPV 16 | 2.76 (1.28, 5.94) 0.0213 | 2.20 (0.92, 5.23) 0.1127 | 2.11 (0.96, 4.66) 0.1236 |
| HPV 18 | 4.07 (1.71, 9.68) 0.0068 | 3.59 (1.27, 10.18) 0.0430 | 2.31 (0.69, 7.73) 0.2318 |
The mode l was not adjusted for covariates. Model II was adjusted for Age, Gender, Race. Model III was adjusted for Age, Gender, Race, Education, Street drug. NHANES National Health and Nutrition Examination Survey, OR odds ratio, CI confidence interval
Table 3.
The association between HPV and depression in different age group
| Subgroup | HPV 6-positive | HPV 11-positive | HPV 16-positive | HPV 18-positive |
|---|---|---|---|---|
| OR (95%CI) P-value | ||||
| Age < 30 | 11.06 (3.65, 33.44) 0.0011 | 3.37 (0.46, 24.73) 0.2554 | 8.03 (2.31, 27.97) 0.0067 | 14.08 (2.27, 87.26) 0.0148 |
| Age ≥ 30 | 1.19 (0.40, 3.59) 0.7616 | 1.81 (0.39, 8.39) 0.4468 | 1.17 (0.38, 3.62) 0.7940 | 2.28 (0.76, 6.84) 0.1666 |
| P-interaction | 0.0090 | 0.2749 | 0.0277 | 0.1090 |
OR odds ratio, CI confidence interval
Enrichment analysis
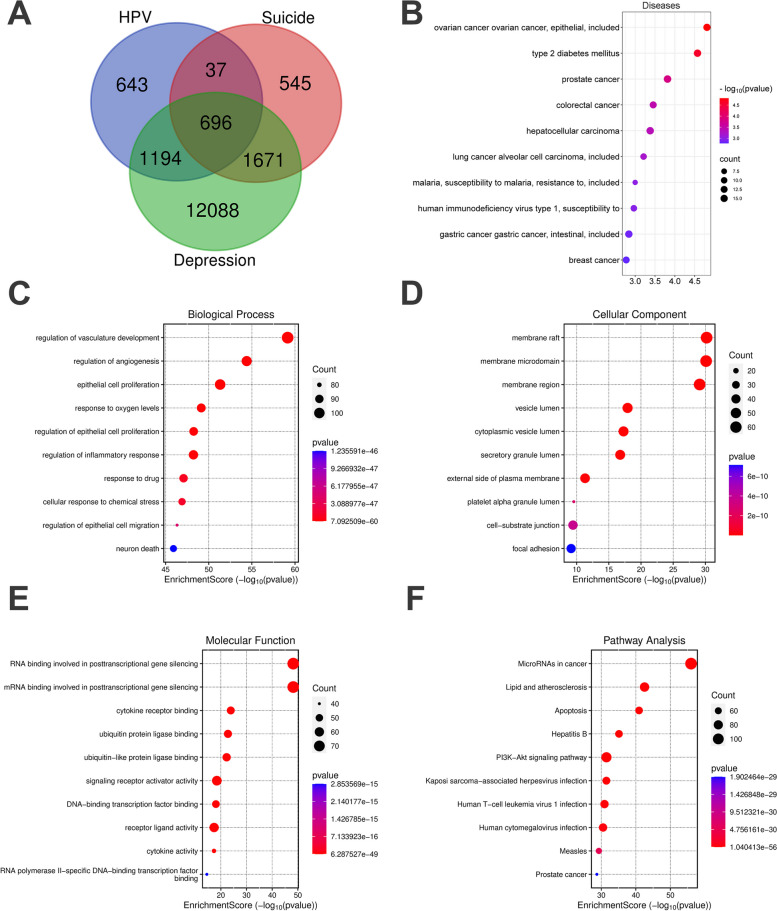
Functional enrichment analysis further revealed shared gene functions between HPV and both depression and self-harm. It was identified a total of 696 common genes (Fig. 4A). BP enrichment primarily highlighted epithelial cell proliferation, regulation of inflammatory responses, and neuronal death (Fig. 4C); CC enrichment results mostly pointed to membrane rafts, membrane microdomains, and membrane regions (Fig. 4D); MF enrichment indicated signal transduction activities such as signaling receptor activator, DNA-binding transcription factor binding, and receptor-ligand activity (Fig. 4E). In KEGG analysis, pathways related to Kaposi sarcoma-associated herpesvirus infection, Human T-cell leukemia virus 1 infection, and Human cytomegalovirus infection were implicated (Fig. 4F). On the other hand, DO enrichment analysis suggested that common genes are primarily involved in ovarian cancer, breast cancer, and human immunodeficiency virus type 1 et al. (Fig. 4B).
Fig. 4.
Enrichment analysis. A The common genes of HPV, depression and suicide. B The top 10 items of diseases enrichment (C) The top 10 items of GO biological process. D The top 10 items of GO cellular component. E The top 10 items of GO molecular function. F The top 10 signal pathways
Discussion
STDs and psychological disorders are significant public health issues that warrant attention and prevention globally [25]. Notably, in the routine daily clinical consultations, we observe that patients with STIs exhibit varying degrees of emotional problems [26]; and effective disorders are also risk factors for high-risk sexual behavior [27]. However, there is currently no genetic evidence linking them. Therefore, this study aims to investigate the exact causal relationship between STIs and depression/self-harm using methods such as MR, thereby shedding light on this pressing clinical issue.
To our knowledge, this research is the first to analyze the bidirectional association between STIs and the risks of depression and self-harm by combining large-scale genetic data with extensively observational research data. In this study, our MR results indicate that STIs can increase the risk of depression and self-harm. And in the findings from forward MR and MVMR, self-harm, as an exposure factor, can also increase the risk of STIs. It was supported in previous research too. Wu D. et al. reported that a clinical trial focusing on men who have sex with men (MSM) revealed that this population exhibits elevated levels of anxiety and depression. Furthermore, anxiety was found to correlate with lower educational attainment, limited knowledge regarding HIV, and additional factors such as a history of STIs [28]. A retrospective study from Boston, Massachusetts, involving 368 female participants, noted that women diagnosed with STDs had increased odds of bipolar disorder and utilization of outpatient mental health counseling services, compared to whom with no history of STDs [29]. But our study benefits from a large sample size, minimizing the impact of confounding factors on the results. It is important that in this study, we included IVs with F-statistics greater than 10 to avoid weak instrument bias. Additionally, several sensitivity analyses, including MR-Egger, horizontal pleiotropy, MR-PRESSO, and leave-one-out analysis, were employed to validate the reliability of our results. Interestingly, depression showed no correlation with STIs in our results. However, we also noted that previous research has offered differing viewpoints: a cross-sectional survey targeting non-institutionalized U.S. civilians aged 18–25 indicated that individuals with a history of depression have an increased risk of acquiring STDs [30]. Potential factors contributing to this discrepancy include: first, the variation in ethnic groups studied; our research focused on individuals of European descent rather than those of American descent. Additionally, adolescents represent a specific demographic, and we did not further stratify by age.
To further explore the connection between STIs and depression, we conducted a two-step mediation MR analysis. Our findings suggest that CD62L- HLA DR + + monocyte cells may facilitate the impact of STIs on depression, with a mediation proportion of 7.7%. Current insights into psychiatric inflammation indicate that immune disturbances triggered by infections can particularly promote psychopathology, increasing psychological stress related to potentially fatal illnesses, as well as inflammation associated with stress [31–33]. The interaction between the innate and adaptive immune systems and neurotransmitters is recognized as a mechanism underlying emotional disorders, psychosis, and depression [34, 35]. In STIs, such as HPV and HIV infections, the patients’ innate immune defenses are activated, with monocytes/macrophages being key components [36]. In the treatment of genital warts, imiquimod works by inducing monocyte/macrophage secretion of cytokines (interferon-α, interleukin-12, tumor necrosis factor-α) to inhibit viral replication [37]. On the other hand, a prospective cohort study from IRCCS San Raffaele Hospital assessed psychiatric symptoms in 402 COVID-19 survivors, revealing that the baseline systemic immune inflammation index (SII) was positively correlated with depression and anxiety scores at follow-up [38]. These highlights the role of monocytes in emotional disorders. Thus, combining our results, we conclude that monocytes may mediate the impact of STIs on depression. However, further empirical studies are needed to elucidate the specific molecular mechanisms involved.
Our cross-sectional analysis results further support the aforementioned conclusions from MR. Weighted regression analysis indicates that HPV (types 6, 11, and 18) is a risk factor for depression. HPV, a well-known DNA virus family, significantly contributes to sexually transmitted diseases, leading to conditions such as genital warts, cervical cancer, and skin warts [39]. Graziottin et al. has highlighted that the personal psychological vulnerability increases with the frequency of HPV infections, and simultaneously depression, anxiety, and anger will be the most commonly reported emotional responses [40]. A cross-sectional study on other STIs has drawn similar conclusions. Type 2 herpes simplex virus is associated with an increased risk of depression, and this is also true within HIV-susceptible populations [41, 42]. Moreover, to verify the robustness of the results, we conducted a subgroup analysis. Although HPV infection increases the risk of depression, this association is statistically significant only in the age < 30 group; however, the trend is consistent across both groups, indicating the generalizability of the findings. But we advocate for greater attention to younger populations on this issue, particularly adolescents. They are sexually active and tend to be relatively psychologically immature, making them more susceptible to harm.
Additionally, we conducted bioinformatics analyses to investigate the shared genetic mechanisms between HPV, depression, and self-harm. Enrichment results indicated that shared genes are primarily involved in viral infectious diseases (including HIV) and urogenital tumors (such as ovarian cancer). This is understandable and indicates that the identified genes are indeed relevant to this topic. Furthermore, in terms of signaling pathways, these genes significantly relate to the PI3K-Akt signaling pathway and infectious viral signaling pathways (like cytomegalovirus). Studies have shown that the PI3K/AKT/mTOR signaling cascade plays a critical role in the interplay between HPV-positive cancers and viral host cells, particularly with the activation of AKT and mTOR by HPV’s E6 and E7 oncogenes [43]. Additionally, research suggests that women co-infected with HIV-1 can replicate PI3K-AKT pathway mutations to enhance the carcinogenic effects of HPV compared with those not infected with HIV [44]. On the other hand, the PI3K-AKT signaling pathway is essential in the brain and may contribute to the pathogenesis of depression through its involvement in anti-inflammatory processes, neurogenesis, and synaptic plasticity [45]. These findings align with our results, indicating possible directions for the future foundational research, such as the contribution of HPV to depression via PI3K-AKT signaling pathway.
Limitations
However, our study has some limitations. Firstly, our findings are based solely on European and American populations, which restricts the generalizability to other demographics. But it is worth mentioning that it is the acknowledged limitation in many previous studie [46, 47]. P < 5e-6 IVs in MR may be more bias towards the null from estimation error (since the ratio of effect size to variance is smaller than it is when using P < 5e-8), but that this will not affect their power to detect a causal effect [48, 49]. Additionally, this study only encompasses a collection of STIs. Given the extensive spectrum of the STDs, our study seeks to investigate the clinical issue from a macro-level and a holistic perspective. Future research should involve large-scale and rich datasets, as well as prospective cohort studies to achieve more comprehensive results.
Conclusion
In conclusion, STIs could increase the risk of depression and self-harm, while self-harm also serves as a significant risk factor for STIs. Furthermore, monocytes might play a role in enhancing the impact of STIs on depression. Therefore, in clinical practice, we should conduct mental health assessments, monitor patients with STIs, and provid psychological education and guidance. Additionally, it is essential to offer necessary sexual education to populations with psychological disorders to prevent STIs.
Supplementary Information
Acknowledgements
We would like to express our gratitude to Professor Tao Feng from Hunan University, as well as the reviewers and editor for their contributions to this study.
Abbreviations
- STIs
Sexually transmitted infections
- STDs
Sexually transmitted diseases
- AIDS
Acquired immune deficiency syndrome
- HIV
Human immunodeficiency virus
- MR
Mendelian randomization
- SNPs
Single nucleotide polymorphisms
- IV
Instrumental variable
- MVMR
Multivariable mendelian randomization
- NHANES
National health and nutrition examination survey
- NCHS
National center for health statistics
- GWAS
Genome-wide association studies
- LD
Linkage disequilibrium
- IVW
Inverse variance-weighted
- OR
Odds ratios
- CI
Confidence intervals
- HPV
Human papilloma virus
- GO
Gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- BP
Biological process
- CC
Cellular component
- MF
Molecular function
- DO
Disease ontology
- SII
Systemic immune inflammation index
- CIDI
Composite international diagnostic interview
Authors’ contributions
W. Y. and X. H. designed the study and do for conceptualization and investigation. S. P. performed the formal and data analysis. J. D. writed the manuscript. Y. Z., Y. L. and Z. C. collated the data and literature. S. P. and J.D. contributed equally to this work. All authors reviewed the manuscript and agree to submit the article for publication.
Funding
Nonoe.
Data availability
The data are available in the Supporting Information of this article, and also can be downloaded from Internet datasets.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shixiong Peng and Jia Deng contributed equally to this work.
Contributor Information
Wenjie Yan, Email: ywj716@qq.com.
Xi Huang, Email: 1352215665@qq.com.
References
- 1.Kuehn BM. Resurgence of sexually transmitted diseases in the US. JAMA. 2022;327:1951. 10.1001/jama.2022.7483. [DOI] [PubMed] [Google Scholar]
- 2.Schneede P, Tenke P, Hofstetter AG. Sexually transmitted diseases (STDs)--a synoptic overview for urologists. Eur Urol. 2003;44:1–7. 10.1016/s0302-2838(03)00193-3. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs W, Brockmeyer NH. Sexually transmitted infections. J Der Deutschen Dermatologischen Gesellschaft = J German Soc Dermatology: JDDG. 2014;12:451–63. 10.1111/ddg.12310. [DOI] [PubMed] [Google Scholar]
- 4.Samkange-Zeeb FN, Spallek L, Zeeb H. Awareness and knowledge of sexually transmitted diseases (STDs) among school-going adolescents in Europe: a systematic review of published literature. BMC Public Health. 2011;11: 727. 10.1186/1471-2458-11-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adolescent TLC. Youth STIs: an epidemic fuelled by shame. Lancet Child Adolesc Health. 2022;6:353. 10.1016/s2352-4642(22)00128-6. [DOI] [PubMed] [Google Scholar]
- 6.Ginieri-Coccossis M, Triantafillou E, Papanikolaou N. Quality of life and depression in chronic sexually transmitted infections in UK and Greece: the use of WHOQOL-HIV/STI BREF. Psychiatriki. 2018;29(3):209–19. 10.22365/jpsych.2018.293.209. PMID: 30605425. [DOI] [PubMed] [Google Scholar]
- 7.Dannenberg AL, McNeil JG, Brundage JF, Brookmeyer R. Suicide and HIV infection. Mortality follow-up of 4147 HIV-seropositive military service applicants. JAMA. 1996;276:1743–6. 10.1001/jama.276.21.1743. [DOI] [PubMed] [Google Scholar]
- 8.Rukundo GZ, Levin J, Mpango RS, Patel V, Kinyanda E. Effect of suicidality on clinical and behavioural outcomes in HIV positive adults in Uganda. PLoS One. 2021;16(8): e0254830. 10.1371/journal.pone.0254830. PMID: 34415901; PMCID: PMC8378732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vayngortin T, Clark K, Hollenbach K. Sexual history documentation and screening in adolescent females with suicidal ideation in the emergency department. Int J Environ Res Public Health. 2022;19:19. 10.3390/ijerph192013018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeitoun T, El-Sohemy A. Using mendelian randomization to study the role of iron in health and disease. Int J Mol Sci. 2023;24(17): 13458. 10.3390/ijms241713458. PMID: 37686261; PMCID: PMC10487635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory biomarkers and risk of schizophrenia: a 2-sample mendelian randomization study. JAMA Psychiatry. 2017;74(12):1226–33. 10.1001/jamapsychiatry.2017.3191. PMID: 29094161; PMCID: PMC6583386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrology: JASN. 2016;27:3253–65. 10.1681/asn.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson SC, Butterworth AS, Burgess S. Mendelian randomization for cardiovascular diseases: principles and applications. Eur Heart J. 2023;44:4913–24. 10.1093/eurheartj/ehad736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birney E. Mendelian randomization. Cold Spring Harbor Perspect Med. 2022;12. 10.1101/cshperspect.a041302. [DOI] [PMC free article] [PubMed]
- 15.Weng H, Li H, Zhang Z, Zhang Y, et al. Association between uric acid and risk of venous thromboembolism in east Asian populations: a cohort and mendelian randomization study. Lancet Reg Health West Pac. 2023;39: 100848. 10.1016/j.lanwpc.2023.100848. PMID: 37565068; PMCID: PMC10410163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harbor Perspect Med. 2021;11. 10.1101/cshperspect.a038984. [DOI] [PMC free article] [PubMed]
- 17.Carter AR, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36:465–78. 10.1007/s10654-021-00757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, et al. Association between periodontitis and temporomandibular joint disorders. Arthritis Res Ther. 2023;25. 10.1186/s13075-023-03129-0. [DOI] [PMC free article] [PubMed]
- 19.Cai Y, et al. Association of mTORC1–dependent circulating protein levels with cataract formation: a mendelian randomization study. BMC Genomics. 2022;23:719. 10.1186/s12864-022-08925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orrù V, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52:1036–45. 10.1038/s41588-020-0684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouchard MF, Bellinger DC, Weuve J, Matthews-Bellinger J, Gilman SE, Wright RO, Schwartz J, Weisskopf MG. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults. Arch Gen Psychiatry. 2009;66(12):1313–9. 10.1001/archgenpsychiatry.2009.164. PMID: 19996036; PMCID: PMC2917196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Z, et al. Causality between allergic diseases and kidney diseases: a two-sample mendelian randomization study. Front Med. 2024;11: 1347152. 10.3389/fmed.2024.1347152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang R, Yan L, Lei Y. The relationship between high-density lipoprotein cholesterol (HDL-C) and glycosylated hemoglobin in diabetic patients aged 20 or above: a cross-sectional study. BMC Endocr Disord. 2021;21(1):198. 10.1186/s12902-021-00863-x. PMID: 34635098; PMCID: PMC8507179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang BF, Xu SL, Yang Z, Xu P. Early admission is better-the time to admission (TTA) is associated with one-year mortality in hip fracture. Int J Surg (London England). 2024. 10.1097/js9.0000000000001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang DL, et al. Neighborhood environment, sexual risk behaviors and acquisition of sexually transmitted infections among adolescents diagnosed with psychological disorders. Am J Community Psychol. 2010;46:303–11. 10.1007/s10464-010-9352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu L, et al. Longitudinal trajectories of emotional problems and unmet mental health needs among people newly diagnosed with HIV in China. J Int AIDS Soc. 2019;22: e25332. 10.1002/jia2.25332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adan Sanchez AY, et al. High-risk sexual behaviour in young people with mental health disorders. Early Interv Psychiat. 2019;13:867–73. 10.1111/eip.12688. [DOI] [PubMed] [Google Scholar]
- 28.Wu D, et al. Study on anxiety and depression of men who have sex with men: an application of group-based trajectory model. Front Psychol. 2022;13: 857203. 10.3389/fpsyg.2022.857203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reisner SL, et al. Sexually transmitted disease (STD) diagnoses and mental health disparities among women who have sex with women screened at an urban community health center, Boston, MA, 2007. Sex Transm Dis. 2010;37(5–12). 10.1097/OLQ.0b013e3181b41314. [DOI] [PMC free article] [PubMed]
- 30.Jenkins WD, Botchway A. Young adults with depression are at increased risk of sexually transmitted disease. Prev Med. 2016;88:86–9. 10.1016/j.ypmed.2016.03.020. Epub 2016 Apr 4 PMID: 27058942. [DOI] [PubMed] [Google Scholar]
- 31.Mellon SH, Gautam A, Hammamieh R, Jett M, Wolkowitz OM. Metabolism, metabolomics, and inflammation in posttraumatic stress disorder. Biol Psychiatry. 2018;83:866–75. 10.1016/j.biopsych.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:9. 10.3389/fnins.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat Rev Neurol. 2019;15:317–28. 10.1038/s41582-019-0174-4. [DOI] [PubMed] [Google Scholar]
- 34.Lauten TH, Natour T, Case AJ. Innate and adaptive immune system consequences of post-traumatic stress disorder. Auton Neurosci. 2024;252: 103159. 10.1016/j.autneu.2024.103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medina-Rodriguez EM, Lowell JA, Worthen RJ, Syed SA, Beurel E. Involvement of innate and adaptive immune systems alterations in the pathophysiology and treatment of depression. Front Neurosci. 2018;12: 547. 10.3389/fnins.2018.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nijhawan P, et al. Systemic, mucosal immune activation and psycho-sexual health in ART-Suppressed women living with HIV: evaluating biomarkers and environmental stimuli. Viruses. 2023;15:15. 10.3390/v15040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hengge UR, Cusini M. Topical immunomodulators for the treatment of external genital warts, cutaneous warts and molluscum contagiosum. Br J Dermatol. 2003;149:15–9. 10.1046/j.0366-077x.2003.05623.x. [DOI] [PubMed] [Google Scholar]
- 38.Mazza MG, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain M, et al. Immuno-pathogenesis, immune escape mechanisms and vaccine evaluation for HPV-associated carcinogenesis. Pathogens. 2023;12. 10.3390/pathogens12121380. [DOI] [PMC free article] [PubMed]
- 40.Graziottin A, Serafini A. HPV infection in women: psychosexual impact of genital warts and intraepithelial lesions. J Sex Med. 2009;6:633–45. 10.1111/j.1743-6109.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 41.Pratt LA, Xu F, McQuillan GM, Robitz R. The association of depression, risky sexual behaviours and herpes simplex virus type 2 in adults in NHANES, 2005–2008. Sex Transm Infect. 2012;88(1):40–4. 10.1136/sextrans-2011-050138. Epub 2011 Nov 5. PMID: 22057015. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Huang Y, Peng J, Tang R, Luo B, Xia Z. Association between depression and HIV infection vulnerable populations in United States adults: a cross-sectional analysis of NHANES from 1999 to 2018. Front Public Health. 2023;11: 1146318. 10.3389/fpubh.2023.1146318. PMID: 37325316; PMCID: PMC10267355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bossler F, Hoppe-Seyler K, Hoppe-Seyler F. PI3K/AKT/mTOR signaling regulates the virus/host cell crosstalk in HPV-positive cervical cancer cells. Int J Mol Sci. 2019;20. 10.3390/ijms20092188. [DOI] [PMC free article] [PubMed]
- 44.Olwal CO, et al. Network modeling suggests HIV infection phenocopies PI3K-AKT pathway mutations to enhance HPV-associated cervical cancer. Mol Omics. 2023;19:538–51. 10.1039/d3mo00025g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo N, et al. PI3K/AKT signaling pathway: molecular mechanisms and therapeutic potential in depression. Pharmacol Res. 2024;206: 107300. 10.1016/j.phrs.2024.107300. [DOI] [PubMed] [Google Scholar]
- 46.Ran B, Zhang Y, Wu Y, Wen F. Association between depression and COPD: results from the NHANES 2013–2018 and a bidirectional mendelian randomization analysis. Expert Rev Respir Med. 2023;17(11):1061–8. 10.1080/17476348.2023.2282022. Epub 2023 Dec 26 PMID: 38085600. [DOI] [PubMed] [Google Scholar]
- 47.Wang M, Jian Z, Ma Y, Jin X, Li H, Wang K. Depression increases the risk of kidney stone: results from the national health and nutrition examination survey 2007–2018 and mendelian randomization analysis. J Affect Disord. 2022;312:17–21 Epub 2022 Jun 9. PMID: 35691420. [DOI] [PubMed] [Google Scholar]
- 48.Lorincz-Comi N, Yang Y, Li G, Zhu X. MRBEE: A bias-corrected multivariable Mendelian randomization method. HGG Adv. 2024;5(3):100290. 10.1016/j.xhgg.2024.100290. [DOI] [PMC free article] [PubMed]
- 49.Ye T, Shao J, Kang H. Debiased inverse-variance weighted estimator in two-sample summary-data mendelian randomization. Anna Stat. 2021;49(4):2079–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available in the Supporting Information of this article, and also can be downloaded from Internet datasets.