Abstract
Background
Schistosomiasis and soil-transmitted helminth (STH) infections occurring during pregnancy may pose adverse health consequences to the mother and the developing baby. This study aims to determine the prevalence of Schistosoma mansoni and STHs, and their association with adverse birth outcomes among pregnant women in Jimma Town.
Methods
A cross-sectional study involving 314 pregnant women was conducted in Jimma Town, Southwest Ethiopia. The pregnant women were recruited from selected public health facilities during their antenatal care (ANC) visits from August to December 2021. Data on demographic characteristics and factors associated with S. mansoni and STH infections were collected using a pretested questionnaire. Moreover, during the third trimester, stool specimen of each pregnant woman was examined using Kato-Katz technique, and hemoglobin was measured using a HemoCue analyzer. Data on adverse birth outcomes were collected during delivery. Data were analyzed using STATA-MP_12 (StataCorp., TX, USA).
Results
The overall prevalence of intestinal helminthic infections was 26.1%. Soil-transmitted helminths and S. mansoni were detected in 20.4% (95%CI: 15.9–24.8) and 5.7% (95%CI: 3.2–8.3) of the pregnant women, respectively. The magnitude of low birth weight was 6.4%, higher in those with late-term delivery and maternal anemia (p < 0.05). Maternal anemia was also associated with post-partum bleeding (p < 0.05). Pregnant women who carry out laundry activities in the river were significantly more infected by S. mansoni (AOR 10.7, 95% CI: 2.2–51.9).
Conclusions
This study sheds light on the burden of maternal STH and S. mansoni infections in Jimma Town. A quarter of the pregnant women were infected with STHs and S. mansoni. It is recommended that pregnant women in the area avoid washing clothes in the river to reduce the risk of S. mansoni infection. Screening for intestinal parasitic infections should be conducted for pregnant women living in endemic areas during their ANC follow-up.
Keywords: S. mansoni, Soil-transmitted helminths, Prevalence, Pregnant women, Ethiopia
Background
Schistosomiasis and soil-transmitted helminthiasis (STH) are caused by blood flukes (trematodes) and intestinal roundworms (nematodes), respectively. Two forms of schistosomiasis exist - urogenital schistosomiasis (caused by Schistosoma haematobium) and intestinal schistosomiasis (caused by S. mansoni, S. japonicum, S. mekongi, S. intercalatum and S. guineensis). Globally, the majority of schistosomiasis is caused by S. mansoni and S. haematobium [1]. Schistosomiasis may be asymptomatic and symptomatic with acute and chronic manifestations [2]. On the other hand, STH is caused by four species of nematodes, Ascaris lumbricoides, Trichuris trichiura and hookworms (Necator americanus and Ancylostoma duodenale). The clinical symptoms of STH include abdominal pain, diarrhea, rectal prolapse, malabsorption of nutrients, and concomitant physical and cognitive impairments [1, 3]. Both schistosomiasis and STH are neglected tropical diseases (NTDs). The estimated global disability-adjusted life years lost due to schistosomiasis and STH in 2019 was 1.6 million and 2.8 million, respectively [4, 5]. Intestinal schistosomiasis caused by S. mansoni and STH are endemic in all regions of Ethiopia, while urogenital schistosomiasis is relatively restricted in its geographical distribution [6].
Pregnant women resident in endemic regions of schistosomiasis and STH could be vulnerable to these infections, often with severe consequences. To address this, the 2030 targets of the global NTDs road map 2021–2030 included reaching adolescent girls, pregnant women and lactating mothers with preventive chemotherapy (PC) [7–9]. The 2011–2020 road map focused on school-age children (SAC) [10, 11]. In 2019, an estimated 17 million pregnant women were dewormed globally for schistosomiasis and STH during maternal and child health services [5].
Maternal and child health is of great health concern, especially in low-income countries, including Ethiopia. Certain peri-natal infections may predispose to low-birth-weight infants, also affecting maternal health [12, 13]. Moreover, poor health infrastructure and low utilization of antenatal care (ANC) services in developing countries may deleteriously affect maternal and child health outcomes. Infants with low birth weight are at increased risk of death and compromised developmental issues later in life [14]. Despite the endemicity of S. mansoni and STHs in Ethiopia, emphasis is not given to screening the infections during ANC visits.
Lack of integration of schistosomiasis and STH control programs with maternal health services may affect maternal and child health. Schistosomiasis during pregnancy is associated with anemia, adverse birth outcomes, pre-term labor, dyspareunia, and menstrual disorders [15, 16]. Similarly, STH during pregnancy has adverse consequences on birth outcomes (low birth weight, abortion and pre-term labor) and maternal health (anemia, malnutrition and morbidities) [17]. Despite the endemicity of intestinal schistosomiasis and STH in Jimma town, there is paucity of data on the burden and impact of these infections on birth outcomes in the area. Therefore, this study aimed to determine the prevalence of schistosomiasis and STH, and its association with birth outcomes among pregnant women attending public health facilities in Jimma Town, Southwest Ethiopia.
Methods
Study setting and design
A cross-sectional study was conducted among pregnant women attending ANC service in selected public health facilities of Jimma Town from August to December 2021. Jimma Town is located 352 km Southwest of Addis Ababa, with an estimated population of 227,499 in 2020. According to the information obtained from Jimma Town Health Office, there are six public health facilities (Jimma Medical Center, Shenen General Gibe Hospital and four health centers), three private primary hospitals, and 49 private medium clinics in the town. Although most of these health facilities provide ANC services, only the six public health facilities, the three private primary hospitals and four private medium clinics had delivery services in 2021. Five public health facilities (Shenen Gibe General Hospital and the four health centers) were included in this study. The health centers were Becho Bore Health Center, Jimma Health Center, Jimma Higher-2 Health Center and Mendera Kochi Health Center.
Sample size and sampling technique
The five public health facilities were selected purposively based on their distribution in the town and presence of both ANC and delivery services within the health facility. Jimma Medical Center (Jimma University) was excluded from the study as it gives much of delivery services for referral cases from the surrounding districts and zones.
The sample size was calculated using single population proportion formula:
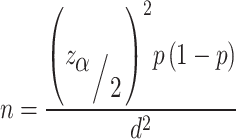 |
where ‘n’ is the minimum sample size, ‘z’ is the level of confidence according to the standard normal distribution (at 95% CI, (Zα/2)2 = 1.96), ‘p’ is the estimated prevalence, and ‘d’ is the margin of error (5%). After comparing the prevalence (p) of schistosomiasis (3%) [18] and STHs (25.2%) [19], p = 25.2% was used to calculate the minimum sample size required for the study. Accordingly, considering 10% for anticipated non-response and dropout rates, the final sample size obtained was 319 pregnant women. The sample size was allocated to each health facility proportionally based on the number of pregnant women recorded for ANC service in the previous year. Accordingly, the number of pregnant women allocated for each health center was 80 for Becho Bore Health Center, 22 for Jimma Higher-2 Health Center, 66 for Mendera Kochi Health Center, 63 for Jimma Health Center and 88 for Shenen Gibe General Hospital.
Eligibility
Inclusion
All pregnant women in their third-trimester gestational age who attended the selected public health facilities during the study period, and residents of Jimma Town.
Exclusion
Pregnant women who were not permanent residents of Jimma Town (not lived ≥ six months), who received anthelminthic drugs in the past four weeks, and who have not attended their respective health facility for delivery or referral.
Data collection
Questionnaire data
Questionnaire data including socio-demographic, associated factors and related morbidities were collected using a semi-structured questionnaire. The questionnaire was collected from pregnant women who attended the health facilities during their third trimester of gestation by two trained midwives from each health facility. The questionnaire was first prepared in English and translated into local languages (Afan Oromo and Amharic).
Laboratory data
A stool sample was collected from each consented pregnant woman during the third-trimester visit using a clean, leak-proof, labeled, and marked stool cup. The study participants were instructed to provide stool sample up to the designated mark on the stool cup, ensuring it was not contaminated with urine and other debris. The collected stool samples were stored in a refrigerator for no longer than four hours in the health facility and transported to Jimma University NTDs laboratory using a cold box, temperature maintained at 2-80 C, for examination. A single Kato-Katz smear [20] was deployed to detect ova of S. mansoni, STHs and other intestinal helminths, and the examination was done after 30 min of smear preparation for STHs (particularly, for hookworms), and after 24 h for S. mansoni. Sample processing and laboratory examinations were done by experienced laboratory experts at the Jimma University NTDs laboratory following standard operating procedures. The egg counts per gram of stool for both S. mansoni and STHs were classified into light, moderate, and heavy infection intensities based on the classification guidelines of World Health Organization [21].
Finger-prick blood sample was obtained from each participant pregnant woman and hemoglobin was measured using machine (HemoCue® Hb 301, Angelholm, Sweden), following the manufacturer’s instructions. The hemoglobin was measured by trained midwives in each health facility.
Anthropometric measurements
The weight and mid-upper arm circumference (MUAC) of the pregnant women were measured by the attending midwives during data collection using weight scale available at the ANC service room and MUAC measurement tape, respectively.
Birthoutcome data
Cards and ANC logbooks of the study participants were labeled with unique study identification code, and the required data was collected during delivery. The birth-outcome data including birth weight, postpartum bleeding (defined as estimated blood loss of > 500 ml within 24 h of delivery), and status of birth (live/stillbirth) were collected during delivery by the midwives. In referral cases, the principal investigator (PI) of the project was informed, the PI communicated with the referral health facility (Jimma Medical Center), and collected the required data from the mothers’ cards and physicians on duty at the time of delivery.
Data quality
Data collectors were trained onsite about the research objectives, how to collect and store stool samples, how to collect blood samples and test for hemoglobin using HemoCue analyzer, and how to keep and report data. Questionnaires were prepared in English, translated to local languages (Afan Oromo and Amharic), and back-translated to English by language experts to maintain the context. Adjustments and corrections to the questionnaire were made following a pre-test conducted on 5% of the sample size (n = 16) at Jimma University Medical Center. All collected questionnaire data were checked for completeness during the data handover and incomplete data were re-filled during subsequent visits or by phone. Randomly selected 10% of the Kato-Katz smears were re-checked by another laboratory expert who was blinded for the first reading.
Data management and analysis
Hard copies of the data were received from the health facility midwives. The data were entered, cleaned, and coded using Microsoft Excel, and exported to a statistical software package STATA-MP_12 (StataCorp., TX, USA) for analysis. Descriptive statistics were computed to summarize the socio-demographic profile of the study participants and parasite infection intensities. Chi-Square (χ2) test was used to measure the association of study variables with birth outcomes and clinical characteristics. Associations of the dependent S. mansoni and STH infections and the independent variables were analyzed using bivariate and multivariable logistic regression. Statistically significant association was considered at p ≤ 0.05.
Results
Socio-demographic characteristics
A total of 319 pregnant women were approached during their third-trimester visits at the selected health facilities and five were missed due to not visiting their respective health facilities for delivery. Thus, 314 pregnant women completed the study. About half of the pregnant women were in the age group of 25–34 years, and most of them were multigravida. The socio-demographic characteristics of the study participants were summarized in Table 1.
Table 1.
Socio-demographic characteristics of the pregnant women in selected public health facilities in Jimma Town, Southwest Ethiopia, 2021
| Characteristics | Frequency n (%) | |
|---|---|---|
| Age group (years) | 16–24 | 117(37.3) |
| 25–34 | 154(49.0) | |
| 35–45 | 43(13.7) | |
| Residence | Urban | 263(83.8) |
| Suburb | 51(16.2) | |
| Family size | < 5 | 172(54.8) |
| ≥ 5 | 142(45.2) | |
| Educational status | Secondary and above | 75(23.9) |
| Primary (1–8 grades) | 129(41.1) | |
| No formal education | 110(35.0) | |
| Occupation | Employed | 47(15.0) |
| Housewife | 218(69.4) | |
| Daily laborer | 27(8.6) | |
| Others* | 22(7.0) | |
| Attended health facility | Shenen Gibe Hospital | 85(27.1) |
| Becho Bore Health Center | 80(25.5) | |
| Mendera Kochi Health Center | 66(21.0) | |
| Jimma Health Center | 63(20.1) | |
| Jimma Higher- 2 Health Center | 20(6.3) | |
| Gravida a | Primigravida b | 64(20.4) |
| Multigravida c | 250(79.6) | |
| Latrine ownership | Yes | 303(96.5) |
| No | 11(3.5) | |
*Farmers, students & private workers
a Gravida = number of pregnancies, b Primigravida = first pregnancy, c Multigravida = multiple pregnancy
Prevalence of S. mansoni and STHs
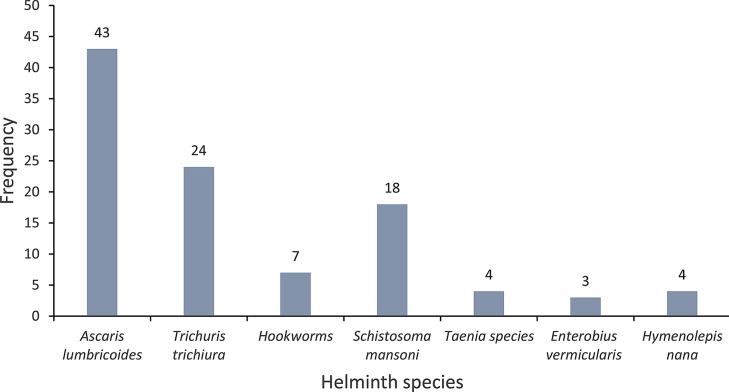
Seven species of intestinal helminths were detected in the stool samples of the study participants with overall prevalence of 26.1% (82/314). The prevalence of STHs and S. mansoni was 20.4% (64/314) and 5.7% (18/314), respectively. From the STHs group, A. lumbricoides accounted 13.7% (43/314) followed by T. trichiura – 7.6% (24/314) and hookworms – 2.2% (7/314). Multiple (triple and double) infections of intestinal helminths were identified in 7% (22/314) of the pregnant women. From these multiple infections, one had triple infections of A. lumbricoides, T. trichiura and E. vermicularis, six had double infections with A. lumbricoides and T. trichiura, two had double infections of A. lumbricoides and hookworms, two had double infections of T. trichiura and hookworms, and four harbored S. mansoni and STH infections. The frequency of intestinal helminthic infections is summarized in Fig. 1.
Fig. 1.
Frequency of intestinal helminthic infections among pregnant women in selected public health facilities in Jimma Town, Southwest Ethiopia, 2021
Infection intensities of S. mansoni and STHs
Infection intensities of S. mansoni and STHs among the pregnant women range from light to heavy intensities. Accordingly, for A. lumbricoides, 74.4% (32/43) light, 20.9% (9/43) moderate and 4.7% (2/43) heavy intensities. All the T. trichiura infections (n = 24) were with light intensities. Of the seven hookworm infections, six and one were light and moderate intensities, respectively. Similarly, most of the S. mansoni infections (83.3%) were with light intensity. Moderate and heavy S. mansoni infection intensities were detected in one and two of the infections, respectively. Geometric mean fecal egg per gram (epg) for A. lumbricoides was 881.3, interquartile range (IQR) = 4092 (168–4260) The corresponding mean epg for the remaining helminths were T. trichiura − 85.2 (IQR = 78 (48–126)), hookworms 168.9 (IQR = 300 (48–348)), and S. mansoni − 68.7 (IQR = 66 (30–96)).
Factors associated with STH infections
There was a significant difference in the prevalence of STHs by the health facility attended. Accordingly, pregnant women who attended Shenen Gibe General Hospital were four times more likely to be infected with STHs (AOR: 3.7; 95%CI: 1.4–9.5; p < 0.01) compared to the study participants from Becho Bore Health Center. Factors associated with STH infections among the pregnant women are presented in Table 2.
Table 2.
Factors associated with STH infections among pregnant women in selected public health facilities in Jimma Town, Southwest Ethiopia, 2021
| Characteristics | Positive n (%) | COR (95%CI) | p-value | AOR (95%CI) | p-value | |
|---|---|---|---|---|---|---|
| Age group (years) | 16–24 | 28(23.9) | 1.4(0.8–2.4) | 0.31 | 1.5(0.8–3.1) | 0.19 |
| 25–34 | 29(18.8) | 1 | 1 | |||
| 35–45 | 7(16.3) | 0.8(0.3–2.1) | 0.70 | 0.6(0.2–1.7) | 0.33 | |
| Residence | Urban | 56(21.6) | 1 | 1 | ||
| Suburb | 8(15.7) | 0.7(0.3–1.5) | 0.36 | 0.5(0.2–1.4) | 0.18 | |
| Educational status | Secondary and above | 14(18.7) | 0.8(0.4–1.7) | 0.61 | 0.8(0.4–1.8) | 0.62 |
| Primary (1–8 grades) | 28(21.7) | 1 | 1 | |||
| No formal education | 22(20.0) | 0.9(0.5–1.7) | 0.75 | 0.8(0.4–1.7) | 0.65 | |
| Occupation | Employed | 7(14.9) | 0.6(0.3–1.4) | 0.23 | 0.5(0.2–1.3) | 0.17 |
| Housewife | 50(22.9) | 1 | 1 | |||
| Daily laborer | 4(14.8) | 0.6(0.2–1.8) | 0.34 | 0.4(0.1–1.4) | 0.17 | |
| Others | 3(13.6) | 0.5(0.2–1.9) | 0.32 | 0.5(0.1–2.4) | 0.41 | |
| Attended health facility | Becho Bore HC | 11(13.8) | 1 | 1 | ||
| Jimma Higher-2 HC | 4(20.0) | 1.6(0.4–5.6) | 0.49 | 1.2(0.3–4.7) | 0.76 | |
| Mendera Kochi HC | 17(25.8) | 2.2(0.9–5.1) | 0.07 | 2.1(0.8–5.2) | 0.12 | |
| Jimma HC | 12(19.1) | 1.5(0.6–3.6) | 0.39 | 1.0(0.4–2.8) | 0.98 | |
| Shenen Gibe Hospital | 20(23.5) | 1.9(0.9–4.3) | 0.11 | 3.7(1.4–9.5) | < 0.01* | |
| Open defecation | Never | 51(18.9) | 1 | 1 | ||
| Sometimes | 13(29.6) | 1.8(0.9–3.7) | 0.11 | 2.0(0.8–5.1) | 0.12 | |
| Water source | Protected a | 51(19.3) | 1 | 1 | ||
| Unprotected b | 13(26.0) | 1.5(0.7-3.0) | 0.28 | 1.4(0.5–3.6) | 0.53 | |
| Walk barefoot | Never | 45(18.8) | 1 | 1 | ||
| Sometimes | 19(25.3) | 1.5(0.8–2.7) | 0.22 | 1.4(0.7–3.2) | 0.36 | |
aProtected = pipe water and water sources covered by concrete or other materials to prevent contamination
bUnprotected = water sources with no barriers to protect water from contamination
*Significant at p < 0.05
Abbreviations: CI, confidence interval; COR, crude odds ratio; AOR, adjusted odds ratio
Factors associated with S. mansoni infections
Schistosoma mansoni had significant association with washing clothes in the river (AOR: 10.7; 95%CI: 2.2–51.9; p < 0.01). Pregnant women who attended Mendera Kochi Health Center had a significantly higher odds of S. mansoni infections compared to Shenen Gibe General Hospital attendees (AOR: 23.5; 95% CI: 1.7–317; p = 0.02). Factors associated with S. mansoni infections are presented in Table 3.
Table 3.
Factors associated with S. mansoni infections among pregnant women in selected public health facilities in Jimma Town, Southwest Ethiopia, 2021
| Characteristics | Positive n(%) | COR (95%CI) |
p-value | AOR (95%CI) | p-value | |
|---|---|---|---|---|---|---|
| Age group (years) | 16–24 | 10(8.6) | 1 | 1 | ||
| 25–34 | 7(4.6) | 0.3(0.03–2.1) | 0.20 | 0.8(0.1–8.5) | 0.86 | |
| 35–45 | 1(2.3) | 0.5(0.2–1.4) | 0.18 | 1.0(0.3–3.1) | 0.98 | |
| Residence | Urban | 14(5.3) | 1 | 1 | ||
| Suburb | 4(7.8) | 1.5(0.5–4.8) | 0.48 | 2.7(0.6–11.2) | 0.18 | |
| Attended health facility | Becho Bore HC | 2(2.5) | 2.2(0.2–24.2) | 0.53 | 3.9(0.3–59.2) | 0.33 |
| Jimma Higher-2 HC | 0(0.0) | |||||
| Mendera Kochi HC | 10(15.2) | 15(1.9-120.5) | 0.01 | 23.5(1.7–317) | 0.02* | |
| Jimma HC | 5(7.9) | 7.2(0.8–63.6) | 0.07 | 7.5(0.6–97.1) | 0.12 | |
| Shenen Gibe Hospital | 1(1.2) | 1 | 1 | |||
| Walk barefoot | Never | 10(4.2) | 1 | 1 | ||
| Sometimes | 8(10.7) | 2.7(1.0-7.2) | 0.04 | 1.1(0.2–5.9) | 0.93 | |
| Cross river barefoot | Never | 9(3.7) | 1 | 1 | ||
| Sometimes | 9(12.3) | 3.6(1.4–9.5) | 0.01 | 1.1(0.2–6.7) | 0.90 | |
| Bath in river | Never | 10(4.2) | 1 | 1 | ||
| Sometimes | 8(10.8) | 2.8(1.1–7.3) | 0.04 | 3.8(0.8–18.0) | 0.09 | |
| Wash cloth in river | Never | 5(2.3) | 1 | 1 | ||
| Sometimes | 13(13.7) | 6.8(2.4–19.6) | 0.00 | 10.7(2.2–51.9) | < 0.01* | |
*Significant at p < 0.05
Abbreviations: CI, confidence interval; COR, crude odds ratio; AOR, adjusted odds ratio; HC, Health Center
Clinical characteristics associated with S. mansoni and STH infections
The presence of blood in stool and abdominal pain in the preceding two weeks prior to the survey had statistically significant association with S. mansoni and STH infections (p < 0.05), respectively. However, self-reported diarrhea in the preceding two weeks had no statistically significant association with both S. mansoni and STH infections (p > 0.05). Table 4 summarizes the statistical association of the clinical characteristics with S. mansoni and STHs infection using χ2 test.
Table 4.
Clinical characteristics associated with S. mansoni and STH infections among pregnant women in selected public health facilities in Jimma Town, Southwest Ethiopia, 2021
| Characteristics | S. mansoni infections | STH infections | |||||
|---|---|---|---|---|---|---|---|
| Yes % | χ2 | p-value | Yes % | χ2 | p-value | ||
| Blood in stool (past two weeks) | No | 4.4 (12/271) | 1 | 19.2 (52/271) | 1 | ||
| Yes | 14.0 (6/43) | 6.231 | 0.013* | 27.9 (12/43) | 1.738 | 0.187 | |
| Abdominal pain (past two weeks) | No | 5.9 (11/188) | 1 | 16.0 (30/188) | 1 | ||
| Yes | 5.6 (7/126) | 0.012 | 0.912 | 26.0 (34/126) | 5.652 | 0.017* | |
| Diarrhea the past two weeks | No | 5.7 (15/265) | 1 | 19.6 (52/265) | |||
| Yes | 6.1 (3/49) | 0.016 | 0.898 | 24.9 (12/49) | 0.604 | 0.437 | |
*Significant at P < 0.05
χ2: Chi-Square test
Birth outcomes and associated factors
The prevalence of low birth weight was 6.4% (20/314), and three stillbirths (1%) were recorded. Twelve (3.8%) of the mothers experienced postpartum bleeding. Pre-term delivery was observed in 2.9% (9/314) of the mothers. Low birth weight was significantly higher among late-term deliveries compared to full-term deliveries (p < 0.05). Anemia was significantly higher among infants with low birth weight (p < 0.01). Similarly, anemia had statistically significant association with maternal postpartum bleeding (p < 0.01). Other study variables including history of abortion, MUAC and infections with S. mansoni and STHs had no statistically significant association with both neonatal low birth weight and postpartum bleeding (p > 0.05). Table 5 summarizes X2 results of associated factors of neonatal birth weight and postpartum bleeding.
Table 5.
Associated factors of neonatal low birth weight and postpartum bleeding among pregnant women in selected public health facilities in Jimma Town, Southwest Ethiopia, 2021
| Characteristics | Low birth weight | Post-partum bleeding | |||||
|---|---|---|---|---|---|---|---|
| Yes % | χ2 | p-value | Yes % | χ2 | p-value | ||
| Gestation during delivery | Full term | 5.2 (13/251) | 1 | 3.6 (9/251) | 1 | ||
| Late term | 13.0 (7/54) | 4.394 | 0.036* | 5.6 (3/54) | 0.456 | 0.499 | |
| History of abortion | No | 6.3 (18/288) | 1 | 3.5 (10/288) | 1 | ||
| Yes | 7.7 (2/26) | 0.083 | 0.773 | 7.7 (2/26) | 1.155 | 0.282 | |
| Anemia | No | 4.5 (12/266) | 1 | 2.3 (6/266) | 1 | ||
| Yes | 16.7 (8/48) | 10.074 | 0.002* | 12.5 (6/48) | 11.61 | 0.001* | |
| Mother’s weight (kg) | ≥ 55 | 6.5 (15/232) | 1 | 2.6 (6/232) | 1 | ||
| 37–54 | 6.1 (5/82) | 0.014 | 0.907 | 7.3 (6/82) | 3.689 | 0.055 | |
| MUAC** | ≥ 23 | 4.4 (8/183) | 1 | 3.8 (7/183) | 1 | ||
| < 23 | 9.2 (12/131) | 2.936 | 0.087 | 3.8 (5/131) | 0 | 0.997 | |
| S. mansoni infection | No | 6.4 (19/296) | 1 | 3.7 (11/296) | 1 | ||
| Yes | 5.6 (1/18) | 0.021 | 0.884 | 5.6 (1/18) | 0.156 | 0.693 | |
| STH infections | No | 6.4 (16/250) | 1 | 4.4 (11/250) | 1 | ||
| Yes | 6.3 (4/64) | 0.002 | 0.965 | 1.6 (1/64) | 1.116 | 0.291 | |
*Significant at p < 0.05
**mid-upper arm circumference
χ2: Chi-Square test
Discussion
Both schistosomiasis and STHs are targeted for elimination of transmission by 2030 in the third strategic plan 2021 − 230 of Ethiopia. Over the past decade, the distribution of schistosomiasis and STH has been mapped nationally, and school-based PC is currently being implemented in endemic districts. From 2014 to 2018, PC to schistosomiasis and STH has been distributed to over 27 million and 92 million SAC, respectively [22]. This might have contributed to the remarkable reduction of the burden of both schistosomiasis and STH recorded in school children [22, 23]. However, the national PC program to control schistosomiasis and STH did not target adolescent girls, pregnant women and lactating mothers.
The prevalence of STH infections among the pregnant women in this study is 20.4% (95%CI: 15.9–24.8). A previous study conducted among pregnant women in the same study area also reported a similar magnitude (19.7%) of STH infections [24]. Studies conducted among school children in the same area revealed a decreasing pattern of STH infection prevalence from 68.6% in 2014 to 19.9% in 2021 [25–28]. It appears the mass drug administration program to control STHs remarkably reduced the burden of STHs among school children. However, the transmission and infection intensities of STHs among pregnant women remain unchanged. A recent report also corroborates this [29]. Failure to address the other at-risk population segments during STH control programs, including adolescent girls, pregnant women and lactating mothers, may result in the reemergence of the disease after reducing the number of PC tablets based on the 2030 targets. Therefore, it is essential to include adolescent girls, pregnant women and lactating mothers to the national PC program based on the 2021–2030 global STHs control programs [7]. In this study, the prevalence of STH infections was significantly higher among pregnant women who attended ANC services at Shenen Gibe General Hospital compared with Becho Bore Health Center. Identifying such high STH transmission areas in pregnant women will help to plan local interventions on increasing awareness on the modes of transmission of STHs and provision of STHs diagnosis and prompt treatment at health facility level.
Transmission of schistosomiasis is often localized, mainly depending on the presence of freshwater bodies and specific intermediate hosts. In this study, the prevalence of S. mansoni is significantly higher among pregnant women who attended ANC services at Mendera Kochi Health Center compared to those attending Shenen Gibe Hospital. Studies conducted elsewhere in Ethiopia also documented varying prevalence rates of schistosomiasis among pregnant women [18, 30, 31]. Mendera Kochi Health Center is located in the suburb of Jimma Town where there are multiple freshwater bodies, which might have contributed to the significantly higher prevalence of S. mansoni among the pregnant women in its catchment area. A previous study done among schoolchildren in Jimma Town also reported a significantly higher prevalence of S. mansoni infection in the locality [25]. Thus, focal intervention is essential to control both the disease and the snail intermediate hosts. Pregnant women washing clothes in the river had a higher likelihood of S. mansoni infections. Studies conducted elsewhere also revealed significant association between washing clothes in the river and S. mansoni infections [32, 33]. Women of reproductive age in developing countries are usually engaged with washing clothes in rivers and fetching water from rivers, which may predispose them to S. mansoni infection [33]. Therefore, it is important to consider women of reproductive age in general and pregnant women in particular in schistosomiasis control programs.
Schistosomiasis and STH infections may result in spectrum of disease manifestations, ranging from asymptomatic to severe abdominal symptoms, mainly depending on the intensity of infection and physiological status of the infected individual, among others. In this study, there was a significant association between STH infections and abdominal pain. Moreover, S. mansoni infection was significantly associated presence of blood in stool. Similar findings were also reported earlier [2, 25]. Due to transient immunological and hormonal changes, and possibly nutritional deficiencies during pregnancy, pregnant women may easily succumb to STH and S. mansoni infections, the infections often adversely affecting birth outcome [13].
The common adverse birth outcomes include preterm birth, low birth weight, postpartum bleeding, and stillbirth. In this study, anemia among the pregnant women was significantly associated with low birth weight and maternal postpartum bleeding compared with non-anemic pregnant women. Moreover, post-term delivery was also associated with low birth weight. Other studies also reported significant association of anemia with low birth weight and postpartum bleeding [34, 35]. Low birth weight adversely effects neonatal health and development. Maternal postpartum bleeding mainly in the presence of anemia may result in complications and maternal deaths [35]. Some previous studies also reported the association of S. mansoni and STH infections with adverse birth outcomes including low birth weight [36, 37]. However, this was not observed in this study. It should be noted that due to the cross-sectional nature of this study, examination and treatment of the parasites took place during the third trimester, and birth outcome was assessed at delivery. Thus, it is not possible to ascertain the duration of infections. Moreover, other factors possibly affecting pregnancy outcomes, including ANC visits and nutritional status of the pregnant women were not assessed in the study. Furthermore, the use of a single Kato-Katz smear for each stool specimen may have limited the sensitivity of detecting low-intensity infections.
Conclusions
More than a quarter of the pregnant women were infected with STHs and S. mansoni. The prevalence of S. mansoni is significantly higher among pregnant women attending ANC at Mendera Kochi Health Center. Washing clothes in the river appears to be the most important factor associated with S. mansoni infection among pregnant women in the area. It is recommended to raise awareness among pregnant women in the area about the transmission methods of S. mansoni in general, and discourage washing clothes in the river. Pregnant women in the area need to be screened for intestinal parasitic infections during their ANC follow-up. The impact of maternal STH and S. mansoni infections on adverse birth outcomes should be studied further. Moreover, due attention should be given to the underlying cause of anemia among pregnant women in the area.
Acknowledgements
We would like to acknowledge Jimma University for funding the project. We thank Jimma Town Health Office for giving information, and the health facility directors and their staff members for their support during fieldwork. We are also grateful to the study participants for taking part in the study.
Abbreviations
- ANC
Antenatal Care
- EPG
Eggs Per Gram
- IQR
Interquartile Range
- MUAC
Mid-upper Arm Circumference
- NTDs
Neglected Tropical Diseases
- PC
Preventive Chemotherapy
- SAC
School-Age Children
- STHs
Soil-transmitted Helminthiases
Author contributions
AT conceived the study. AT, EZ, BAM, HG, DD, and MA involved in the designing of the research and data acquisition. AT and EZ involved in data analysis and drafted the manuscript. ZM critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Funding
The study was financially supported by Jimma University.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from ethical review board of Institute of Health, Jimma University (Ref: IHRPGD/105/21). Permission was sought from administrations of the health facilities. The aim of the study was explained to the study participants and informed consent was obtained from all study participants. The study participants provided written consent prior to enrollment into the study. Confidentiality of individual information was maintained at all steps of the research. Pregnant women infected with any of the intestinal parasites were treated based on the national guidelines. Study participants with anemia (hemoglobin < 11 g/dl) were counseled and treated for improvement of their hemoglobin level.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Schistosomiasis and soil-transmitted helminthiases: progress report, 2021. Geneva: World Health Organization; 2022. [Google Scholar]
- 2.Carbonell C, Rodríguez-Alonso B, López-Bernús A, Almeida H, Galindo-Pérez I, Velasco-Tirado V, et al. Clinical spectrum of schistosomiasis: an update. J Clin Med. 2021;10:5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jourdan PM, Lamberton PH, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet. 2018;391:252–65. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Working to overcome the global impact of neglected tropical diseases. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 5.WHO. Schistosomiasis and soil-transmitted helminthiases: progress report. 2020. https://www.who.int/publications/i/item/who-wer9648-585-595 (Accessed August 25, 2023).
- 6.Chala B, Torben W. An epidemiological trend of urogenital schistosomiasis in Ethiopia. Front Public Health. 2018;6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. 2030 targets for soil-transmitted helminthiases control programmes. https://www.who.int/publications/i/item/9789240000315 (Accessed August 25, 2023).
- 8.WHO. WHO guideline on control and elimination of human schistosomiasis. https://www.who.int/publications/i/item/9789240041608 (Accessed August 25, 2023). [PubMed]
- 9.WHO. Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030. https://www.who.int/publications/i/item/9789240010352 (Accessed Aug 25, 2023).
- 10.WHO. Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation: executive summary. https://iris.who.int/handle/10665/70809 (Accessed September 24, 2023).
- 11.WHO. Eliminating soil-transmitted helminthiases as a public health problem in children. Progress report 2001 – 2010 and strategic plan 2011 – 2020. Avaialbe at https://www.who.int/publications/i/item/9789241503129 (Accessed September 24, 2023).
- 12.Hurt K, Kodym P, Stejskal D, Zikan M, Mojhova M, Rakovic J. Toxoplasmosis impact on prematurity and low birth weight. PLoS ONE. 2022;17:e0262593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ness TE, Agrawal V, Bedard K, Ouellette L, Erickson TA, Hotez P, et al. Maternal hookworm infection and its effects on maternal health: a systematic review and meta-analysis. Am J Trop Med Hyg. 2020;103:1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jana A, Saha UR, Reshmi RS, Muhammad T. Relationship between low birth weight and infant mortality: evidence from National Family Health Survey 2019-21, India. Arch Public Health. 2023;81:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adam I, ALhabardi NA, Al-Wutayd O, Khamis AH. Prevalence of schistosomiasis and its association with anemia among pregnant women: a systematic review and meta-analysis. Parasit Vectors. 2021;14. [DOI] [PMC free article] [PubMed]
- 16.Demeke G, Mengistu G, Abebaw A, Toru M, Yigzaw M, Shiferaw A, et al. Effects of intestinal parasite infection on hematological profiles of pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia: Institution based prospective cohort study. PLoS ONE. 2021;16:e0250990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aderoba AK, Iribhogbe OI, Olagbuji BN, Olokor OE, Ojide CK, Ande AB. Prevalence of helminth infestation during pregnancy and its association with maternal anemia and low birth weight. Int J Gynecol Obstet. 2015;129:199–202. [DOI] [PubMed] [Google Scholar]
- 18.Derso A, Nibret E, Munshea A. Prevalence of intestinal parasitic infections and associated risk factors among pregnant women attending antenatal care center at Felege Hiwot Referral Hospital, northwest Ethiopia. BMC Infect Dis. 2016;16. [DOI] [PMC free article] [PubMed]
- 19.Lebso M, Anato A, Loha E. Prevalence of anemia and associated factors among pregnant women in Southern Ethiopia: a community based cross-sectional study. PLoS ONE. 2017;12:e0188783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Basic laboratory methods in medical parasitology. World Health Organization; 1991.
- 21.WHO. Preventive chemotherapy in human helminthiasis. Geneva: World Health Organization; 2006. [Google Scholar]
- 22.MoH. The Third National Neglected Tropical Diseases Strategic Plan 2021–2025. Addis Ababa: Ministry of Health-Ethiopia: 2021. https://espen.afro.who.int/system/files/content/resources/Third%20NTD%20national%20Strategic%20Plan%202021-2025.pdf
- 23.Maddren R, Phillips A, Ower A, Landeryou T, Mengistu B, Anjulo U et al. Soil-transmitted helminths and schistosome infections in Ethiopia: a systematic review of progress in their control over the past 20 years. Parasit Vectors. 2021;14. [DOI] [PMC free article] [PubMed]
- 24.Getachew M, Yeshigeta R, Tiruneh A, Alemu Y, Dereje E, Mekonnen Z. Soil-transmitted helminthic infections and geophagia among pregnant women in Jimma town health institutions. Southwest Ethiopia EJHS. 2021;31. [DOI] [PMC free article] [PubMed]
- 25.Tiruneh A, Zemene E, Abdissa Mizana B, Girma H, Dereje E, Sharew B, et al. Schistosoma mansoni infections and morbidities among School Children in Hotspot areas of Jimma Town, Southwest Ethiopia: a cross-sectional study. Environ Health Insights. 2023;17:11786302231161047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tadege B, Mekonnen Z, Dana D, Tiruneh A, Sharew B, Dereje E, et al. Assessment of the nail contamination with soil-transmitted helminths in schoolchildren in Jimma Town, Ethiopia. PLoS ONE. 2022;17:e0268792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dana D, Vlaminck J, Mekonnen Z, Ayana M, Vogt F, Verdonck K et al. Diagnostic sensitivity of direct wet mount microscopy for soil-transmitted helminth infections in Jimma Town. Ethiopia JIDC. 2020;14. [DOI] [PubMed]
- 28.Mekonnen Z, Hassen D, Debalke S, Tiruneh A, Asres Y, Chelkeba L, et al. Soil-transmitted helminth infections and nutritional status of school children in government elementary schools in Jimma Town, Southwestern Ethiopia. SAGE Open Med. 2020;8:2050312120954696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gebrehiwet MG, Medhaniye AA, Alema HB. Prevalence and associated factors of soil transmitted helminthes among pregnant women attending antenatal care in Maytsebri primary hospital, North Ethiopia. BMC Res Notes. 2019;12. [DOI] [PMC free article] [PubMed]
- 30.Dagnaw A, Sahlie M, Mulugeta H, Shine S, Bediru W, Zebene A et al. Magnitude of intestinal parasite infection and Associated factors among pregnant women attending Antenatal Care Service in Shewarobit Town Health Facilities, North Shoa Zone, Amhara Region, Ethiopia. Infect Drug Resist. 2021:4921–30. [DOI] [PMC free article] [PubMed]
- 31.Alula GA, Munshea A, Nibret E. Prevalence of intestinal parasitic infections and associated risk factors among pregnant women attending prenatal care in the Northwestern Ethiopia. Biomed Res Int. 2021;2021. [DOI] [PMC free article] [PubMed]
- 32.N’Zi CK, Ouattara M, Assaré RK, Bassa FK, Diakité NR, N’Goran EK. Risk Factors and Spatial Distribution of Schistosoma mansoni Infection among Preschool-Aged Children in Blapleu, Biankouma District, Western Cˆote d’Ivoire. J Trop Med. 2021;2021. [DOI] [PMC free article] [PubMed]
- 33.Mazigo HD, Samson A, Lambert VJ, Kosia AL, Ngoma DD, Murphy R, et al. We know about schistosomiasis but we know nothing about FGS: a qualitative assessment of knowledge gaps about female genital schistosomiasis among communities living in Schistosoma haematobium endemic districts of Zanzibar and Northwestern Tanzania. PLoS Negl Trop Dis. 2021;15(9):e0009789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Figueiredo ACMG, Gomes-Filho IS, Batista JET, Orrico GS, Porto ECL, Cruz Pimenta RM, et al. Maternal anemia and birth weight: a prospective cohort study. PLoS ONE. 2019;14:e0212817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omotayo MO, Abioye AI, Kuyebi M, Eke AC. Prenatal anemia and postpartum hemorrhage risk: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2021;47. [DOI] [PMC free article] [PubMed]
- 36.Gerstenberg J, Mishra S, Holtfreter M, Richter J, Davi SD, Okwu DG, Ramharter M, Mischlinger J, Schleenvoigt BT. Hum Placent Schistosomiasis—A Syst Rev Literature Pathogens. 2024;13:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honkpehedji YJ, Adegbite BR, Zinsou JF, Dejon-Agobé JC, Edoa JR, Zoleko Manego R et al. Association of low birth weight and polyparasitic infection during pregnancy in Lambaréné, Gabon. Trop Med Int Health. 2021;26. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.