Abstract
Background
Gonorrhea, induced by Neisseria gonorrhoeae infection, stands as a prevalent sexually transmitted inflammatory disease globally. Our earlier research illuminated that N. gonorrhoeae-infected macrophages provoke inflammation by activating the intracellular sensor NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome, a pivotal regulator in inflammatory diseases governing the maturation and secretion of interleukin (IL)-1β and IL-18. Nevertheless, effective therapies addressing N. gonorrhoeae-mediated NLRP3 inflammasome activation and ensuing inflammation are currently lacking. This study delves into the impact of the angiotensin II receptor antagonist Candesartan (CS) on N. gonorrhoeae-infected macrophages.
Methods
The protein expression levels were examined through ELISA and Western blotting. Intracellular H2O2 levels, mitochondrial reactive oxygen species, and mitochondrial membrane integrity were evaluated using targeted fluorescent probes and analyzed via flow cytometry. NF-κB transcriptional activity was assessed using NF-κB reporter cells. LC3-knockdown cells were created using CRISPR/Cas9 technology.
Results
CS effectively inhibits the NLRP3 inflammasome, as indicated by the suppression of caspase-1 activation, IL-1β secretion, NLRP3 release, and the release of apoptosis-associated speck-like protein containing a CARD (ASC) in N. gonorrhoeae-infected J774A.1 macrophages. Additionally, CS selectively impedes IL-6 secretion and iNOS expression in both N. gonorrhoeae-infected J774A.1 and RAW264.7 macrophages. Mechanistic insights uncover the inhibition of NF-κB by CS in N. gonorrhoeae-infected J774A.1 macrophages, while intracellular H2O2 generation, mitogen-activated protein kinases phosphorylation, and mitochondrial damage remain unaffected. Notably, our study highlights that CS-induced autophagy contributes partially to its inhibitory effect on the NLRP3 inflammasome.
Conclusions
These results underscore the potential of CS as an anti-inflammatory drug for the treatment of gonorrhea, addressing a critical unmet medical need in combating N. gonorrhoeae-induced inflammation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10208-3.
Keywords: Angiotensin II receptor antagonist, Candesartan, NLRP3 inflammasome, Neisseria gonorrhoeae
Background
Gonorrhea, a disease with origins dating back to the 17th century, currently ranks as the second most prevalent sexually transmitted infection globally, trailing only behind Chlamydia trachomatis infections. The emergence of multidrug-resistant strains of Neisseria gonorrhoeae has posed significant public health challenges worldwide and is identified as a priority pathogen by the WHO [1]. Surprisingly, each year, over 82 million individuals grapple with the burdensome consequences of urethritis and cervicitis resulting from N. gonorrhoeae infections [2]. The transmission of N. gonorrhoeae involves the invasion of columnar epithelial cells lining the genitourinary tract or anorectal mucosa, typically facilitated through intimate sexual contact. This invasion triggers a robust immune response, characterized by the aggregation of polymorphonuclear leukocytes and the release of various defensive substances, including peroxides, lysozymes, antimicrobial peptides, and a variety of cytokines [3]. In men, the intrusion of N. gonorrhoeae leads to acute urethral inflammation, resulting in telltale symptoms such as dysuria, painful urination, and the discharge of pus. In certain cases, complications may arise, including parametritis, prostatitis, or epididymitis. On the other hand, women often experience cervicitis, cervicovaginal discharge, and irregular non-menstrual bleeding as a consequence of N. gonorrhoeae infection. In more severe cases, this can progress to pelvic inflammatory disease, and potentially lead to dire outcomes such as ectopic pregnancy and infertility [4].
Interleukin (IL)-1β, primarily synthesized by activated macrophages, plays a crucial role in coordinating intricate inflammatory responses. Its precursor, proIL-1β, is cleaved into the active IL-1β form by the protease caspase-1. Inflammasomes, including NOD-like receptor family pyrin domain-containing 3 (NLRP3), NOD-like receptor family pyrin domain-containing 1 (NLRP1), NLR family CARD domain containing 4 (NLRC4), and absent in melanoma 2 (AIM2) inflammasomes, regulate this sequence of events [5]. Among these, the NLRP3 inflammasome’s role in the development of inflammatory diseases has garnered significant research attention. Comprising NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1, the full activation of the NLRP3 inflammasome depends on both priming and activation signals. These signals involve various molecules and pathways, including reactive oxygen species (ROS), mitogen-activated protein kinases (MAPKs), NF-κB for priming, and P2 × 7 receptor-mediated potassium efflux, as well as mitochondrial damage for activation [6]. Our previous study revealed that N. gonorrhoeae infection initiates the priming signal, leading to NLRP3 and proIL-1β expression through the NF-κB and MAPKs pathways. Subsequently, N. gonorrhoeae’s role in the activation signal culminates in caspase-1 activation within the NLRP3 inflammasome [7].
Inhibiting the NLRP3 inflammasome or reducing the inflammatory responses caused by N. gonorrhoeae infection has the potential to alleviate the symptoms of gonorrhea. The discovery of drugs or compounds that can mitigate N. gonorrhoeae-induced inflammatory responses has garnered the attention of researchers; however, no promising results have been reported as of yet. Drug repositioning has generated substantial interest among researchers and pharmaceutical industries due to its favorable pharmacokinetic attributes and safety profile [8]. Our prior research demonstrated that candesartan (CS), an angiotensin II receptor antagonist, can inhibit NLRP3 inflammasome activation induced by nigericin and ATP in macrophages, as well as alleviate uric acid crystal-induced peritonitis in mice [9]. Nevertheless, the impact of CS on NLRP3 inflammasome activation and inflammatory responses in N. gonorrhoeae-infected macrophages remains uncertain. This study aims to uncover the anti-inflammatory potential of CS in N. gonorrhoeae-infected macrophages.
Materials and methods
Chemicals and reagents
IL-1β antibody (AB-401-NA) was procured from R&D Systems (Minneapolis, MN). We sourced antibodies against NLRP3 (AG-20B-0014) and caspase-1 (AG-20B-0044) from Adipogen International (San Diego, CA). Antibodies for ASC (SC-22514-R), inducible nitric oxide synthase (iNOS) (sc-7271), cyclooxygenase-2 (COX-2) (sc-19999), actin (SC-47778) and LC3 CRISPR/Cas9 knockout plasmids (sc-426563 and sc-417828-HDR) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). We acquired antibodies against phospho-MAPKs (#9910), IKKβ, phospho-IKKα/β (#2697), IκBα (#4814) and phospho-IκBα (#2859) from Cell Signaling Technology (Danvers, MA). Phorbol 12-myristate 13‐acetate (PMA) (P8139) and Gentamicin (G1397) were sourced from Sigma-Aldrich (St. Louis, MO). Additionally, we procured MitoSOX (M36008), MitoTracker Deep Red (M22426), MitoTracker Green (M7514), 2’,7’-dichlorofluorescein diacetate (DCFH2-DA) (C6827), DiOC2(3) (M34150), ELISA kits for mouse IL-1β (88-7013-88), human IL-1β (88-7261-88), mouse IL-6 (88-7064-88), human IL-6 (88-7066-88), mouse TNF-α (88-7324-88) and human TNF-α (88-7346-88) from Thermo Fisher Scientific (Waltham, MA). Our NF-κB-inducible reporter plasmids (pNiFty2-N-SEAP-Zeo, pnifty2-seap), QUANTI-Blue solution (rep-qb2), 3-Methyladenine (3-MA) (inh-3ma-2), Zeocin (ant-zn-05) and Puromycin (ant-pr-1) were obtained from InvivoGen (Carlsbad, CA). Furthermore, the chocolate agar plates for N. gonorrhoeae growth were supplied by Creative Lifesciences (Taipei, Taiwan).
Cell and bacterial cultures
Mouse macrophage cell lines, including J774A.1 (ATCC TIB-67) and RAW264.7 (ATCC TIB-71) cells, along with human THP-1 monocytes (ATCC TIB-202) and N. gonorrhoeae strain F-18 (piliated) (ATCC 49226), were procured from the American Type Culture Collection (Rockville, MD). To differentiate THP-1 monocytes into macrophages, they were cultured in a medium containing 50 nM PMA for 48 h. NF-κB reporter cells were generated by stably transfecting J774A.1 cells with NF-κB-inducible reporter plasmids and subsequently selecting them using 100 µg/ml of Zeocin. Furthermore, LC3-knockdown cells were produced by stably transfecting J774A.1 cells with LC3 CRISPR/Cas9 knockout plasmids and selecting them with Puromycin. Human peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using the Ficoll-Hypaque density gradient centrifugation method, as previously detailed in our report [10]. All these cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, incubated at 37 °C in a 5% CO2 environment. N. gonorrhoeae was maintained on chocolate agar plates at 37 °C in a 5% CO2 incubator.
The impact of CS on NLRP3 inflammasome activation and the inflammatory response in N. Gonorrhoeae-infected macrophages
For optimal N. gonorrhoeae infection efficiency, bacteria were passaged one day prior to the infection. Subsequently, cells were incubated with CS or a vehicle (0.1% DMSO) for 30 min, followed by infection with N. gonorrhoeae at 50 multiplicities of infection (MOI) for 3 h at 37 °C. Afterward, extracellular bacteria were removed by rinsing with sterile PBS, and the cells were cultured in fresh medium containing 1.6 µg/ml Gentamicin along with CS or the vehicle for an additional 5 h (for NLRP3 and proIL-1β expression in the cell lysates) or 21 h. The expression levels of IL-1β, IL-6, and TNF-α in the conditioned medium were analyzed using ELISA. The expression levels of proIL-1β/IL-1β, pro-caspase-1 (p45)/active caspase-1 (p10), NLRP3, and ASC in the conditioned medium were assessed through Western blotting, using methanol/chloroform-concentrated conditioned medium, as described previously [7]. The expression levels of NLRP3, proIL-1β, iNOS, and COX-2 in the cell lysates were also examined via Western blotting.
The impact of CS on MAPKs phosphorylation and NF-κB activation in N. Gonorrhoeae-infected macrophages
J774A.1 cells were incubated with CS or a vehicle for 30 min, followed by infection with N. gonorrhoeae at a 50 MOI for 4 h at 37 °C. The phosphorylation levels of ERK1/2, JNK1/2, p38, IKKα/β, and IκBα in the cell lysates were analyzed through Western blotting. To investigate the impact of CS on NF-κB transcriptional activity, NF-κB reporter cells were incubated with CS or a vehicle for 30 min, followed by infection with N. gonorrhoeae at a 50 MOI for 3 h at 37 °C. After washing out the extracellular bacteria with sterile PBS, the cells were cultured in fresh medium containing 1.6 µg/ml gentamicin and CS or a vehicle for an additional 21 h. The transcriptional activity of NF-κB was assessed using QUANTI-Blue solution, as detailed in our previous report [7].
The impact of CS on intracellular H2O2 production and mitochondrial dysfunction in N. Gonorrhoeae-infected macrophages
For the intracellular H2O2 production assay, J774A.1 cells were incubated with CS or a vehicle for 30 min, followed by infection with N. gonorrhoeae at a 50 MOI for 4 h at 37 °C. Subsequently, the cells were stained with 2 µM DCFH2-DA for 15 min, and the fluorescence signals were assessed using flow cytometry. To examine mitochondrial ROS production, mitochondrial integrity, and mitochondrial membrane potential, J774A.1 cells were incubated with CS or a vehicle for 30 min, followed by infection with N. gonorrhoeae at a 50 MOI for 3 h at 37 °C. After washing out the extracellular bacteria with sterile PBS, the cells were cultured in fresh medium containing 1.6 µg/ml gentamicin and CS or a vehicle for an additional 5 h. For the analysis of mitochondrial ROS production, the cells were stained with 5 nM MitoSOX for 15 min. To assess mitochondrial integrity, the cells were stained with 25 nM MitoTracker Deep Red and MitoTracker Green for 15 min. To analyze mitochondrial membrane potential, the cells were stained with 50 nM DiOC2(3) for 15 min. Subsequently, the fluorescence signals were examined using flow cytometry.
The impact of CS-mediated autophagy on NLRP3 inflammasome activation and the inflammatory responses in N. Gonorrhoeae-infected macrophages
To analyze CS-mediated autophagy induction, J774A.1 macrophages were incubated with CS or a vehicle, either in the presence or absence of a 5 mM autophagy inhibitor 3-MA, for 30 min. This was followed by infection with N. gonorrhoeae at a 50 MOI for 3 h at 37 °C. Additionally, wild-type and LC3-knockout J774A.1 macrophages were incubated with CS or a vehicle for 30 min, followed by infection with N. gonorrhoeae at a 50 MOI for 3 h at 37 °C. Subsequently, extracellular bacteria were removed by rinsing with sterile PBS, and the cells were cultured in fresh medium containing 1.6 µg/ml Gentamicin, along with either CS or the vehicle, for an additional 21 h. The expression levels of IL-1β, IL-6, and TNF-α in the conditioned medium were analyzed using ELISA, while the expression levels of p45/p10 in the conditioned medium were assessed through Western blotting.
Statistical analysis
Statistical analyses were conducted using two-tailed t-tests for comparisons between two groups and ANOVA with Dunnett’s multiple comparisons test for comparisons involving three or more groups. The error bars in the graphs represent the standard deviation of the data obtained from three separate experiments. In the figures, asterisks (*) denote significance levels, with *, **, and *** indicating p < 0.05, p < 0.01, and p < 0.001, respectively.
Results
CS inhibits NLRP3 inflammasome in N. Gonorrhoeae-infected macrophages
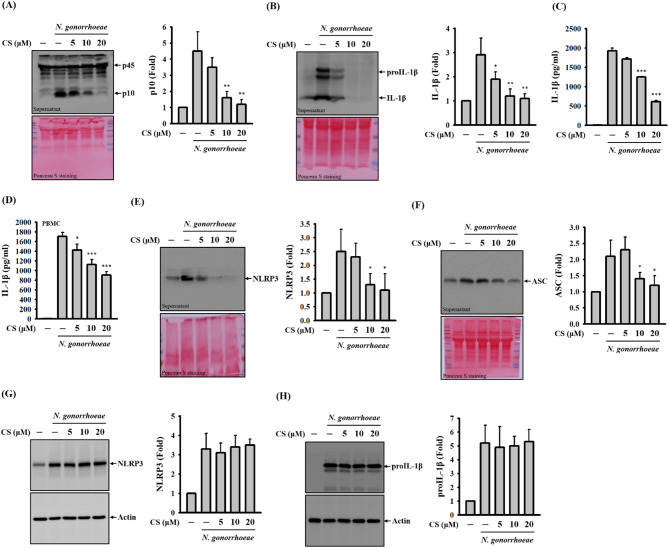
The activation of the NLRP3 inflammasome is characterized by the activation of caspase-1 and the subsequent secretion of IL-1β [6]. To investigate the potential inhibitory effects of CS on NLRP3 inflammasome activation in N. gonorrhoeae-infected macrophages, we embarked on a comprehensive research journey. Notably, CS exhibited a dampening effect on the levels of active caspase-1 (p10) found in the supernatants of N. gonorrhoeae-infected J774A.1 macrophages (Fig. 1A). Moreover, this effect translated into a reduction in IL-1β levels, confirmed by both ELISA measurements (Fig. 1B) and Western blot analysis (Fig. 1C). Encouragingly, the scope of CS’s inhibitory impact extended beyond murine macrophages, as it induced similar reductions in IL-1β levels in the supernatants of N. gonorrhoeae-infected human PBMCs (Fig. 1D). The activation of the NLRP3 inflammasome triggers a series of events, including the formation of plasma membrane pores, leading to increased membrane permeability [11]. Importantly, the extracellular release of inflammasome components from activated cells, especially those stemming from the NLRP3 inflammasome, has been shown to engage adjacent cells, thereby amplifying the inflammatory response [12]. N. gonorrhoeae infection provokes the extracellular release of NLRP3 (Fig. 1E) and ASC (Fig. 1F) within macrophages, initiating a process that could escalate inflammation. However, CS exerts a counteractive effect, reducing the extent of extracellular release. This underscores CS’s role in mitigating membrane permeability in N. gonorrhoeae-infected macrophages, potentially curbing the propagation of inflammatory responses. To further dissect the molecular mechanism behind how CS inhibits the NLRP3 inflammasome in N. gonorrhoeae-infected macrophages, we investigated the effect of CS on the expression of NLRP3 and proIL-1β, which are the end-products of the priming signal for the NLRP3 inflammasome. Following N. gonorrhoeae infection, we observed elevated levels of NLRP3 (Fig. 1G) and proIL-1β (Fig. 1H) in macrophages. Importantly, these effects were not influenced by CS, suggesting that CS’s inhibition of the NLRP3 inflammasome does not result from interference with the priming signal.
Fig. 1.
Effect of CS on the NLRP3 inflammasome in N. gonorrhoeae-infected macrophages. In panels A-C, J774A.1 macrophages were pre-incubated with CS or a vehicle for 0.5 h before N. gonorrhoeae infection, followed by an additional 24-hour incubation period. Levels of pro-caspase-1 (p45)/active caspase-1 (p10) (A) and proIL-1β/IL-1β (B) in the supernatants were assessed using Western blotting, while concentrations of IL-1β in the supernatants were measured through ELISA (C). In panel D, human PBMCs underwent a similar pre-incubation with CS or a vehicle before N. gonorrhoeae infection, followed by an additional 24-hour incubation period. Levels of IL-1β in the supernatants were determined by ELISA. For panels E-H, J774A.1 macrophages were pre-incubated with CS or a vehicle for 0.5 h before N. gonorrhoeae infection, followed by an additional 24-hour (E and F) or 8-hour (G and H) incubation period. Levels of NLRP3 (E) and ASC (F) in the supernatants and levels of NLRP3 (G) and ASC (H) in the cell lysates were assessed via Western blotting. The presented Western blotting images represent individual experiments, and the accompanying histogram illustrates the quantification achieved by analyzing band intensity using ImageJ software, expressed as the mean ± SD for these three experiments. ELISA data are presented as the mean ± SD of three independent experiments. Statistical significance is indicated as *p < 0.05, **p < 0.01, and ***p < 0.001 when compared to N. gonorrhoeae-infected cells
Effect of CS on NLRP3-independent pro-inflammatory mediator expression in N. Gonorrhoeae-infected macrophages
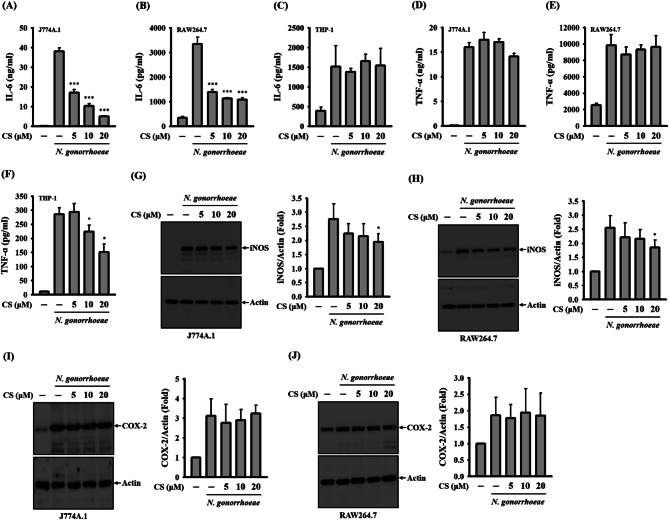
To investigate whether CS’s anti-inflammatory properties are specific to the NLRP3 inflammasome, we examined its influence on the expression of NLRP3-independent pro-inflammatory mediators in N. gonorrhoeae-infected macrophages. Our findings unveiled that N. gonorrhoeae infection led to elevated levels of IL-6 expression in J774A.1 macrophages (Fig. 2A), RAW264.7 macrophages (Fig. 2B), and THP-1 macrophages (Fig. 2C). Notably, CS significantly suppressed IL-6 expression in N. gonorrhoeae-infected J774A.1 macrophages and RAW 264.7 macrophages, while it had no observable effect on THP-1 macrophages. Furthermore, N. gonorrhoeae infection induced an increase in the levels of TNF-α expression in J774A.1 macrophages (Fig. 2D), RAW264.7 macrophages (Fig. 2E), and THP-1 macrophages (Fig. 2F). However, while CS did not exert inhibitory effects on TNF-α expression in N. gonorrhoeae-infected J774A.1 macrophages and RAW264.7 macrophages, it significantly reduced TNF-α expression in THP-1 macrophages. Additionally, Fig. 2G and H demonstrate that N. gonorrhoeae infection led to increased expression levels of iNOS in both J774A.1 macrophages and RAW264.7 macrophages, with the noteworthy finding that these effects were mitigated by exposure to CS. Furthermore, as illustrated in Fig. 2I, J and N. gonorrhoeae infection also resulted in increased expression levels of COX-2 in both J774A.1 macrophages and RAW264.7 macrophages. It is worth noting that CS did not have a discernible impact on COX-2 expression in this context. These results indicate that CS exerts selective anti-inflammatory activity in various cell types.
Fig. 2.
Effect of CS on NLRP3-independent pro-inflammatory mediator expression in N. gonorrhoeae-infected macrophages. In panels A-F, J774A.1 macrophages (A and D), RAW264.7 macrophages (B and E), or THP-1 macrophages (C and F) were pre-incubated with CS or a vehicle for 0.5 h before N. gonorrhoeae infection, followed by an additional 24-hour incubation period. The levels of IL-6 and TNF-α in the supernatants were quantified using ELISA. In panels G-J, J774A.1 macrophages (G and I) or RAW264.7 macrophages (H and J) were pre-incubated with CS or a vehicle for 0.5 h before N. gonorrhoeae infection, followed by an additional 24-hour incubation period. The levels of iNOS and COX-2 in the cell lysates were assessed via Western blotting. ELISA data are presented as the mean ± SD of three independent experiments. The presented Western blotting images represent individual experiments, and the accompanying histogram illustrates the quantification expressed as the mean ± SD for these three experiments. Statistical significance is indicated as *p < 0.05 and ***p < 0.001 when compared to N. gonorrhoeae-infected cells
Effect of CS on ROS production and MAPKs phosphorylation in N. Gonorrhoeae-infected macrophages
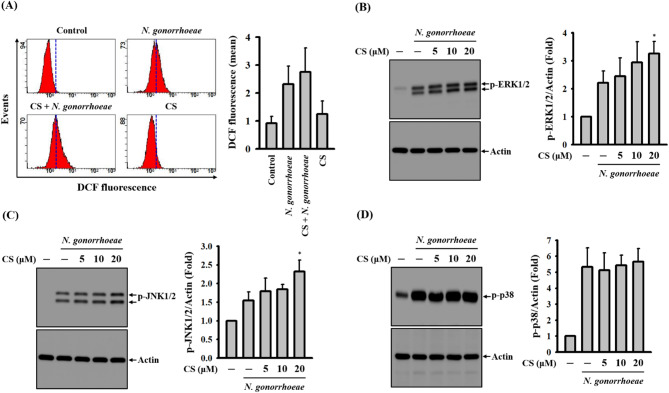
In our previous study, we observed that ROS positively regulate caspase-1 activation and IL-1β secretion in N. gonorrhoeae-infected macrophages [7]. We noted that N. gonorrhoeae infection induced intracellular ROS production, which was evident through DCFH2-DA staining. However, this effect remained unchanged in the presence of CS, as shown in Fig. 3A. Additionally, the inhibition of MAPKs members, namely ERK1/2, JNK1/2, or p38, by specific inhibitors significantly reduced IL-1β secretion in N. gonorrhoeae-infected macrophages [7]. We demonstrated that N. gonorrhoeae infection triggered the phosphorylation of ERK1/2 (Fig. 3B), JNK1/2 (Fig. 3C), and p38 (Fig. 3D). However, in this experimental context, CS was unable to inhibit the phosphorylation of these MAPKs. These results indicate that CS-mediated NLRP3 inflammasome inhibition is not associated with the suppression of ROS or the phosphorylation of MAPKs.
Fig. 3.
Effect of CS on ROS production and MAPKs phosphorylation in N. gonorrhoeae-infected macrophages. J774A.1 macrophages were pre-incubated with CS or a vehicle for 0.5 h before N. gonorrhoeae infection, followed by an additional 4-hour incubation period. The levels of intracellular ROS were evaluated using DCFH2-DA staining (A). The phosphorylation levels of ERK1/2 (B), JNK1/2 (C), and p38 (D) in the cell lysates were assessed through Western blotting. The presented flow cytometry and Western blotting images represent individual experiments, and the accompanying histogram illustrates the quantification expressed as the mean ± SD for these three experiments. Statistical significance is indicated as *p < 0.05 when compared to N. gonorrhoeae-infected cells
Effect of CS on NF-κB activation in N. Gonorrhoeae-infected macrophages
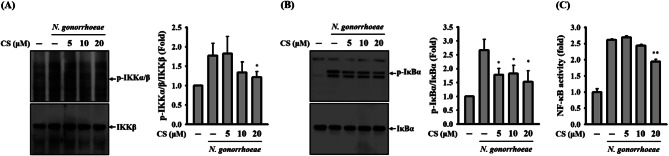
NF-κB activation is initiated by N. gonorrhoeae and, as a result, it positively regulates the secretion of IL-1β in macrophages [11]. In our current investigation, we observed that the levels of phosphorylation of IKKα/β (Fig. 4A) and IκBα (Fig. 4B) induced by N. gonorrhoeae infection were diminished in the presence of CS. Furthermore, CS was found to impede the transcriptional activity of NF-κB, as evidenced by a NF-κB reporter assay (Fig. 4C). This finding confirms the effective inhibition of NF-κB activation by CS.
Fig. 4.
Effect of CS on NF-κB activation in N. gonorrhoeae-infected macrophages. In panels A and B, J774A.1 macrophages were pre-incubated with either CS or a control vehicle for 0.5 h before N. gonorrhoeae infection, followed by an additional 4-hour incubation period. The phosphorylation levels of IKKα/β (A) and IκBα (B) in the cell lysates were evaluated through Western blotting. In panel C, J-Blue cells were pre-treated with CS or a control vehicle for 0.5 h before N. gonorrhoeae infection, followed by an additional 24-hour incubation period. The transcriptional activity of NF-κB was quantified using an NF-κB reporter assay. The presented Western blotting images represent individual experiments, and the accompanying histogram illustrates the quantification expressed as the mean ± SD for these three experiments. NF-κB reporter assay data are presented as the mean ± SD of three independent experiments. Statistical significance is indicated as *p < 0.05 and **p < 0.01 when compared to N. gonorrhoeae-infected cells
Effect of CS on mitochondrial dysfunction in N. Gonorrhoeae-infected macrophages
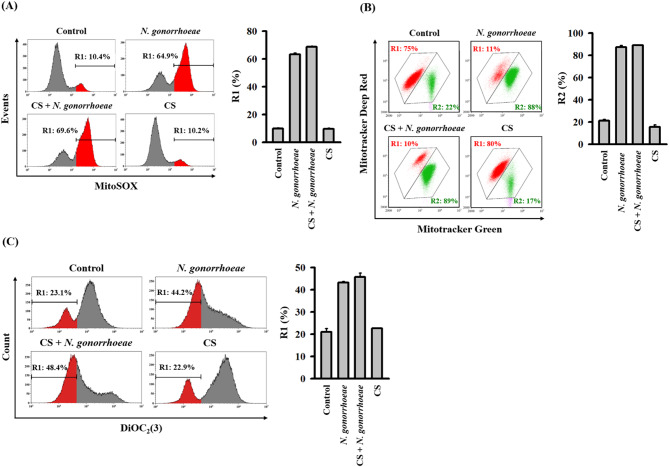
The ROS generated by damaged mitochondria can exacerbate further mitochondrial impairment, including the loss of mitochondrial membrane integrity and membrane potential [13]. DNA released from these damaged mitochondria may undergo oxidation due to mitochondrial ROS and bind to NLRP3 in the cytosol, leading to NLRP3 inflammasome activation [14]. In our prior research, we demonstrated that N. gonorrhoeae infection triggered NLRP3 inflammasome activation via mitochondrial damage in macrophages [7]. In the current study, we confirmed that N. gonorrhoeae infection induced the production of mitochondrial ROS. However, it was observed that CS did not mitigate this effect (Fig. 5A). Using double staining with Mitotracker Deep Red and Mitotracker Green, we also observed that the loss of mitochondrial membrane integrity caused by N. gonorrhoeae infection remained unaffected by CS (Fig. 5B). Furthermore, DiOC2(3) staining revealed that CS did not prevent the loss of mitochondrial membrane potential (Fig. 5C). These findings suggest that the inhibitory effect of CS on NLRP3 inflammasome activation in N. gonorrhoeae-infected macrophages was not mediated through the alleviation of mitochondrial dysfunction.
Fig. 5.
Effect of CS on mitochondrial dysfunction in N. gonorrhoeae-infected macrophages. J774A.1 macrophages were pre-incubated with either CS or a control vehicle for 0.5 h before N. gonorrhoeae infection, followed by an additional 8-hour incubation period. In panel A, mitochondrial ROS production was analyzed using MitoSOX staining. Panel B depicts the analysis of mitochondrial membrane integrity using MitoTracker Deep Red and MitoTracker Green staining. In panel C, the mitochondrial membrane potential was assessed using staining with DiOC2(3). The presented flow cytometry images represent individual experiments, and the accompanying histogram illustrates the quantification expressed as the mean ± SD for these three experiments
CS-mediated autophagy induction inhibits the NLRP3 inflammasome in N. Gonorrhoeae-infected macrophages
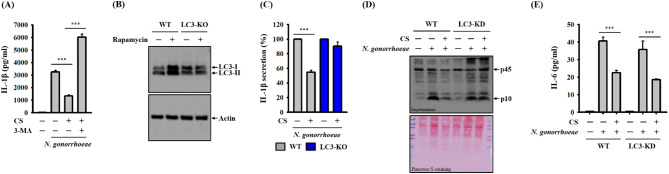
Autophagy serves as a protective cellular process by limiting the activation of the NLRP3 inflammasome. This protective mechanism involves the removal of damaged mitochondria and the promotion of degradation for NLRP3 inflammasome components [15]. In a previous study, we demonstrated that CS increased the expression of LC-3 while reducing the expression of p62. Additionally, CS induced the formation of autophagolysosomes and acidic organelles in macrophages, providing clear evidence of CS’s ability to enhance autophagy. This autophagy induced by CS effectively inhibited the activation of the NLRP3 inflammasome triggered by nigericin in macrophages [8]. In the current study, we investigated whether the autophagy induction mediated by CS plays a role in its inhibitory effect on the NLRP3 inflammasome in N. gonorrhoeae-infected macrophages. Our findings revealed that the reduction of IL-1β mediated by CS could be reversed when an autophagy inhibitor, 3-MA, was employed (Fig. 6A). Moreover, we used CRISPR/Cas9 technology to generate LC3 knockdown macrophages, confirming their autophagic state with LC3 induction by the autophagy inducer, rapamycin (Fig. 6B). In this context, we observed that the IL-1β inhibitory effect of CS was evident in wild-type macrophages. However, in LC3 knockdown cells, CS failed to suppress IL-1β secretion in N. gonorrhoeae-infected macrophages (Fig. 6C). Furthermore, CS also failed to inhibit caspase-1 activation in N. gonorrhoeae-infected LC3 knockdown macrophages (Fig. 6D). Notably, the inhibitory activity of CS on N. gonorrhoeae-mediated IL-6 secretion was not affected by LC3 knockdown (Fig. 6E). These results underscore that CS exerts its inhibitory influence on the NLRP3 inflammasome in N. gonorrhoeae-infected macrophages through the induction of autophagy.
Fig. 6.
Effect of autophagy on CS-mediated NLRP3 Inflammasome inhibition in N. gonorrhoeae-infected macrophages. In panel A, J774A.1 macrophages were pre-incubated with CS or a vehicle in the presence or absence of 5 mM 3-MA for 0.5 h before N. gonorrhoeae infection, followed by an additional 24-hour incubation period. Levels of IL-1β in the supernatants were measured through ELISA. In panel B, wild-type or LC3-knockdown J774A.1 macrophages were incubated with 100 nM rapamycin or a vehicle for 4 h, and the levels of LC3 in the cell lysates were assessed via Western blotting. In panels C-E, wild-type or LC3-knockdown J774A.1 macrophages were pre-incubated with either CS or a control vehicle for 0.5 h before N. gonorrhoeae infection, followed by an additional 24-hour incubation period. The concentrations of IL-1β (C) and IL-6 (E) in the supernatants were measured through ELISA, while the levels of caspase-1 in the supernatants were assessed through Western blotting (D). The ELISA data are presented as the mean ± SD of three independent experiments. The presented Western blotting images represent individual experiments. Statistical significance is indicated as ***p < 0.001 as indicated
Discussion
Drug repositioning, also known as drug repurposing or reprofiling, involves identifying and developing new therapeutic applications for existing drugs, unveiling innovative solutions for treating a variety of diseases. This approach has garnered attention in the pharmaceutical industry and scientific community due to several advantages, such as cost and time efficiency, reduced risk of unexpected adverse effects, provision of new treatment options for unmet medical needs, and extension of the patent life of existing drugs [16]. In our prior study, we presented evidence demonstrating that CS inhibits the NLRP3 inflammasome in macrophages activated by ATP or the bacterial toxin nigericin, and it mitigates uric acid crystal-mediated peritonitis in mice [8]. In the current study, we have uncovered a novel function of CS: it inhibits the activation of the NLRP3 inflammasome and selectively reduces the expression of pro-inflammatory mediators in N. gonorrhoeae-infected macrophages. Additionally, CS exhibits promising therapeutic effects on diseases associated with the NLRP3 inflammasome in animal models. For instance, CS ameliorates Alzheimer’s disease in a mouse model by reducing microglia activation in the hippocampus and diminishing LPS-induced iNOS and COX-2 expression in microglia in vitro [17]. CS alleviates palmitic acid-induced insulin resistance by reducing lipid accumulation and calcium influx in human HepG2 cells, and it improves insulin resistance, hepatic function, and steatosis in high-fat diet-fed mice [18]. Furthermore, CS prevents Cisplatin-induced lung injury in a rat model by attenuating pro-inflammatory cytokine levels and increasing anti-inflammatory cytokine and anti-oxidative enzyme expression in the lungs [19]. Additionally, CS suppresses renal injury in a spontaneously hypertensive rat model by reducing inflammatory cell infiltration and chemokine expression in the kidney [20]. These findings suggest that CS holds the potential for repositioning in the treatment of NLRP3 inflammasome-associated disorders in the future.
Autophagy is a vital cellular process essential for maintaining cellular equilibrium by eliminating damaged or unnecessary cellular components. Beyond its fundamental roles in cellular repair, energy balance, and stress adaptation, autophagy has been identified as a key player in regulating infectious diseases and inflammatory responses [21, 22]. Notably, it is considered a significant negative regulator of the NLRP3 inflammasome [15]. Research into autophagy inducers has generated interest in potential therapeutic interventions to modulate the NLRP3 inflammasome for addressing various health challenges [23–25]. In a previous study, we identified CS as an autophagy inducer in macrophages [8]. Another study also observed that CS increased autophagy-related genes in the brain tissue of d-galactose-treated rats [26]. In our current investigation, we discovered that inhibiting autophagy either through pharmaceutical inhibitors or genetic approaches abolishes the CS-mediated inhibition of the NLRP3 inflammasome in N. gonorrhoeae-infected macrophages. These findings suggest that CS partially inhibits the NLRP3 inflammasome through autophagy induction. Studies indicate that autophagy inhibits NLRP3 inflammasome activation by eliminating damaged mitochondria or destroying the component proteins of the NLRP3 inflammasome [27–30]. However, despite CS inducing autophagy in macrophages, it did not reduce intracellular ROS generation, mitochondrial damage, or the expression of NLRP3 and proIL-1β in N. gonorrhoeae-infected macrophages. Further investigation is required to understand how autophagy inhibits the NLRP3 inflammasome in N. gonorrhoeae-infected macrophages.
Although autophagy induced by CS contributes to inhibiting the NLRP3 inflammasome in N. gonorrhoeae-infected macrophages, it is crucial to explore additional potential mechanisms of action. Previous research has indicated that elevated intracellular calcium levels promote NLRP3 inflammasome activation, whereas intracellular cyclic adenosine monophosphate (cAMP) inhibits activation by directly binding to NLRP3, preventing inflammasome assembly [31]. Earlier studies have demonstrated that CS significantly reduces intracellular calcium levels induced by 1 S, 3R-1‐aminocyclopentane‐trans‐1,3‐dicarboxylic acid in astrocytic endfeet [32]. Furthermore, it diminishes angiotensin II-mediated up-regulation of intracellular calcium concentration in renal vascular smooth muscle cells [33]. Additionally, CS elevates intracellular cAMP levels in dopamine D2 receptor-activated HEK-293T cells coexpressing angiotensin II type 1 and dopamine D2 receptors. The same effect was observed in dopamine D2 receptor agonist quinpirole-treated rat primary cultured striatal neurons [34]. Our previous study showed that N. gonorrhoeae infection caused IL-1β and TNF-α secretion through Toll-like receptor (TLR)-2 in macrophages [7], and another study had a similar finding that the Lip lipoprotein from N. gonorrhoeae stimulates IL-6 expression through TLR-2 in epithelial cells [35]. TLR-2/TLR-4 depletion reduced the expression of NLRP3, prostaglandin E2, and TNF-α in N. gonorrhoeae-infected human endometrial epithelial cells, suggesting that inhibiting TLR2/TLR4 might serve as a treatment to reduce N. gonorrhoeae-mediated inflammation [36]. Notably, CS has been demonstrated to reduce Pam3CSK4- or LPS-induced TLR-2 and TLR-4 expression in human monocytes [37]. Additionally, oral administration of CS reduced TLR-2 and TLR-4 expression in ischemic brain tissue in mice [38]. These findings suggest that CS may inhibit the NLRP3 inflammasome in N. gonorrhoeae-infected macrophages by either decreasing intracellular calcium levels and TLR-2/TLR-4 expression or increasing intracellular cAMP levels. However, these hypotheses require further investigation.
In our study, we observed that macrophages infected with N. gonorrhoeae not only activate the NLRP3 inflammasome but also lead to the up-regulation of IL-6, TNF-α, iNOS, and COX-2. Similarly, epithelial cells infected with N. gonorrhoeae demonstrate increased expression levels of IL-6 and TNF-α [39, 40]. Elevated plasma levels of IL-6 and TNF-α were observed in both male and female individuals positive for N. gonorrhoeae [41]. NF-κB positively regulates IL-6 and TNF-α expression in N. gonorrhoeae-infected mucosal epithelial cells [40]. It also positively regulates IL-1β and NLRP3 expression in N. gonorrhoeae-infected macrophages, indicating the crucial role of NF-κB in the inflammatory response to N. gonorrhoeae [7]. Our study revealed that CS inhibits NF-κB activation in N. gonorrhoeae-infected J774A.1 macrophages, suggesting that the CS-mediated down-regulation of IL-6 and IL-1β may be associated with reduced NF-κB activation. However, CS did not reduce the expression of NLRP3, TNF-α, iNOS, and COX-2, which may also be regulated by NF-κB. Moreover, mucosal epithelial cells infected with N. gonorrhoeae induce the expression of transcription factor ATF3 through MAPKs. Knockdown of ATF3 leads to the up-regulation of IL-6 expression during infection, indicating that ATF3 negatively regulates IL-6 expression in N. gonorrhoeae-infected mucosal epithelial cells [42]. Interestingly, in our study, CS did not inhibit the phosphorylation of MAPKs but instead further increased the phosphorylation of ERK1/2 and JNK1/2, suggesting a potential activity to enhance ATF3 in macrophages. Significantly, it has been noted that autophagy inhibits the secretion of IL-6 and TNF-α in macrophages, as supported by studies [43, 44]. Conversely, conflicting reports suggest that autophagy may promote IL-6 and TNF-α secretion in macrophages according to other studies [45, 46]. The contradictory role of autophagy in the inflammatory response appears to be contingent on experimental conditions. In our study, although the inhibition of autophagy reversed the inhibitory effect of CS on N. gonorrhoeae-mediated NLRP3 inflammasome activation, it had no effect on IL-6 expression. These findings underscore the need for further investigations to comprehensively understand the impact of CS in N. gonorrhoeae-infected macrophages.
Mouse and mouse-derived cell lines are the preferred experimental tools for many immunologists, as studies of their immune responses have provided valuable insights into human immune system functioning. However, these models might not accurately replicate the interactions observed in human cells [47]. Due to the specificity of N. gonorrhoeae towards humans and certain primates, research using mouse cell lines faces notable limitations. In our study, we discovered that CS significantly suppressed IL-6 expression in N. gonorrhoeae-infected mouse macrophages J774A.1 and RAW 264.7 cells, but showed no observable effect on human THP-1 macrophages. Conversely, while CS did not inhibit TNF-α expression in N. gonorrhoeae-infected mouse macrophages J774A.1 and RAW 264.7 cells, it significantly suppressed TNF-α expression in human THP-1 macrophages. These findings indicate that the effects of CS may depend on the cell type and species involved. It has been demonstrated that murine and human macrophages exhibit different metabolic responses to inflammatory stimulation. Human peripheral blood monocyte-derived macrophages utilize oxidative phosphorylation rather than glycolysis for ATP generation in response to LPS. In contrast, LPS activation increases glycolysis and decreases oxidative phosphorylation in mouse bone marrow-derived macrophages. Moreover, mitochondrial bioenergetics after LPS stimulation remain unchanged in human macrophages but are significantly impaired in mouse macrophages [48]. These differences in cellular responses, along with other relevant factors, may influence the study outcomes in human and murine macrophages. Such differences should be taken into account when using mouse cell lines as experimental models for human disease.
One limitation of this study is that only one strain of N. gonorrhoeae was tested. Although dozens of N. gonorrhoeae strains are available in the American Type Culture Collection, strain F-18 was selected for this study. The reasons for choosing N. gonorrhoeae strain F-18 are as follows: This strain [F-18, 89-018314, CDC 10,001, P935] is a whole-genome sequenced bacterium, well-suited for susceptibility disk testing, media testing, and research on sexually transmitted diseases. According to the American Type Culture Collection website, N. gonorrhoeae strain F-18 has been cited 319 times, making it one of the most popular strains in scientific literature. Additionally, it serves as a standard reference strain at Taipei City Hospital for diagnosing N. gonorrhoeae in patients. To further understand the impact of CS on gonorrhea, future studies should use clinically isolated N. gonorrhoeae from patients to infect macrophages. Due to the temporary closure of the animal facility, we are currently unable to conduct the animal model for N. gonorrhoeae infection. This poses a limitation to our study as it prevents us from assessing the in vivo impact of CS on N. gonorrhoeae infection. Previous research has indicated that mice infected with N. gonorrhoeae experienced neutrophil infiltration and increased expression of NLRP3, IL-1β, IL-6, and TNF-α in genital tissues [49]. Exploring the potential effects of orally administering CS on NLRP3 inflammasome activation and cytokine production in N. gonorrhoeae-infected mice is a worthwhile avenue for future investigation.
Conclusions
The current study presents substantial direct evidence of CS’s capability to impede the NLRP3 inflammasome and regulate the inflammatory response in N. gonorrhoeae-infected macrophages, shedding light on a newfound role for CS in modulating infectious diseases. The autophagy induction and NF-κB inhibitory properties of CS play a crucial role in its therapeutic benefits, particularly in mitigating the N. gonorrhoeae-induced activation of the NLRP3 inflammasome. These findings offer a novel perspective on CS, indicating that its therapeutic potential extends beyond addressing high blood pressure and congestive heart failure. To fully grasp and harness the potential of CS in treating Gonorrhea or other infectious diseases involving NLRP3 inflammasome activation, additional research and clinical studies will be indispensable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- NLRP3
The intracellular sensor NACHT, LRR, and PYD domain-containing protein 3
- IL
Interleukin
- CS
Candesartan
- ASC
The extracellular release of NLRP3 and apoptosis-associated speck-like protein containing a CARD
- NLRP1
NOD-like receptor family pyrin domain-containing 1
- NLRC4
NLR family CARD domain containing 4
- AIM2
Absent in melanoma 2
- ROS
Reactive oxygen species
- MAPKs
Mitogen-activated protein kinases
- PMA
Phorbol 12-myristate 13‐acetate
- DCFH2-DA
2’,7’-dichlorofluorescein diacetate
- 3-MA
3-Methyladenine
- PBMCs
Peripheral blood mononuclear cells
- MOI
Multiplicities of infection
- cAMP
Intracellular cyclic adenosine monophosphate
- TLR
Toll-like receptor
Author contributions
L.-H. L. and K.-F. H. is the guarantor of the article. L.-H. L. and K.-F. H. conceived and designed the study. W.-Y. L., J.-L. T., H.-W. C., W.-T. W., C.-H. W., S.-P. Y. and Y. R. performed the experiments and analyzed the data. C.-L. H. assisted with some experiments. H.-T. H, A. C., C.-C. W., C.-H. H. and O. C. contributed to critical revision of the manuscript. W.-Y. L., K.-F. H. and L.-H. L. wrote and finished the manuscript. All authors participated in revising the manuscript and approved the final version.
Funding
This research work is supported by the funding from the National Science and Technology Council, Taiwan (MOST 111-2628-B-197-001-MY3, NSTC 112-2313-B-197-002 and NSTC 113-2313-B-197-002 to K.-F. H.; MOST 111-2811-B-197-001 and NSTC 112-2811-B-197-002 to H.-W. C.), Tri-Service General Hospital, Taipei, Taiwan (TSGH-D-113215 to W.-Y. L.) and Teh-Tzer Study Group for Human Medical Research Foundation (B1101034 to K.-F. H.).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
The acquisition of whole blood strictly followed the guidelines and regulations set forth and sanctioned by the Institutional Review Board of the Tri-Service General Hospital, National Defense Medical Center, as detailed in references TSGH-IRB-2-106-05-190 and TSGH-IRB-2-106-05-009. Prior to the initiation of the study, informed consent was obtained from all participating individuals, and the documentation thereof was securely maintained within the Department of Laboratory Medicine at the Linsen, Chinese Medicine, and Kunming Branch of the Taipei City Hospital in Taipei, Taiwan. The bacterial infection experiments were conducted with the approval of the Taiwan CDC, under the designated approval number 098013.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kuo-Feng Hua, Email: kuofenghua@gmail.com.
Lan-Hui Li, Email: A1525@tpech.gov.tw.
References
- 1.WHO Bacterial Priority Pathogens List. 2024 update.
- 2.Mlynarczyk-Bonikowska B, Kowalewski C, Krolak-Ulinska A, et al. Molecular mechanisms of Drug Resistance and Epidemiology of Multidrug-resistant variants of Neisseria gonorrhoeae. Int J Mol Sci. 2022;23(18):10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol. 2018;16(4):226–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darville T. Pelvic inflammatory Disease due to Neisseria gonorrhoeae and Chlamydia trachomatis: Immune Evasion mechanisms and pathogenic Disease pathways. J Infect Dis. 2021;224(12 Suppl 2):S39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–20. [DOI] [PubMed] [Google Scholar]
- 6.Fu J, Wu H. Structural mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu Rev Immunol. 2023;41:301–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li LH, Lin JS, Chiu HW, et al. Mechanistic insight into the activation of the NLRP3 inflammasome by Neisseria gonorrhoeae in macrophages. Front Immunol. 2019;10:1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kale MA, Shamkuwar PB, Mourya VK, et al. Drug repositioning: a Unique Approach to Refurbish Drug Discovery. Curr Drug Discov Technol. 2022;19(1):e140122192307. [DOI] [PubMed] [Google Scholar]
- 9.Lin WY, Li LH, Hsiao YY, et al. Repositioning of the angiotensin II receptor antagonist Candesartan as an anti-inflammatory Agent with NLRP3 inflammasome inhibitory activity. Front Immunol. 2022;13:870627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernikov OV, Wong WT, Li LH, et al. A GalNAc/Gal-specific lectin from the sea mussel Crenomytilus Grayanus modulates immune response in macrophages and in mice. Sci Rep. 2017;7(1):6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baroja-Mazo A, Martín-Sánchez F, Gomez AI, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15(8):738–48. [DOI] [PubMed] [Google Scholar]
- 13.Kepp O, Galluzzi L, Kroemer G. Mitochondrial control of the NLRP3 inflammasome. Nat Immunol. 2011;12(3):199–200. [DOI] [PubMed] [Google Scholar]
- 14.Xian H, Watari K, Sanchez-Lopez E, et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55(8):1370–e13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol. 2020;11:591803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58. [DOI] [PubMed] [Google Scholar]
- 17.Torika N, Asraf K, Apte RN, et al. Candesartan ameliorates brain inflammation associated with Alzheimer’s disease. CNS Neurosci Ther. 2018;24(3):231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JW, Gu HO, Jung Y, et al. Candesartan, an angiotensin-II receptor blocker, ameliorates insulin resistance and hepatosteatosis by reducing intracellular calcium overload and lipid accumulation. Exp Mol Med. 2023;55(5):910–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atwa AM, Abd El-Ghafar OAM, Hassanein EHM, et al. Candesartan attenuates Cisplatin-Induced Lung Injury by modulating oxidative stress, inflammation, and TLR-4/NF-κB, JAK1/STAT3, and Nrf2/HO-1 signaling. Pharmaceuticals (Basel). 2022;15(10):1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Ge Y, Si J, et al. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int. 2008;74(9):1128–38. [DOI] [PubMed] [Google Scholar]
- 21.Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54(3):437–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Y, Cai W. Autophagy and bacterial infection. Adv Exp Med Biol. 2020;1207:413–23. [DOI] [PubMed] [Google Scholar]
- 23.Wu CH, Gan CH, Li LH, et al. A synthetic small molecule F240B decreases NLRP3 inflammasome activation by Autophagy Induction. Front Immunol. 2020;11:607564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh CY, Li LH, Lam Y, et al. Synthetic 4-Hydroxy auxarconjugatin B, a Novel Autophagy Inducer, attenuates gouty inflammation by inhibiting the NLRP3 inflammasome. Cells. 2020;9(2):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong WT, Li LH, Rao YK, Yet al, et al. Repositioning of the beta-blocker carvedilol as a Novel Autophagy Inducer that inhibits the NLRP3 inflammasome. Front Immunol. 2018;9:1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khedr NF, Werida RH, Abo-Saif MA. Candesartan protects against d-galactose induced - neurotoxicity and memory deficit via modulation of autophagy and oxidative stress. Toxicol Appl Pharmacol. 2022;435:115827. [DOI] [PubMed] [Google Scholar]
- 27.Zhou R, Yazdi AS, Menu P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. [DOI] [PubMed] [Google Scholar]
- 28.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris J, Hartman M, Roche C, et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J Biol Chem. 2011;286(11):9587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chuang SY, Yang CH, Chou CC, et al. LR-induced PAI-2 expression suppresses IL-1β processing via increasing autophagy and NLRP3 degradation. Proc Natl Acad Sci U S A. 2013;110(40):16079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee GS, Subramanian N, Kim AI, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2 + and cAMP. Nature. 2012;492(7427):123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boily M, Li L, Vallerand D, et al. Angiotensin II disrupts neurovascular coupling by potentiating calcium increases in Astrocytic Endfeet. J Am Heart Assoc. 2021;10(17):e020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuller AJ, Hauschild BC, Gonzalez-Villalobos R, et al. Calcium and chloride channel activation by angiotensin II-AT1 receptors in preglomerular vascular smooth muscle cells. Am J Physiol Ren Physiol. 2005;289(4):F760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Pinilla E, Rodríguez-Pérez AI, Navarro G, et al. Dopamine D2 and angiotensin II type 1 receptors form functional heteromers in rat striatum. Biochem Pharmacol. 2015;96(2):131–42. [DOI] [PubMed] [Google Scholar]
- 35.Fisette PL, Ram S, Andersen JM, et al. The lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a toll-like receptor 2-dependent manner. J Biol Chem. 2003;278(47):46252–60. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Liu S, Liu J, et al. Inhibition of TLR2/TLR4 alleviates the Neisseria gonorrhoeae infection damage in human endometrial epithelial cells via Nrf2 and NF-Kβsignaling. J Reprod Immunol. 2020;142:103192. [DOI] [PubMed] [Google Scholar]
- 37.Dasu MR, Riosvelasco AC, Jialal I. Candesartan inhibits toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis. 2009;202(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barakat W, Safwet N, El-Maraghy NN, et al. Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. Eur J Pharmacol. 2014;724:43–50. [DOI] [PubMed] [Google Scholar]
- 39.Płaczkiewicz J, Adamczyk-Popławska M, Kozłowska E, et al. Both Neisseria gonorrhoeae and Neisseria sicca Induce Cytokine Secretion by Infected Human cells, but only Neisseria gonorrhoeae Upregulates the expression of long non-coding RNAs. Pathogens. 2022;11(4):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naumann M, Wessler S, Bartsch C, et al. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor kappaB and activator protein 1 and the induction of inflammatory cytokines. J Exp Med. 1997;186(2):247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maina A, Mureithi M, Kiiru J, et al. Systemic and mucosal concentrations of nine cytokines among individuals with Neisseria gonorrhoeae infection in Nairobi Kenya. AAS Open Res. 2022;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calton CM, Wade LK, So M. Upregulation of ATF3 inhibits expression of the pro-inflammatory cytokine IL-6 during Neisseria gonorrhoeae infection. Cell Microbiol. 2013;15(11):1837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pun NT, Subedi A, Kim MJ, et al. Globular adiponectin causes tolerance to LPS-Induced TNF-α expression via Autophagy Induction in RAW 264.7 macrophages: involvement of SIRT1/FoxO3A Axis. PLoS ONE. 2015;10(5):e0124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Liang T, Yang W, et al. Astragalus Membranaceus Injection suppresses production of Interleukin-6 by activating autophagy through the AMPK-mTOR pathway in Lipopolysaccharide-Stimulated Macrophages. Oxid Med Cell Longev. 2020;2020:1364147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang S, Wang R, Han L, et al. Activation of autophagy reverses gemcitabine-induced immune inhibition of RAW264.7 macrophages by promoting TNF-α, IL-6 and MHC-II expression. Immunol Res. 2021;69(4):352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Y, Chen J, Ren G, et al. Punicalagin prevents inflammation in LPS-Induced RAW264.7 macrophages by inhibiting FoxO3a/Autophagy signaling pathway. Nutrients. 2019;11(11):2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–8. [DOI] [PubMed] [Google Scholar]
- 48.Vijayan V, Pradhan P, Braud L, et al. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide - A divergent role for glycolysis. Redox Biol. 2019;22:101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, Zhang Y, Gan L, et al. Progesterone suppresses Neisseria gonorrhoeae-Induced inflammation through inhibition of NLRP3 inflammasome pathway in THP-1 cells and murine models. Front Microbiol. 2021;12:570093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.