Abstract
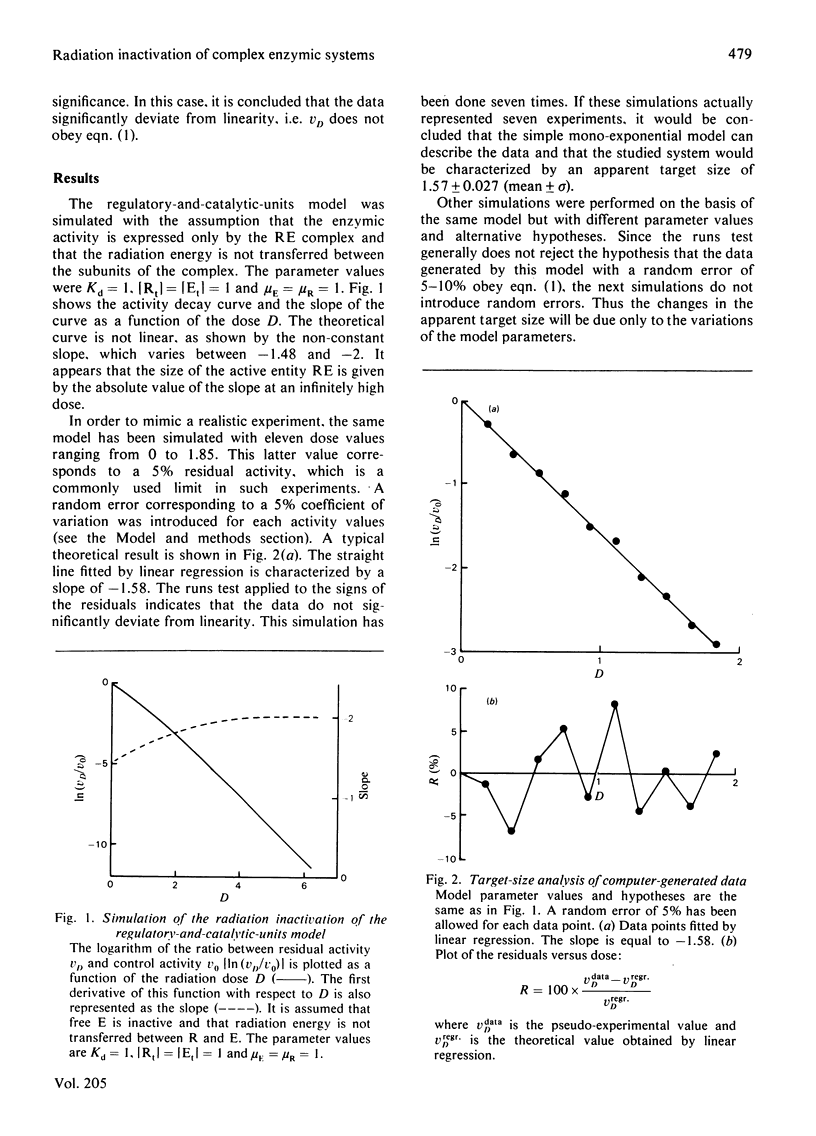
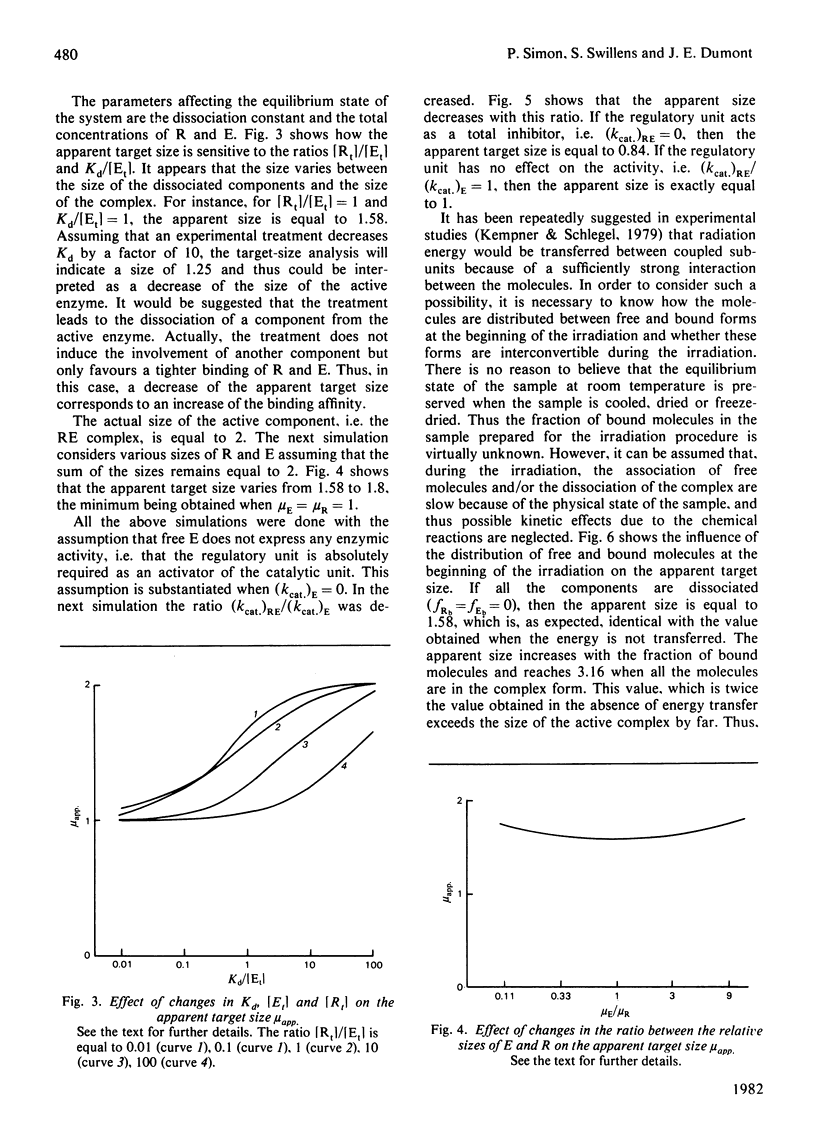
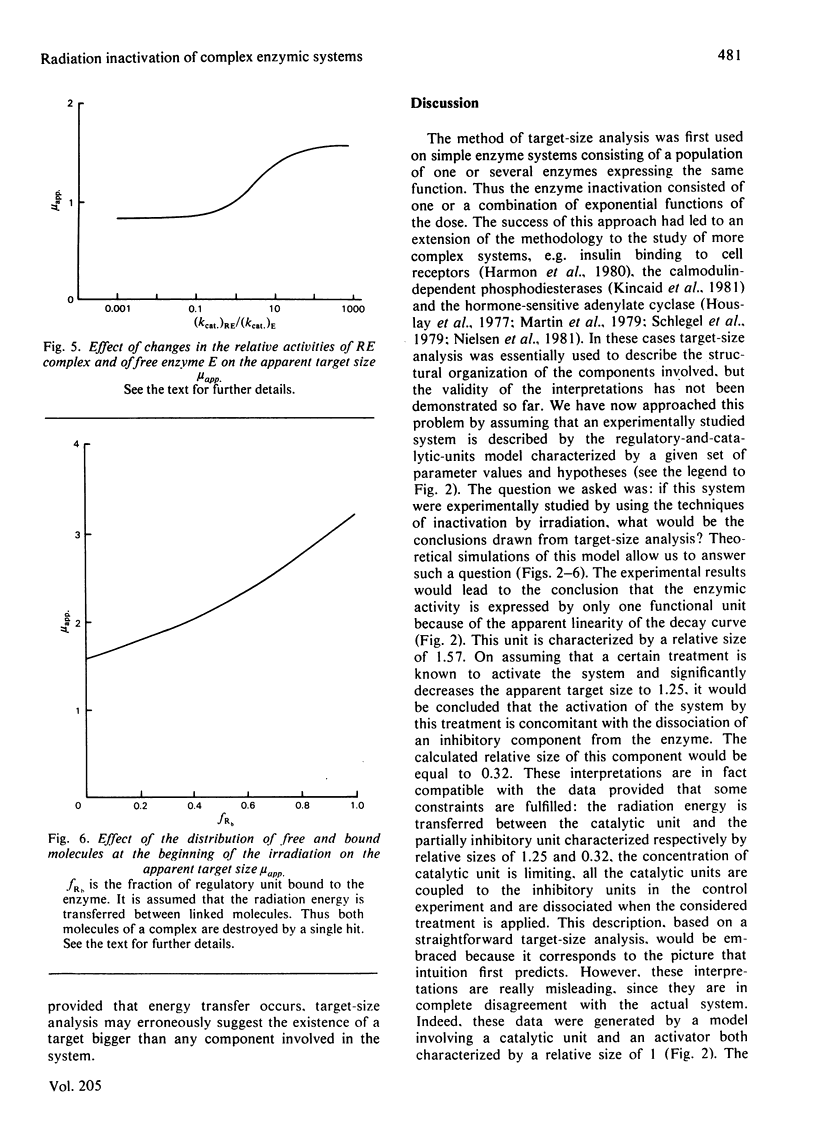
Radiation inactivation of complex enzymic systems is currently used to determine the enzyme size and the molecular organization of the components in the system. We have simulated an equilibrium model describing the regulation of enzyme activity by association of the enzyme with a regulatory unit. It is assumed that, after irradiation, the system equilibrates before the enzyme activity is assayed. Our theoretical results show that the target-size analysis of these numerical data leads to a bad estimate of the enzyme size. Moreover, some implicit assumptions such as the transfer of radiation energy between non-covalently bound molecules should be verified before interpretation of target-size analysis. It is demonstrated that the apparent target size depends on the parameters of the system, namely the size and the concentration of the components, the equilibrium constant, the relative activities of free enzyme and enzymic complex, the existence of energy transfer, and the distribution of the components between free and bound forms during the irradiation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harmon J. T., Kahn C. R., Kempner E. S., Schlegel W. Characterization of the insulin receptor in its membrane environment by radiation inactivation. J Biol Chem. 1980 Apr 25;255(8):3412–3419. [PubMed] [Google Scholar]
- Houslay M. D., Ellory J. C., Smith G. A., Hesketh T. R., Stein J. M., Warren G. B., Metcalfe J. C. Exchange of partners in glucagon receptor-adenylate cyclase complexes. Physical evidence for the independent, mobile receptor model. Biochim Biophys Acta. 1977 Jun 2;467(2):208–219. doi: 10.1016/0005-2736(77)90197-3. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Schlegel W. Size determination of enzymes by radiation inactivation. Anal Biochem. 1979 Jan 1;92(1):2–10. doi: 10.1016/0003-2697(79)90617-1. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Kempner E., Manganiello V. C., Osborne J. C., Jr, Vaughan M. Calmodulin-activated cyclic nucleotide phosphodiesterase from brain. Relationship of subunit structure to activity assessed by radiation inactivation. J Biol Chem. 1981 Nov 10;256(21):11351–11355. [PubMed] [Google Scholar]
- Martin B. R., Stein J. M., Kennedy E. L., Doberska C. A., Metcalfe J. C. Transient complexes. A new structural model for the activation of adenylate cyclase by hormone receptors (guanine nucleotides/irradiation inactivation). Biochem J. 1979 Nov 15;184(2):253–260. doi: 10.1042/bj1840253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T. B., Lad P. M., Preston M. S., Kempner E., Schlegel W., Rodbell M. Structure of the turkey erythrocyte adenylate cyclase system. Proc Natl Acad Sci U S A. 1981 Feb;78(2):722–726. doi: 10.1073/pnas.78.2.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Kempner E. S., Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J Biol Chem. 1979 Jun 25;254(12):5168–5176. [PubMed] [Google Scholar]
- Swillens S., Dumont J. E. A pitfall in the interpretation of data on adenylate cyclase inactivation by irradiation. FEBS Lett. 1981 Nov 2;134(1):29–31. doi: 10.1016/0014-5793(81)80543-1. [DOI] [PubMed] [Google Scholar]


