Abstract
Background
The aim of this study is to summarize our center’s experience with patent ductus arteriosus (PDA) ligation during extracorporeal membrane oxygenation (ECMO) treatment in newborns with severe respiratory failure due to persistent pulmonary hypertension of the newborn (PPHN).
Methods
We retrospectively collected and analyzed clinical data from five newborns with severe respiratory failure due to PPHN who underwent PDA ligation during ECMO treatment at our hospital between January 2021 and August 2023.
Results
All five patients had large PDAs, measuring 10 mm, 6 mm, 6 mm, 7 mm, and 6 mm, respectively. Significant left-to-right shunting through the PDA was observed after 29 h, 14 h, 3 h, 7 h, and 5 h of ECMO treatment, respectively, at which point successful PDA ligation was performed. The surgical durations were 52 min, 45 min, 55 min, 50 min, and 40 min, respectively. Post-ligation, blood lactate levels significantly decreased compared to preoperative values. Four patients were successfully weaned off ECMO, with ECMO support durations of 64 h, 92 h, 70 h, and 87 h, respectively. After ECMO removal, mechanical ventilation was discontinued after 5.2 days, 7.2 days, 9.5 days, and 5.5 days, respectively. None of the four surviving patients experienced complications such as residual shunting, bleeding, chylothorax, neurologic injury, pneumothorax, poor wound healing, or sepsis.
Conclusion
During ECMO treatment for PPHN in newborns with large PDAs, the direction of blood flow through the PDA should be closely monitored. PDA ligation is a feasible and reasonable intervention when pulmonary artery pressure decreases and left-to-right shunting through the PDA becomes evident.
Keywords: Newborns, ECMO, PDA ligation, Early results
Introduction
Persistent pulmonary hypertension of the newborn (PPHN) is characterized by a sustained increase in pulmonary vascular resistance after birth, leading to right-to-left shunting of blood at the atrial and/or ductal levels, resulting in severe hypoxemia and a high mortality rate [1, 2]. The conventional treatment for PPHN includes mechanical ventilation support, maintenance of normal systemic circulatory pressure, administration of vasodilators, and inhaled nitric oxide. However, in cases of severe PPHN that do not respond to standard treatments and meet the criteria for extracorporeal membrane oxygenation (ECMO) application, ECMO support should be considered [3–6]. Newborns with PPHN often experience severe hypoxemia, acidosis, and pulmonary hypertension, which can lead to heart failure and circulatory instability. Venoarterial (V-A) ECMO provides both respiratory and cardiac support, making it the preferred mode of treatment for patients with PPHN [7, 8].
Patent ductus arteriosus (PDA) is a common vascular anomaly in newborns, where a connection persists between the descending aorta and the pulmonary artery [9, 10]. The presence of a PDA can lead to blood shunting, particularly in cases of large PDAs with significant left-to-right shunting, which may result in pulmonary congestion, pulmonary hemorrhage, reduced systemic blood volume, inadequate systemic perfusion, elevated blood lactate levels, necrotizing enterocolitis, and other related complications [11, 12]. These patients often require surgical closure of the PDA. Newborns with severe respiratory failure due to PPHN frequently have a concomitant PDA, which may present as a right-to-left or bidirectional shunt. When ECMO treatment is initiated, pulmonary artery pressure may decrease, resulting in left-to-right shunting through the PDA. In cases where the shunt volume through the PDA is large, it can lead to hemodynamic instability during ECMO treatment, necessitating surgical closure of the PDA. However, there is limited literature reporting on the experience of PDA ligation during ECMO treatment, with most accounts consisting of case reports. This study summarizes the experience of PDA ligation during ECMO treatment in five cases of severe respiratory failure due to PPHN at our center and reports early clinical outcomes.
Methods and materials
Clinical data were collected from five newborns with severe respiratory failure due to PPHN who underwent PDA ligation during ECMO treatment at our hospital between January 2021 and August 2023. This study was approved by the hospital’s ethics committee, and informed consent was obtained from the patients’ families.
ECMO cannulation technique
All patients underwent bedside cannulation for ECMO in our cardiac intensive care unit. Right carotid artery and right internal jugular vein cannulation were performed in all cases. The arterial cannulas (Medtronic, USA) were all 8 French (F), and the venous cannulas (Medtronic, USA) were all 10 F.
PDA ligation technique
After anesthesia, patients were carefully positioned in the right lateral decubitus position. Special attention was given to maintaining ECMO flow, and the ECMO circuit was securely fastened. A 2 cm incision was made below the outer edge of the left scapula, extending 3–4 cm in an arc toward the spine. The skin, subcutaneous tissue, and muscles were sequentially dissected, and the chest was entered through the fourth intercostal space. Respirator settings, including positive end-expiratory pressure (PEEP) and tidal volume, were reduced to allow for lung collapse. The mediastinal pleura was incised longitudinally from the origin of the descending aorta near the left pulmonary artery to the pulmonary hilum, and it was then retracted. The ductus arteriosus was identified near the origin of the descending aorta, close to the left pulmonary artery, and was carefully dissected free at its upper and lower poles, revealing a ductus arteriosus approximately 1 cm wide and 1 cm long (Fig. 1). After separating the space between the upper and lower margins of the PDA with angled forceps, two 1 − 0 sutures were placed around the PDA, one above and one below. The PDA was ligated, when sodium nitroprusside was applied to reduce the systolic blood pressure to 40-50mmHg. After confirming that there was no bleeding, the mediastinal pleura was sutured to achieve hemostasis, and a chest tube was left in place. The chest was closed layer by layer, including the muscle, subcutaneous tissue, and skin. Patients were then carefully repositioned, and the ECMO circuit was re-secured.
Fig. 1.
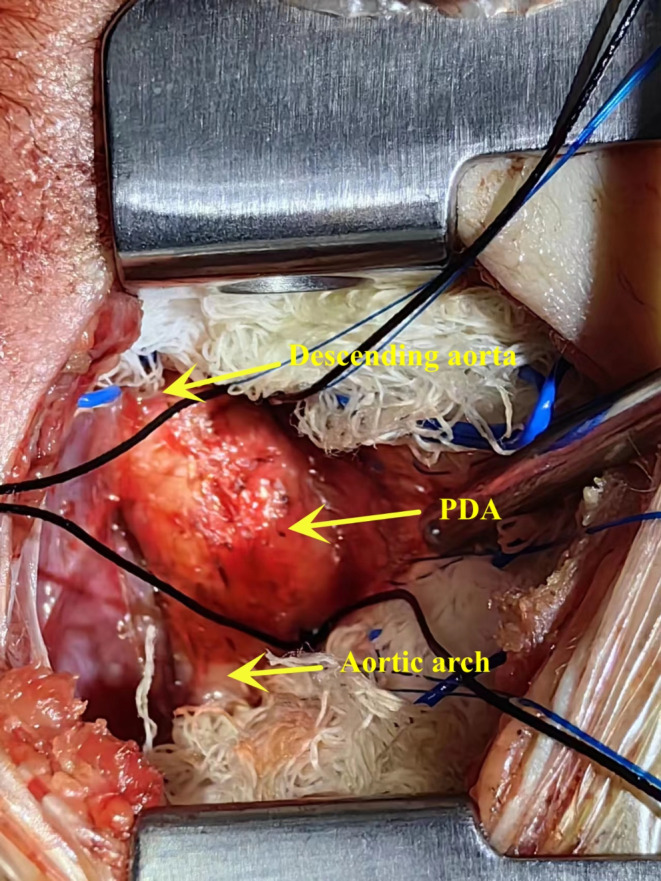
The exposure of PDA
Results
The gestational ages of the five newborns in this study were 39.7 weeks, 37.7 weeks, 39.6 weeks, 38.4 weeks, and 35.3 weeks, respectively, and their birth weights were 3280 g, 2740 g, 2375 g, 3805 g, and 2700 g, respectively. All presented with PPHN shortly after birth, leading to severe hypoxemia and heart failure. ECMO was initiated at 1 day, 1 day, 3 days, 2 days, and 5 days, respectively. All five cases were complicated by large PDAs, with sizes of 10 mm, 6 mm, 6 mm, 7 mm, and 6 mm, respectively. The pulmonary artery pressures before ECMO were 65 mmHg, 78 mmHg, 66 mmHg, 70 mmHg, and 69 mmHg, respectively, and lactate levels were 8 mmol/L, significantly elevated, 12 mmol/L, 9 mmol/L, and 13 mmol/L, respectively. The direction of blood flow through the PDA was a right-to-left shunt, and no pulmonary hemorrhage was observed (Table 1).
Table 1.
Clinical data of patient during ECMO period
| Gestational age (week) |
Birth weight (g) |
Age (d) | Pulmonary artery pressure (mmHg) | Size of PDA (mm) |
Pneum- orrhagia before PDA ligation |
Blood flow direction of PDA | Lactic acid (mmol/L) | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 39.7 | 3280 | 1 | 65 | 10 | No | right -to- left shunt | 8 |
| Patient 2 | 37.7 | 2740 | 1 | 78 | 6 | No | right -to- left shunt | High |
| Patient 3 | 39.6 | 2375 | 3 | 66 | 6 | No | right -to- left shunt | 12 |
| Patient 4 | 38.4 | 3805 | 2 | 70 | 7 | No | right -to- left shunt | 9 |
| Patient 5 | 35.3 | 2700 | 5 | 69 | 6 | No | right -to- left shunt | 13 |
After ECMO support, pulmonary artery pressure decreased in all patients, and the left-to-right shunt through the PDA gradually increased, while lactic acid levels did not decrease. During this period, three patients developed symptoms of pulmonary hemorrhage. Significant left-to-right shunting through the PDA was observed in all patients after 29 h, 14 h, 3 h, 7 h, and 5 h of ECMO treatment, respectively, prompting the decision to perform PDA ligation. The surgical durations were 52 min, 45 min, 55 min, 50 min, and 40 min, respectively. Before PDA ligation, the patients’ platelet counts were 67 × 109/L, 82 × 109/L, 178 × 109/L, 129 × 109/L, and 328 × 109/L, respectively. The activated clotting times (ACT) were 198 s, 200 s, 186 s, 190 s, and 210 s, respectively. Intraoperative bleeding volumes were 10 ml, 10 ml, 2 ml, 5 ml, and 5 ml, respectively. After PDA ligation, all patients showed a significant decrease in blood lactate levels compared to preoperative values. Four patients were successfully weaned off ECMO, with ECMO support durations of 64 h, 92 h, 70 h, and 87 h, respectively. After ECMO removal, mechanical ventilation was discontinued after 5.2 days, 7.2 days, 9.5 days, and 5.5 days, respectively. One patient experienced intracranial hemorrhage on the second day after PDA ligation, and the family decided to discontinue further treatment, resulting in the cessation of care (Table 2).
Table 2.
Clinical data of patient in perioperative period of patent ductus arteriosus ligation
| Pulmonary artery pressure before PDA ligation (mmHg) | Pneum- orrhagia before PDA ligation |
Blood flow direction of PDA | Platelet before PDA ligation (/L) | ACT before PDA ligation (s) |
Operation time of PDA ligation (min) | Amount of bleeding (ml) | Lactic acid before PDA ligation (mmol/L) | Lactic acid in 1 h after PDA ligation (mmol/L) | Lactic acid in 3 h after PDA ligation (mmol/L) | Pneum- Orrhagia after PDA ligation |
Time of ECMO treatment (h) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 55 | Yes | left-to- right shunt | 67*10^9 | 198 | 52 | 10 | 10.4 | 6.6 | 5.1 | Reduction | 64 |
| Patient 2 | 65 | Yes | left-to- right shunt | 82*10^9 | 200 | 45 | 10 | High | High | 29 | Reduction | Abandon treatment |
| Patient 3 | 53 | Yes | left-to- right shunt | 178*10^9 | 186 | 55 | 2 | 15 | 11.9 | 10.5 | Reduction | 92 |
| Patient 4 | 60 | No | left-to- right shunt | 129*10^9 | 190 | 50 | 5 | 11.1 | 4.5 | 2.7 | - | 70 |
| Patient 5 | 59 | No | left-to- right shunt | 328*10^9 | 210 | 40 | 5 | 15 | 7.8 | 4.8 | - | 87 |
None of the four surviving patients experienced complications such as residual shunt, major bleeding, chylothorax, neurological injury, pneumothorax, poor wound healing, or sepsis.
Discussion
Pulmonary artery hypertension occurs in 2–5 of every 1,000 live births [13]. Acute pulmonary hypertension, which leads to hypoxemic respiratory failure during the transitional period, is characterized by a failure of the normal postnatal decline in pulmonary vascular resistance. The hypoxemia is related to impaired pulmonary blood flow and/or right ventricular dysfunction secondary to elevated pulmonary artery pressure and increased right ventricular afterload [14]. Consequently, severe PPHN in newborns often leads to profound hypoxemia, ultimately resulting in life-threatening circulatory and respiratory failure, with a mortality rate of approximately 50%[15, 16]. If respiratory failure persists despite standard treatment, ECMO therapy becomes necessary [17].
Cardiac dysfunction is more pronounced in neonates due to an underdeveloped contractile system with decreased compliance, reduced adaptability to changes in afterload, and an increased risk of diastolic dysfunction [18]. In the context of severe acute pulmonary hypertension, where there is significant right ventricular dysfunction and/or extremely low cardiac output, a continuous right-to-left shunt through the PDA can play a supportive role by reducing right ventricular pressure. The PDA should remain open during this time [19]. As treatment progresses and pulmonary vascular resistance and pulmonary artery pressure decrease, the PDA may develop a left-to-right shunt. Numerous studies have shown that when the PDA exhibits hemodynamically significant left-to-right shunting, closure of the PDA is necessary, as it can lead to complications such as pulmonary hemorrhage, renal failure, necrotizing enterocolitis, and even neonatal mortality [20, 21]. Hemodynamically significant PDA (HsPDA) is typically defined as a PDA with significant left-to-right shunting through the ductus arteriosus, confirmed by echocardiography and clinical evidence of systemic hypoperfusion and pulmonary overcirculation [22, 23]. For newborns with large PDAs, during ECMO treatment, when pulmonary artery pressure decreases, a large PDA can result in significant left-to-right shunting, leading to pulmonary congestion, hemorrhage, and reduced systemic circulation, among other adverse effects. Therefore, surgical closure of the PDA should be considered in newborns with severe PPHN and concomitant large PDAs during ECMO support. However, performing PDA ligation during ECMO carries a high risk. This study summarizes our clinical experience with performing PDA ligation during ECMO therapy in newborns.
Since PPHN in newborns is often self-limiting, pulmonary artery pressure decreases as the infant ages. Therefore, when ECMO-assisted therapy reduces pulmonary artery pressure to the point where left-to-right shunting occurs through the PDA, surgical closure of the PDA should be considered. If the PDA is large at this point, it can result in significant left-to-right shunting, leading to pulmonary congestion, pulmonary hemorrhage, and adverse effects on the already compromised lung function and the management of pneumonia, which can hinder successful ECMO removal [24, 25]. A significant reduction in systemic circulation can also lead to inadequate systemic perfusion, tissue ischemia, hypoxia, and complications such as elevated lactate levels and necrotizing enterocolitis [26]. Therefore, for newborns with severe respiratory failure and large PDAs undergoing ECMO-assisted therapy, closure of the PDA should be considered when pulmonary artery pressure decreases to the point where left-to-right shunting begins [24]. If significant pulmonary congestion, pulmonary hemorrhage, or elevated lactate levels occur, PDA ligation should be performed promptly. In the cases discussed in this study, all five patients had PDAs measuring 6 mm to 10 mm, which were close to or even exceeded the diameter of the aorta. Before PDA ligation, all five patients exhibited continuously rising lactate levels, and three experienced pulmonary hemorrhages. After PDA ligation, lactate levels significantly decreased, and pulmonary hemorrhages gradually resolved.
Due to the invasive nature of PDA ligation surgery and the need for heparin anticoagulation during ECMO therapy, the risk of intraoperative bleeding is relatively high [27]. To prevent bleeding, careful surgical techniques, avoidance of vascular and unnecessary tissue injury, and meticulous hemostasis are essential. We followed the approach recommended by Wang G, et al., [28] which involves maintaining the ACT within the range of 180–220 s, ensuring platelet counts above 60 × 10⁹/L, and using sodium nitroprusside to control the systolic blood pressure at 40–50 mmHg during PDA ligation. None of the five patients in our study experienced severe bleeding complications, with intraoperative bleeding volumes not exceeding 10 ml.
To improve the success rate of the surgery and minimize complications, we summarized the following key points from our experience: optimal exposure and a clear surgical field were crucial for the procedure’s success. Since ECMO support was available for cardiopulmonary function, we temporarily suspended mechanical ventilation during chest entry, retaining PEEP, and resumed ventilation only after completing the PDA ligation. This approach prevented respiratory movements from obstructing the surgical field, significantly improving visibility without causing circulatory changes. It is important to note that this brief pause in ventilation did not lead to lung perfusion issues, as the surgery duration was short. Newborns often had significant tissue edema due to their young age, heart failure, and ECMO support, making the aorta and PDA tissue very thin and susceptible to damage. Therefore, it was essential to perform the surgery meticulously and gently. The incision into the mediastinal pleura was extended as close as possible to the subclavian artery at the upper end and down to the pulmonary hilum, ensuring full exposure, with prompt application of electrocautery for hemostasis to minimize bleeding. To minimize the risk of damaging the PDA during ligation, we employed a technique where, after separating the upper and lower margins of the PDA with angled forceps, the PDA plane was freed from the outer posterior aspect of the descending aorta. We then inserted two 1 − 0 sutures, one above and one below the PDA margins, by passing them through the posterior aspect of the descending aorta. This approach simplified the procedure and minimized the risk of tissue damage during PDA ligation.
Our study had several limitations. It was a single-center retrospective study with a small sample size. This report reflects the early experience of our center and serves as an exploratory study. More objective and accurate conclusions will require studies with larger sample sizes in the future.
Conclusion
During ECMO treatment for newborns with PPHN and large PDA, the direction of blood flow in the PDA should be closely monitored. PDA ligation is a feasible and reasonable intervention when pulmonary artery pressure decreases and a left-to-right shunt through the PDA becomes evident. However, performing PDA ligation during ECMO therapy still carries a high risk, necessitating close perioperative monitoring.
Acknowledgements
We highly acknowledge the following researchers’ contributions: Shun-Min Wang, Xiu-Xuan Ruan, Zeng-Chun Wang, and Ling-Shan Yu.
Abbreviations
- PDA
Patent Ductus Arteriosus
- ECMO
Extracorporeal Membrane Oxygenation
- PPHN
Persistent Pulmonary Hypertension
- V-A
Venoarterial
- PEEP
Positive End Expiratory Pressure
- ACT
Activated Clotting Time
- F
French
Author contributions
ZQL and CQ designed the study, performed the statistical analysis, participated in the operation, and drafted the manuscript. LYN and ZYT collected the clinical data. ZYR supervised the study. All authors read and approved the final manuscript.
Funding
There is no funding.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Fujian Children’s Hospital and strictly adhered to the tenets of the Declaration of Helsinki. The parents or guardians of the patients gave written informed consent for their respective minors to participate in the study.
Consent for publication
No individual data.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi-Rong Zheng and Qiang Chen authors shared the corresponding authorship.
Contributor Information
Yi-Rong Zheng, Email: zhengyirong2021@163.com.
Qiang Chen, Email: chenqiang2228@163.com.
References
- 1.Sankaran D, Lakshminrusimha S. Pulmonary hypertension in the newborn- etiology and pathogenesis. Semin Fetal Neonatal Med. 2022;27(4):101381. [DOI] [PubMed] [Google Scholar]
- 2.Fei Q, Pan J, Zhang F, Lin Y, Yuan T. Comparison of different treatments of persistent pulmonary hypertension of the Newborn: a systematic review and network Meta-analysis. Crit Care Med. 2024;52(6):e314–22. [DOI] [PubMed] [Google Scholar]
- 3.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL, American Heart Association Council on Cardiopulmonary., Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037-99. [DOI] [PubMed]
- 4.Yang ZH, Ning BT, Zhang CM, Lin R, Ye S, Liu T. Clinical application of extracorporeal membrane oxygenation in children with refractory cardiopulmonary failure. World J Pediatr. 2016;12(3):364–7. [DOI] [PubMed] [Google Scholar]
- 5.Doymaz S, Zinger M, Sweberg T. Risk factors associated with intracranial hemorrhage in neonates with persistent pulmonary hypertension on ECMO. J Intensive Care. 2015;3(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye LF, Fan Y, Shu Q, Lin R. Management of persistent pulmonary hypertension in newborns with ECMO support: a single center’s experience. World J Pediatr. 2019;15(1):100–3. [DOI] [PubMed] [Google Scholar]
- 7.Macchini F, Di Cesare A, Morandi A, Ichino M, Raffaeli G, Conigliaro F, Sorrentino G, Neri S, Mosca F, Leva E, Cavallaro G. Surgical Expertise in neonatal extracorporeal membrane oxygenation (ECMO): a single Center experience. Front Pediatr. 2019;7:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter JL, Yu YR, Cass DL, Olutoye OO, Thomas JA, Burgman C, Fernandes CJ, Lee TC. Use of venovenous ECMO for neonatal and pediatric ECMO: a decade of experience at a tertiary children’s hospital. Pediatr Surg Int. 2018;34(3):263–8. [DOI] [PubMed] [Google Scholar]
- 9.Susheel Kumar TK. Surgical management of patent ductus arteriosus. Congenit Heart Dis. 2019;14(1):57–9. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande P, Baczynski M, McNamara PJ, Jain A. Patent ductus arteriosus: the physiology of transition. Semin Fetal Neonatal Med. 2018;23(4):225–31. [DOI] [PubMed] [Google Scholar]
- 11.Weisz DE, McNamara PJ. Patent ductus arteriosus ligation and adverse outcomes: causality or bias? J Clin Neonatol. 2014;3(2):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed MA, El-Dib M, Alqahtani S, Alyami K, Ibrahim AN, Aly H. Patent ductus arteriosus in premature infants: to treat or not to treat? J Perinatol. 2017;37(6):652–7. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig EB, Bates A, Mullen MP, Abman SH, Austin ED, Everett A, Fineman J, Feinstein J, Hopper RK, Kinsella JP, Krishnan US, Lu M, Mandl KD, Raj JU, Varghese N, Yung D, Handler SS, Sleeper LA. Cardiac catheterization and Hemodynamics in a Multicenter Cohort of Children with Pulmonary Hypertension. Ann Am Thorac Soc. 2022;19(6):1000–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruoss JL, Moronta SC, Bazacliu C, Giesinger RE, McNamara PJ. Management of cardiac dysfunction in neonates with pulmonary hypertension and the role of the ductus arteriosus. Semin Fetal Neonatal Med. 2022;27(4):101368. [DOI] [PubMed] [Google Scholar]
- 15.Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, Ivy DD, Berger RMF. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53(1):1801916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sluiter I, van Heijst A, Haasdijk R, Kempen MB, Boerema-de Munck A, Reiss I, Tibboel D, Rottier RJ. Reversal of pulmonary vascular remodeling in pulmonary hypertensive rats. Exp Mol Pathol. 2012;93(1):66–73. [DOI] [PubMed] [Google Scholar]
- 17.Hirakawa E, Ibara S, Tokuhisa T, Maede Y, Kuwahara T, Ishihara C, Noguchi H, Naitou Y, Yamamoto M, Kibe M, Yamamoto T, Kurimoto T, Kamitomo M, Cho K, Minakami H. Extracorporeal membrane oxygenation in 61 neonates: single-center experience. Pediatr Int. 2017;59(4):438–42. [DOI] [PubMed] [Google Scholar]
- 18.Critser PJ, Levy PT. Risk Assessment and Monitoring of right ventricular function in congenital diaphragmatic hernia. Ann Am Thorac Soc. 2020;17(11):1380–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siefkes HM, Lakshminrusimha S. Management of systemic hypotension in term infants with persistent pulmonary hypertension of the newborn: an illustrated review. Arch Dis Child Fetal Neonatal Ed Jul. 2021;106(4):446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehgal A, McNamara PJ. International perspective on management of a patent ductus arteriosus: lessons learned. Semin Fetal Neonatal Med. 2018;23:278–84. [DOI] [PubMed] [Google Scholar]
- 21.Pavlek LR, Slaughter JL, Berman DP, Backes CH. Catheter-based closure of the patent ductus arteriosus in lower weight infants. Semin Perinatol. 2018;42(4):262–8. [DOI] [PubMed] [Google Scholar]
- 22.Lee JA. Practice for preterm patent ductus arteriosus; focusing on the hemodynamic significance and the impact on the neonatal outcomes. Korean J Pediatr. 2019;62(7):245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Jin H, Jiang Y, Du J. Prediction of therapeutic response to cyclooxygenase inhibitors in Preterm infants with Patent Ductus Arteriosus. Pediatr Cardiol. 2018;39(4):647–52. [DOI] [PubMed] [Google Scholar]
- 24.Tschuppert S, Doell C, Arlettaz-Mieth R, Baenziger O, Rousson V, Balmer C, Prêtre R, Dodge-Khatami A. The effect of ductal diameter on surgical and medical closure of patent ductus arteriosus in preterm neonates: size matters. J Thorac Cardiovasc Surg. 2008;135(1):78–82. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg JG, Evans FJ, Burns KM, Pearson GD, Kaltman JR. Surgical ligation of patent ductus arteriosus in premature infants: trends and practice variation. Cardiol Young. 2016;26(6):1107–14. [DOI] [PubMed] [Google Scholar]
- 26.Rong X, Ye Q, Wang Q, Wang J, Zhu Q, Chen Y, Wu R. Post-interventional evaluation and Follow-Up in children with patent Ductus Arteriosus Complicated with moderate to severe pulmonary arterial hypertension: a retrospective study. Front Cardiovasc Med. 2021;8:693414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buscher H, Vukomanovic A, Benzimra M, Okada K, Nair P. Blood and anticoagulation management in extracorporeal membrane oxygenation for Surgical and Nonsurgical patients: a single-Center Retrospective Review. J Cardiothorac Vasc Anesth. 2017;31(3):869–75. [DOI] [PubMed] [Google Scholar]
- 28.Hui Wang G, Wang X, Hong Y, Liu J, Zhao G, Zhou. One case of neonatal ligation arterial catheter next to bed during extracorporeal membrane oxygenation treatment. Chin J Pediatr Surg. 2021;42(4):365–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


