Abstract
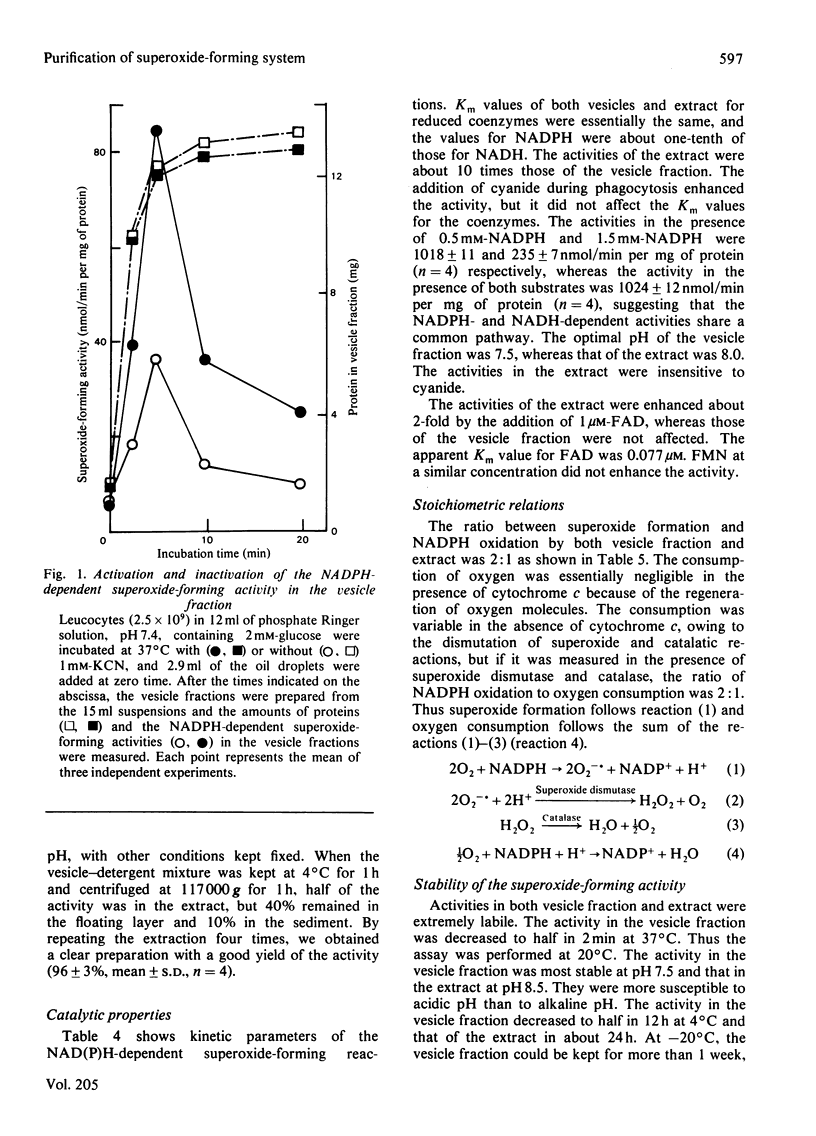
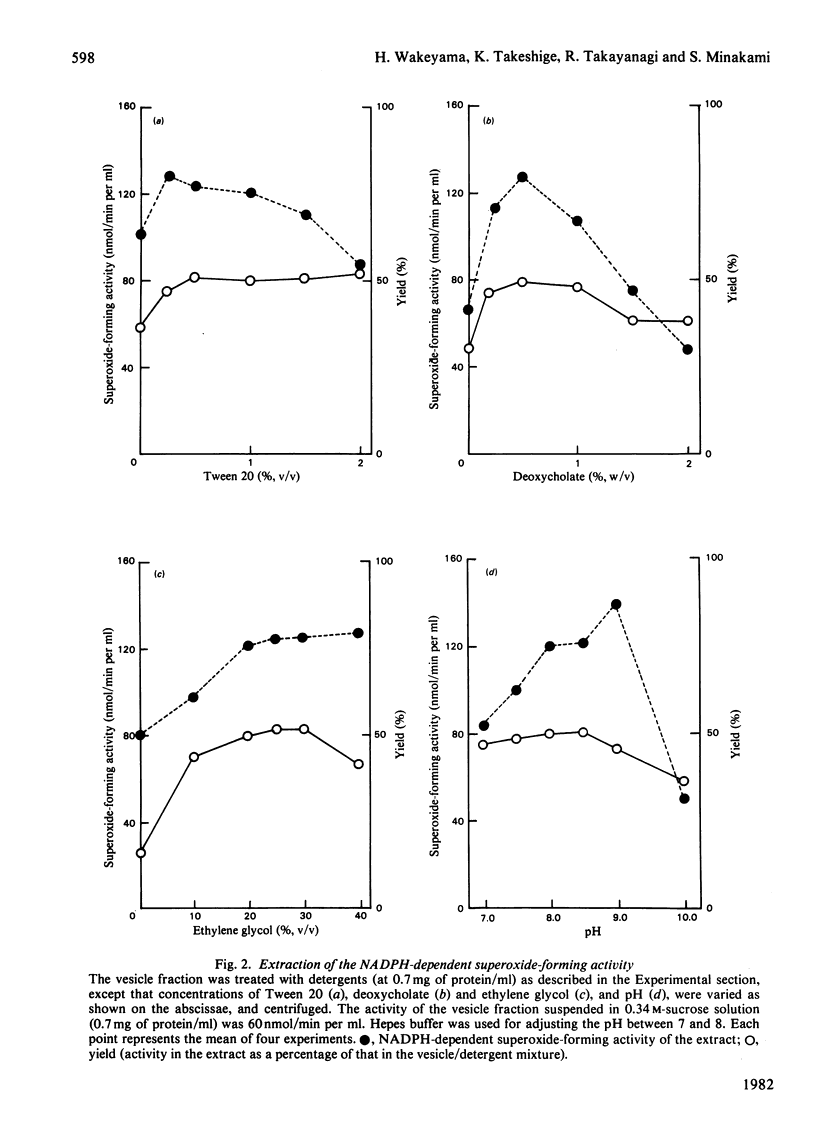
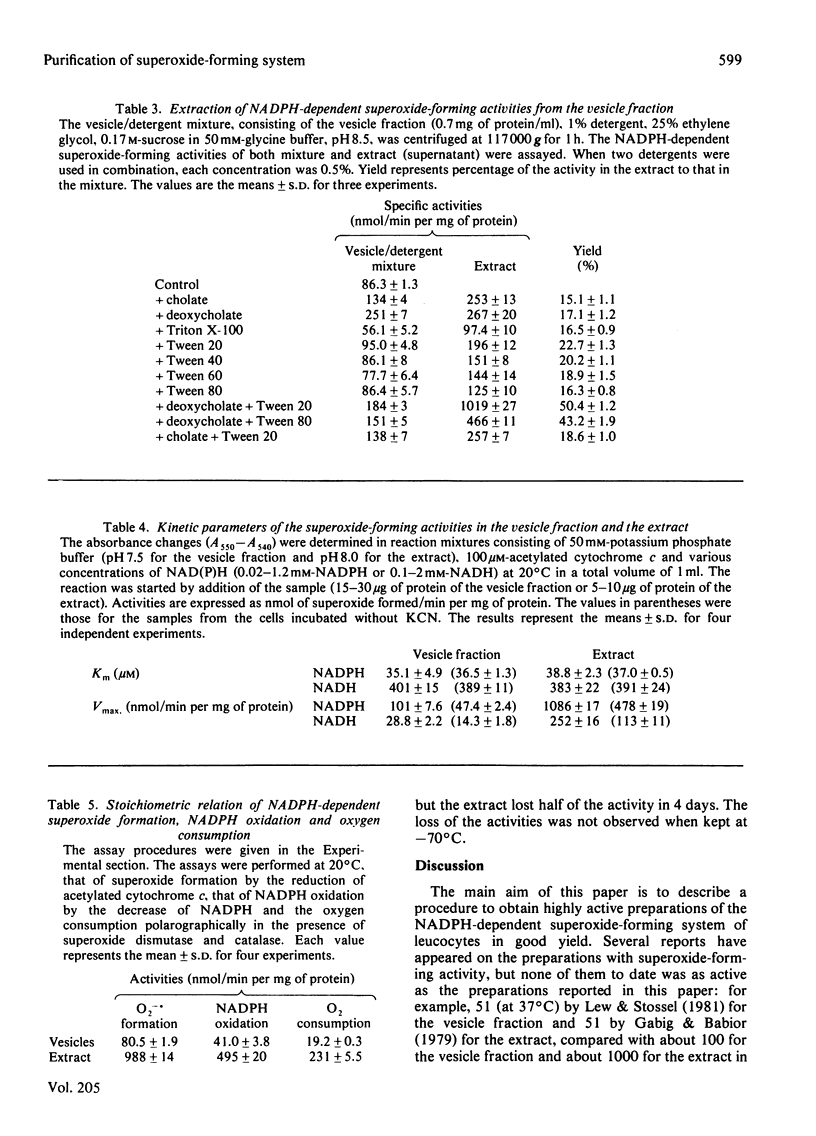
A phagocytic vesicle fraction with high NADPH-dependent superoxide-forming activity was obtained in large quantity from pig blood polymorphonuclear leucocytes, phagocytosing oil droplets in the presence of cyanide. The activity of the homogenate of the phagocytosing cells was 40 times that of the resting cells, and 70% of the activity in the homogenate was recovered in the phagocytic vesicle fraction. Essentially all of the superoxide-forming activity was extracted by repeated extraction with a mixture containing deoxycholate and Tween 20. The extract had a superoxide-forming activity of 1 mumol/min per mg of protein with NADPH, and one-fifth of this with NADH, Km values being similar to those of the vesicle fraction (40 microM for NADPH and 400 microM for NADH). A stoichiometric relationship of 1:2 for NADPH oxidation and superoxide formation was obtained, in agreement with the reaction NADPH +2O2 leads to NADP+ + 2O2 -. + H+. The activity of the extract was enhanced 2-fold by the addition of FAD, suggesting that the flavin is a component of the enzyme system. The Km value for FAD was 0.077 microM. The activities in both vesicle fraction and extract were labile even on refrigeration, but could be kept for several months at -70 degrees C.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano F., Mizuno D. Fc-receptor modulates the fusion between phagocytic vesicles and lysosomes in guinea pig polymorphonuclear leukocytes. FEBS Lett. 1980 Jun 30;115(2):193–196. doi: 10.1016/0014-5793(80)81166-5. [DOI] [PubMed] [Google Scholar]
- Aust S. D., Roerig D. L., Pederson T. C. Evidence for superoxide generation by NADPH-cytochrome c reductase of rat liver microsomes. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1133–1137. doi: 10.1016/0006-291x(72)90952-7. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr, Nathan D. G. Comparative study of the metabolic and bactericidal characteristics of severely glucose-6-phosphate dehydrogenase-deficient polymorphonuclear leukocytes and leukocytes from children with chronic granulomatous disease. J Reticuloendothel Soc. 1972 Aug;12(2):150–169. [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E., Davies W. A. Activation of the guinea pig granulocyte NAD(P)H-dependent superoxide generating enzyme: localization in a plasma membrane enriched particle and kinetics of activation. Blood. 1980 Mar;55(3):355–363. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gabig T. G., Babior B. M. The O2(-) -forming oxidase responsible for the respiratory burst in human neutrophils. Properties of the solubilized enzyme. J Biol Chem. 1979 Sep 25;254(18):9070–9074. [PubMed] [Google Scholar]
- Hirschhorn R., Hirschhorn K., Weissmann G. Appearance of hydrolase rich granules in human lymphocytes induced by phytohemagglutinin and antigens. Blood. 1967 Jul;30(1):84–102. [PubMed] [Google Scholar]
- Iverson D., DeChatelet L. R., Spitznagel J. K., Wang P. Comparison of NADH and NADPH oxidase activities in granules isolated from human polymorphonuclear leukocytes with a fluorometric assay. J Clin Invest. 1977 Feb;59(2):282–290. doi: 10.1172/JCI108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma K., Minakami S. Effects of fatty acids on superoxide radical generation in leukocytes. Biochim Biophys Acta. 1978 Jan 3;538(1):50–59. doi: 10.1016/0304-4165(78)90251-9. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Mikkelsen R. B., Corfman D. H., André-Schwartz J. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 1980 Jul;86(1):21–28. doi: 10.1083/jcb.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Karnovsky M. J., Karnovsky M. L. The distributions of some granule-associated enzymes in guinea-pig polymorphonuclear leucocytes. Biochem J. 1970 Jan;116(2):207–216. doi: 10.1042/bj1160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi Z. F., Takeshige K., Hatae T., Minakami S. Hydrogen peroxide formation of polymorphonuclear leukocytes stimulated with cytochalasin D. Exp Cell Res. 1979 Dec;124(2):293–300. doi: 10.1016/0014-4827(79)90205-2. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Baxter C. R., Masters B. S. Simultaneous demonstration of phagocytosis-connected oxygen consumption and corresponding NAD(P)H oxidase activity: direct evidence for NADPH as the predominant electron donor to oxygen in phagocytizing human neutrophils. Biochem Biophys Res Commun. 1981 Feb 12;98(3):743–751. doi: 10.1016/0006-291x(81)91175-x. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Reduction and subsequent oxidation of a cytochrome b of human neutrophils after stimulation with phorbol myristate acetate. Biochem Biophys Res Commun. 1979 May 14;88(1):130–134. doi: 10.1016/0006-291x(79)91706-6. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. The role of the phagocyte in host-parasite interactions. VII. Di- and triphosphopyridine nucleotide kinetics during phagocytosis. Biochim Biophys Acta. 1967 Jul 25;141(2):243–249. doi: 10.1016/0304-4165(67)90097-9. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Lehrer R. I. NAD(P)H oxidase activity in human neutrophils stimulated by phorbol myristate acetate. J Clin Invest. 1980 Dec;66(6):1409–1418. doi: 10.1172/JCI109994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K., Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem J. 1979 Apr 15;180(1):129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. W., Powars D. R., Hochstein P. New evidence for the role of NADH oxidase in phagocytosis by human granulocytes. Biochem Med. 1975 May;13(1):83–88. doi: 10.1016/0006-2944(75)90142-8. [DOI] [PubMed] [Google Scholar]


