Abstract
Purpose
To investigate the safety and effectiveness of naltrexone-bupropion in Korean adults with obesity.
Patients and methods
This was a prospective, observational multicenter study from April 29, 2016, to April 28, 2022. Individuals with obesity with a body mass index of ≥30 kg/m2 or ≥27 kg/m2 who had obesity-related comorbidities were included. The naltrexone-bupropion dose was gradually titrated weekly from 8/90 to 32/360 mg and maintained at the maximum tolerated dose. In total, 612 and 300 individuals were evaluated for safety and effectiveness, respectively.
Results
In total, 41.34% individuals reported drug-related adverse reactions, such as nausea (19.12%), headache (7.68%), and dizziness (5.23%). Older age and comorbidities were significantly associated with adverse events. At 12 weeks after reaching the maintenance dose, naltrexone-bupropion 32/360 mg resulted in the greatest weight reduction (−7.21%) compared with other doses, which persisted at week 24 (−7.69%). The naltrexone-bupropion 16/180 mg resulted in significant weight reduction, achieving −5.99% and −9.18% reductions at weeks 12 and 24, similar to that with naltrexone-bupropion 32/360 mg. Young age and no comorbidities were significantly associated >5% weight reduction.
Conclusion
Naltrexone-bupropion demonstrated marked stability and weight loss effectiveness, particularly in young individuals with obesity without comorbidities. Therefore, individualized treatment is necessary when prescribing naltrexone-bupropion.
Keywords: naltrexone-bupropion, obesity management, post-marketing survey, weight loss efficacy, adverse events
Introduction
Obesity is a global health epidemic and its prevalence steadily increased over the past few decades.1 The prevalence of obesity in Korea has also shown a continuous increase, rising from 30.2% in 2012 to 38.4% in 2021.2 It is closely associated with various metabolic disorders, including hypertension, type 2 diabetes mellitus, and dyslipidemia, which exacerbate the disease burden and eventually lead to mortality.3,4 Lifestyle modifications, such as diet and exercise, remain the cornerstone of obesity management;5 however, these interventions alone may not provide sufficient and sustained weight loss for many individuals.6
Pharmacotherapy has emerged as an adjunct treatment option for individuals struggling with obesity. Long-term treatment with anti-obesity medications is essential for effective obesity management, necessitating the establishment of drug safety and efficacy over extended periods.7 Although many drugs have been developed for obesity, only a few have proven long-term safety, one of which is naltrexone-bupropion.8,9 Naltrexone-bupropion was approved by the FDA (Food and Drug Administration) for long-term weight management in patients with obesity in 2014. Naltrexone, an opioid receptor antagonist, and bupropion, a dopamine and norepinephrine reuptake inhibitor, act synergistically to modulate appetite and energy balance.10 This combination therapy has demonstrated efficacy in promoting weight loss by reducing food intake, enhancing energy expenditure, and improving metabolic parameters in clinical trials.11–13
Despite the promising outcomes observed in randomized controlled trials (RCTs) and meta-analysis,9,11–14 post-marketing surveillance is imperative for assessing the real-world effectiveness and safety profile of naltrexone-bupropion therapy. According to a study conducted in the United Kingdom, real-world evidence suggests that patients with type 2 diabetes using GLP-1 (Glucagon-Like Peptide-1) receptor agonists experience lower weight loss effects than those reported in clinical trials, with only a small proportion of patients achieving clinically significant weight reduction.15 RCTs of anti-obesity medications may not fully reflect the diverse and dynamic conditions encountered in everyday life, thus potentially limiting their applicability to real-world settings where multiple variables interact and emphasizing the importance of real-world evidence.16 However, a few studies have analyzed the effects and side effects of naltrexone-bupropion, and to the best of our knowledge, none of these studies have been conducted in Asian populations. Therefore, we conducted this study to evaluate the effectiveness and safety of naltrexone-bupropion in Korean adults with obesity using post-marketing surveillance.
Methods
Study Design
This prospective, real-world, single country, non-interventional post-marketing surveillance study was conducted from April 29, 2016 to April 28, 2022. Naltrexone-bupropion (Contrave®) was approved by the FDA for long-term obesity treatment on September 10, 2014. It was approved by the Korean MFDS (Ministry of Food and Drug Safety) on April 29, 2016, for weight management in individuals with overweight or obesity. This study adhered to the Standard for Re-examination of New Drugs as specified in the Pharmaceutical Affairs Act, Regulation on the Safety of Medicinal Products, and Korean MFDS guidelines. To meet the sample size requirement for the reexamination of new drugs, as stipulated in MFDS Notification No. 2015–79 (dated October 30, 2015), the study aimed to recruit more than 600 individuals.17
Naltrexone-bupropion was initiated with a titration phase, starting from the lowest dose (8 mg of naltrexone and 90 mg of bupropion per day) and adjusted to a maintenance dose based on tolerance. The dosages of naltrexone-bupropion were gradually increased on a weekly basis, starting from an initial 8/90 mg dose to a maintenance dose of 32/360 mg, based on individual tolerance. Specifically, in the first week, patients were instructed to take one 8/90 mg tablet in the morning with breakfast. In the second week, the dosage was increased to one tablet in the morning and one in the evening. During the third week, patients were advised to take two tablets in the morning and one in the evening. By the fourth week, patients reached the final recommended dosage of two tablets twice daily. However, if patients experienced side effects that made it difficult to increase the dosage before reaching four tablets per day, they were instructed to maintain the highest tolerable dose. This titration was aimed at minimizing the adverse effects of early treatment, while achieving optimal therapeutic effectiveness. If individuals experienced adverse effects and consequently, could not up-titrate, we allowed them to maintain maximum tolerable doses.
Participants
Participants were recruited from 54 healthcare centers specializing in obesity treatment across various regions as part of a post-marketing surveillance study. The study enrolled participants with obesity aged 19 to 74 with a body mass index (BMI) ≥ 30 kg/m2, as well as individuals with overweight with a BMI of 27 kg/m2 to <30 kg/m2 who had at least one additional cardiovascular risk factors, such as hypertension, dyslipidemia, or type 2 diabetes. Eligible participants were those who had not previously received naltrexone-bupropion; the drugs were prescribed to them as part of routine clinical care after the contract date with the respective hospitals. Exclusion criteria included uncontrolled hypertension, a history of glaucoma, or psychological disorders that could contraindicate the medication. Participants were also excluded if they had a seizure disorder, central nervous system tumors, recent alcohol discontinuation, or a history of bulimia or anorexia nervosa. Additional exclusions applied to those with opioid dependence, acute hepatitis or liver failure, severe hepatic impairment, end-stage renal disease, pregnancy, breastfeeding, or those under 18 or over 75 years of age.
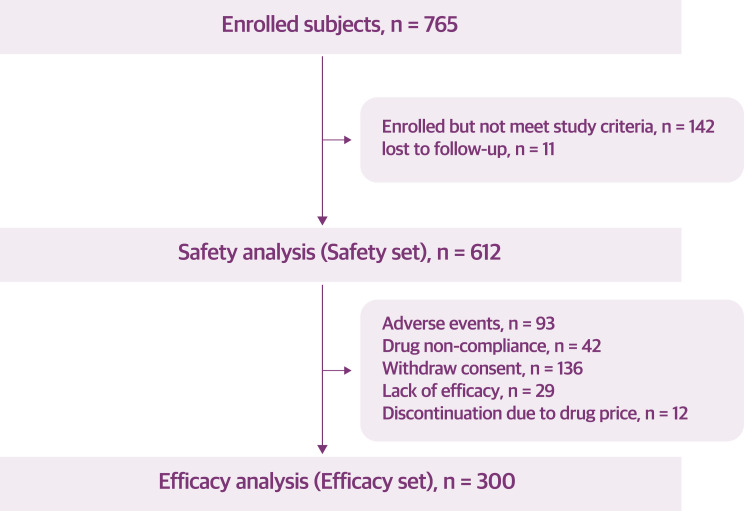
A total of 765 participants were initially enrolled in the study (Figure 1). After enrollment, 142 participants were excluded because they did not meet the study criteria, and an additional 11 participants were lost to follow-up. This resulted in a total of 612 individuals eligible for safety analysis, as they had taken the medication at least once; these individuals were in line with the regulatory requirements for re-evaluation purposes. However, due to the nature of a post-marketing study, there were some missing values for certain variables. As a result, the sample size varies for some variables, with responses for smoking and alcohol history available from 610 participants and weight data from 608 participants.
Figure 1.
Flowchart of the enrollment of participants.
Among the individuals included in the safety analysis, those who took the medication for more than three months and had weight loss effectiveness data were included in the effectiveness analysis. A total of 312 individuals were excluded from the effectiveness analysis due to dropping out of the study for various reasons: 93 individuals experienced adverse events, 71 individuals were noncompliant, 136 individuals withdrew consent, and 12 individuals discontinued treatment because of the cost of the drug. This left 300 individuals for the effectiveness analysis, which constituted the effectiveness set used to evaluate the primary outcomes of the study.
The clinical characteristics, medical history, comorbidities, side effects, and naltrexone-bupropion doses were recorded. Based on the clinical practice guidelines for obesity by the Korean Society for the Study of Obesity, the participants were classified according to their BMI as follows:5 Class I (BMI 25–30), Class II (BMI 30–35), and Class III (BMI ≥35). Smoking history was categorized as current smokers, ex-smokers (previously smoked but stopped), and non-smokers. Alcohol consumption was recorded as “Yes” if the individual drank alcohol at the time and “No” if they did not. Comorbidities were divided into the following categories: hypertension, diabetes, dyslipidemia, chronic liver failure, chronic kidney disease, arthritis, malignancy, hypothyroidism, and hyperthyroidism. Participants with comorbid conditions such as hypertension, diabetes, and dyslipidemia were permitted to continue their prescribed medications for these conditions alongside naltrexone-bupropion.
Safety and Effectiveness Assessment
The safety evaluation of this study was conducted on participants who had received at least one dose of the study drug and were monitored for the occurrence of adverse events following administration. The incidence of adverse events was analyzed across different dosages and individual subgroups to identify patterns or significant differences. Adverse events were documented throughout the study period, with specific attention to drug-related adverse reactions not covered by existing safety guidelines. Safety assessments included monitoring for any significant adverse events, including, but not limited to, serious adverse events requiring hospitalization, persistent disabilities, congenital anomalies, and other medically significant conditions.
Effectiveness data on weight loss were collected from participants who were able to be followed up until the 12-week after reaching the maintenance dose. Among the participants who completed the 12-week follow-up, approximately 60 were randomly selected for further evaluation of long-term weight loss effectiveness and safety. In our study, the effectiveness of naltrexone-bupropion 8/90 mg and 24/270 mg was not described because of the small sample size (20 patients for 8/90 mg and 10 patients for 24/270 mg) and was instead detailed in the Supplementary Data.
Statistical Analysis
Data were analyzed to determine the correlations between treatment outcomes and various demographic and clinical factors. Descriptive statistics were used to report baseline clinical characteristics. The incidence and influencing factors of adverse and severe adverse events were explored using chi-square tests and multiple logistic regression analyses. The covariates included in the multiple logistic regression model were sex, age, weight, smoking status, alcohol consumption, anti-obesity drug history, and naltrexone-bupropion dose.
Differences in weight changes between baseline and follow-up visits were evaluated according to the dosages using t-tests. Multiple logistic regression analyses were conducted to analyze the influencing factors for patients who achieved more than 5% weight loss. All statistical analyses were performed using a two-sided approach, with a significance threshold set at 5%. The analyses were performed using the R statistical software.
Results
Baseline Characteristics
Our study included 612 participants with varying demographic and clinical profiles (Table 1). The mean age of the patients was approximately 45 years. The cohort consisted predominantly of females (70.64%) with an average BMI of 32.14 kg/m2. The obesity classifications were Class I (22.68%), II (58.99%), and III (18.27%). The most prevalent comorbidities were hypertension (33.82%), diabetes (21.08%), and dyslipidemia (39.54%). The participants received different final doses of naltrexone-bupropion as follows: 8/90 mg (98 participants), 16/180 mg (176 participants), 24/270 mg (52 participants), and 32/360 mg (286 participants).
Table 1.
Baseline Characteristics of Study Populations (Safety Analysis Set, n=612)
| Category | Number | Statistic |
|---|---|---|
| Female | 612 | 432 (70.64) |
| Age, years | 607 | 44.95 (11.97) |
| <30 | 72 (11.84) | |
| ≥30 and <40 | 140 (23.03) | |
| ≥40 and <50 | 191 (31.41) | |
| ≥50 and <60 | 135 (22.24) | |
| ≥60 | 69 (11.35) | |
| Weight, kg | 608 | 86.30 (14.93) |
| BMI, kg/m2 | 612 | 32.14 (3.83) |
| Obesity classa | 612 | |
| Class I | 139 (22.68) | |
| Class II | 361 (58.99) | |
| Class III | 112 (18.27) | |
| Smoking history | 610 | |
| Non-smoker | 495 (81.01) | |
| Ex-smoker | 33 (5.41) | |
| Current-smoker | 82 (13.42) | |
| Alcohol consumption (Yes) | 610 | 183 (30.00) |
| Comorbidityb | 612 | |
| Hypertension | 207 (33.82) | |
| Diabetes | 129 (21.08) | |
| Dyslipidemia | 242 (39.54) | |
| Chronic liver failure | 63 (10.29) | |
| Chronic kidney disease | 6 (0.98) | |
| Arthritis | 24 (3.92) | |
| Malignancy | 13 (2.12) | |
| Hypothyroidism | 19 (3.10) | |
| Hyperthyroidism | 4 (0.65) | |
| Previous anti-obesity drug history | 612 | 107 (17.46) |
| Dose of NBSR (milligram per day) | 612 | |
| NBSR 8/90 | 98 (15.99) | |
| NBSR 16/180 | 176 (28.71) | |
| NBSR 24/270 | 52 (8.48) | |
| NBSR 32/360 | 286 (46.66) |
Notes: Values are expressed as either number (percentage) or mean ± standard deviation. aObesity is defined as obese class I (25 kg/m2 ≤ BMI < 30 kg/m2), obese class II (30 kg/m2 ≤ BMI < 35 kg/m2), or obesity class III (BMD ≥ 35 kg/m2). bComorbidity was reported by self-report of patients.
Abbreviations: BMI, body mass index; NBSR, naltrexone SR/bupropion SR combination.
Adverse Events
Table 2 shows the adverse events observed in the present study. Overall, 41.34% of participants experienced adverse effects. Specifically, gastrointestinal disorders, such as nausea (19.12%), constipation (3.92%), and vomiting (3.59%), were reported. Nervous system disorders were also frequently reported, including headaches (7.68%) and dizziness (5.23%).
Table 2.
Adverse Events of Naltrexone and Bupropion Combination
| Event Type | Total (n = 612) |
|---|---|
| Number | |
| NBSR 8/90 | 98 (16.01) |
| NBSR 16/180 | 176 (28.76) |
| NBSR 24/270 | 52 (8.50) |
| NBSR 32/360 | 286 (46.73) |
| Gastrointestinal disorders | |
| Nausea | 117 (19.12) |
| Vomiting | 22 (3.59) |
| Constipation | 24 (3.92) |
| Diarrhea | 6 (0.98) |
| Abdominal pain | 11 (1.80) |
| Abdominal discomfort | 22 (3.59) |
| Cardiovascular disorders | |
| Chest pain | 1 (0.16) |
| Palpitation | 9 (1.47) |
| Elevated blood pressure | 3 (0.49) |
| Nervous system disorders | |
| Headache | 47 (7.68) |
| Dizziness | 32 (5.23) |
| Tremor | 7 (1.14) |
| Sleep disturbance | 5 (0.82) |
| Memory disturbance | 1 (0.16) |
| Depression | 1 (0.16) |
| Other adverse events | |
| General weakness | 15 (2.45) |
| Fatigue | 4 (0.65) |
| Thirst | 2 (0.33) |
| Edema | 2 (0.33) |
| Diaphoresis | 3 (0.49) |
| Urticaria | 6 (0.98) |
Note: Values are presented as number (%).
Abbreviation: NBSR, naltrexone SR/bupropion SR combination.
Table 3 shows the multiple demographic and clinical factors that were significantly associated with the incidence of adverse events. Higher age was significantly associated with a higher likelihood of adverse events, with each additional year increasing the odds by 2.4% (odd ratio [OR] 1.024, 95% confidence interval [CI] 1.006–1.043, p=0.0086). Notably, participants aged over 60 years exhibited a >4–fold increase in adverse event odds compared with those aged 20–30 (OR 3.701, 95% CI 1.624–8.656, p=0.0021). Alcohol consumers had a significantly lower incidence of side effects than alcohol non-consumers (OR 0.547, 95% CI 0.345–0.858, p=0.0094). Comorbidities, such as hypertension, diabetes, and dyslipidemia were significantly associated with an increased risk of adverse events after adjusting for covariant (OR 1.569, 95% CI 1.067–2.310, p=0.0220 for hypertension; OR 1.557, 95% CI 1.017–2.389, p=0.0419 for diabetes; OR 1.524, 95% CI 1.061–2.192, p=0.0227 for dyslipidemia; and OR 1.845, 95% CI 1.049–3.258, p=0.0335 for dyslipidemia, respectively). The naltrexone–bupropion dose did not show a dose–dependent relationship with the occurrence of adverse events.
Table 3.
Multivariate Analysis of Individual Factors Associated with Increased Adverse Events
| Categorya | Odds Ratio (95% CI)b | P-value | |
|---|---|---|---|
| Sex | Female | 1.610 (0.987–2.652) | 0.0583 |
| Male | Reference | ||
| Age, 1 year | 1.024 (1.006–1.043) | 0.0086 | |
| > 60 | 3.701 (1.624–8.656) | 0.0021 | |
| 50–60 | 2.566 (1.262–5.355) | 0.0103 | |
| 40–50 | 1.256 (0.659–2.456) | 0.4956 | |
| 30–40 | 2.004 (1.051–3.929) | 0.0381 | |
| 20-30 | Reference | ||
| BMI, 1 kg/m2 | 1.006 (0.991–1.020) | 0.4402 | |
| Obesity classc | Class III | 2.099 (1.188–3.737) | 0.0110 |
| Class II | 1.793 (1.124–2.891) | 0.0152 | |
| Class I | Reference | ||
| Smoking history | Current-smoker | 1.493 (0.564–4.158) | 0.4277 |
| Ex-smoker | 1.653 (0.903–3.030) | 0.1027 | |
| Non-smoker | Reference | ||
| Alcohol consumption | Yes | 0.547 (0.345–0.858) | 0.0094 |
| No | Reference | ||
| Hypertension | Yes | 1.569 (1.067–2.310) | 0.0220 |
| No | Reference | ||
| Diabetes | Yes | 1.557 (1.017–2.389) | 0.0419 |
| No | Reference | ||
| Dyslipidemia | Yes | 1.524 (1.061–2.192) | 0.0227 |
| No | Reference | ||
| Chronic liver failure | Yes | 1.845 (1.049–3.258) | 0.0335 |
| No | Reference | ||
| Hypothyroidism | Yes | 1.068 (0.407–2.755) | 0.8912 |
| No | Reference | ||
| History of malignancy | Yes | 2.264 (0.719–7.792) | 0.1688 |
| No | Reference | ||
| Previous anti-obesity drug history | Yes | 1.303 (0.824–2.057) | 0.2561 |
| No | Reference | ||
| Dose of NBSR | NBSR 32/360 | 0.583 (0.350–0.968) | 0.0370 |
| NBSR 24/270 | 1.459 (0.701–3.064) | 0.3142 | |
| NBSR 16/180 | 0.622 (0.365–1.055) | 0.0788 | |
| NBSR 8/90 | Reference |
Notes: aObesity is defined as obese class I (25 kg/m2 ≤ BMI < 30 kg/m2), obese class II (30 kg/m2 ≤ BMI < 35 kg/m2), or obesity class III (BMD ≥ 35 kg/m2). bThe reference group for each category is denoted as “Reference” and serves as the baseline for comparison in calculating odds ratios and p-values. cData are adjusted for age, sex, weight, smoking, alcohol drink, previous drug history, dose of naltrexone-bupropion.
Abbreviations: CI, confidence interval; BMI, body mass index; NBSR, naltrexone SR/bupropion SR combination.
Effectiveness
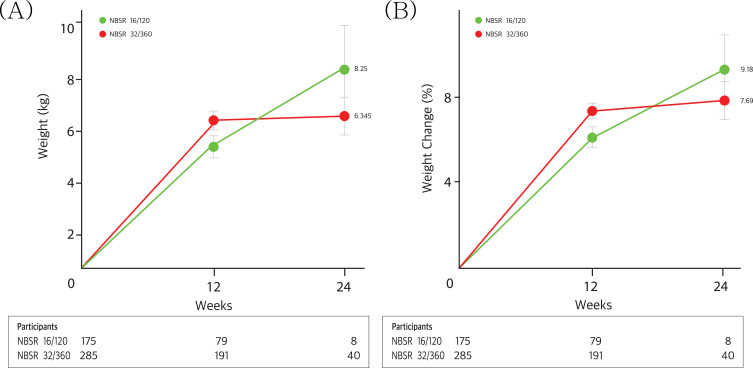
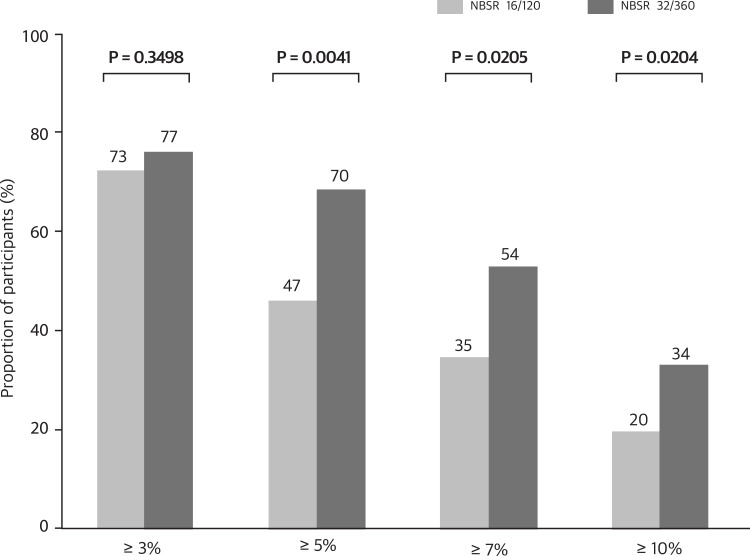
Table 4 and Figures 2 and 3 show the effectiveness, assessed by changes in weight at weeks 12 and 24 from baseline, across different doses of naltrexone-bupropion. At week 12, the greatest average percentage change in weight was observed in the naltrexone-bupropion 32/360 mg group at approximately −7.21%. This weight reduction effectiveness continued until week 24 (−7.69%, p=0.0000). Notably, the naltrexone-bupropion 16/180 mg group also showed a marked weight loss effect of 5.99% at week 12 and 9.18% at week 24 (p=0.0000), demonstrating a weight loss effect comparable to the 32/360 mg dose. Participants who received naltrexone-bupropion 32/360 mg achieved greater weight loss of over 3%, 5%, 7%, and 10% than those who received the 16/180 mg dose.
Table 4.
Effectiveness of Naltrexone and Bupropion Combinations in People with Obesity at Weeks 12 and 24
| NBSR 16/180 | NBSR 32/360 | Total | |
|---|---|---|---|
| Participants | 79 | 191 | 300 |
| Baseline | 86.60±17.88 | 85.41±13.99 | 85.85±14.83 |
| Week 12 | 81.48±17.57 | 79.22±13.50 | 80.16±14.54 |
| Change from baseline at week 12 | −5.12±4.37 | −6.19±4.60 | −5.69±4.51 |
| P | 0.0000 | 0.0000 | 0.0000 |
| Change (%) from baseline at week 12 | −5.99±4.75 | −7.21±5.00 | −6.64±4.94 |
| P | 0.0000 | 0.0000 | 0.0000 |
| Participants | 8 | 40 | 53 |
| Baseline | 84.58±15.21 | 83.35±12.95 | 83.79±13.28 |
| Week 24 | 76.33±10.67 | 77.00±13.29 | 77.32±12.96 |
| Change from baseline at week 24 | −8.25±5.32 | −6.35±4.80 | −6.47±4.81 |
| P | 0.0032 | 0.0000 | 0.0000 |
| Change (%) from baseline at week 24 | −9.18±4.58 | −7.69±5.60 | −7.70±5.39 |
| P | 0.0008 | 0.0000 | 0.0000 |
Abbreviation: NBSR, naltrexone SR/bupropion SR combination.
Figure 2.
Weight change in patients with a 24-week follow-up. Panels A and B show the observed mean percentage change (A) and kilogram (B) from baseline in body weight over time among participants in the full analysis population. I bars indicate one standard errors. The participants are the numbers of participants with available data contributing to the means at each visit.
Figure 3.
Proportion of participants achieving different levels of weight loss with naltrexone and bupropion doses. The p-values indicate the statistical significance of the differences between the two groups for each weight loss category.
Table 5 shows that multiple demographic and clinical factors were significantly associated with a weight reduction >5%. Higher age was significantly negatively associated with a good response to naltrexone-bupropion, with each additional year decreasing the odds by 3.4% (OR 0.976, 95% CI 0.956–0.996, p<0.0219). In the presence of comorbidities, the achievement of a 5% weight loss target was significantly reduced for individuals with diabetes (OR 0.399, 95% CI 0.200–0.784, p=0.0081), dyslipidemia (OR 0.550, 95% CI 0.317–0.951, p=0.0323), and a history of malignancy (OR 0.346, 95% CI 0.182–0.644, p=0.0010) after adjusting for the covariant. Individuals with a previous history of anti–obesity drug treatment exhibited a decreased rate of achieving the target weight loss, with an OR of 0.346 (95% CI 0.182–0.644, p=0.0010), compared with those without such a history. Naltrexone–bupropion 32/360 mg significantly increased the likelihood of achieving the target outcome compared with naltrexone–bupropion 8/90 mg, with an OR of 4.111 (95% CI 1.502–11.860, p=0.0105).
Table 5.
Multivariate Analysis of Individual Factors Associated with Achievement of Weight Reduction Over 5%
| Categorya | Odds Ratio (95% CI)b | P-value | |
|---|---|---|---|
| Sex | Female | 1.123 (0.552–2.263) | 0.7466 |
| Male | Reference | ||
| Age, 1 year | 0.976 (0.953–1.000) | 0.0476 | |
| > 60 | 0.433 (0.137–1.330) | 0.1467 | |
| 50–60 | 0.384 (0.143–0.980) | 0.0496 | |
| 40–50 | 0.617 (0.250–1.440) | 0.2759 | |
| 30–40 | 0.767 (0.292–1.949) | 0.5811 | |
| 20-30 | Reference | ||
| BMI, 1 kg/m2 | 0.960 (0.905-1.018) | 0.1765 | |
| Obesity classc | Class III | 0.843 (0.352–2.008) | 0.7002 |
| Class II | 1.635 (0.847–3.147) | 0.1407 | |
| Class I | Reference | ||
| Smoking history | Current-smoker | 0.426 (0.159–1.112) | 0.0829 |
| Ex-smoker | 0.378 (0.087–1.472) | 0.1721 | |
| Non-smoker | Reference | ||
| Alcohol consumption | Yes | 1.494 (0.780–2.922) | 0.2324 |
| No | Reference | ||
| Hypertension | Yes | 0.739 (0.418–1.315) | 0.3002 |
| No | Reference | ||
| Diabetes | Yes | 0.399 (0.200–0.784) | 0.0081 |
| No | Reference | ||
| Dyslipidemia | Yes | 0.550 (0.317–0.951) | 0.0323 |
| No | Reference | ||
| Chronic liver failure | Yes | 0.798 (0.342–1.878) | 0.6001 |
| No | Reference | ||
| Hypothyroidism | Yes | 0.460 (0.106–1.893) | 0.2770 |
| No | Reference | ||
| History of malignancy | Yes | 0.346 (0.182–0.644) | 0.0010 |
| No | Reference | ||
| Previous anti-obesity drug history | Yes | 0.333 (0.169–0.644) | 0.0012 |
| No | Reference | ||
| Dose of NBSR | NBSR 32/360 | 4.111 (1.502–11.860) | 0.0067 |
| NBSR 24/270 | 0.680 (0.114–3.463) | 0.6502 | |
| NBSR 16/180 | 1.804 (0.642–5.306) | 0.2685 | |
| NBSR 8/90 | Reference |
Notes: aObesity is defined as obese class I (25 kg/m2 < BMI < 30 kg/m2), obese class II (30 kg/m2 < BMI < 35 kg/m2), or obesity class III (BMD ≥ 35 kg/m2). bThe reference group for each category is denoted as “Reference” and serves as the baseline for comparison in calculating odds ratios and p-values. cData are adjusted for age, sex, weight, smoking, alcohol drink, previous drug history, dose of naltrexone-bupropion.
Abbreviations: CI, confidence interval; BMI, body mass index; NBSR, naltrexone SR/bupropion SR combination.
Discussion
This post-marketing survey was conducted to evaluate the safety and effectiveness of naltrexone-bupropion in people with obesity. Among the 612 participants, One-third experienced drug-related adverse effects, most commonly mild symptoms such as nausea, headache, and dizziness, with no serious adverse effects reported. After 12 weeks at a maintenance dose, the average weight loss with naltrexone-bupropion was 6.64%, with weight loss effects of 5.99% for the 16/180 mg dose and 7.21% for the 32/360 mg dose, respectively. Higher age and the presence of underlying diseases were associated with an increased incidence of adverse effects and reduced weight-loss effectiveness, emphasizing the need for an individualized approach in obesity treatment.
Our study revealed that gastrointestinal side effects, such as nausea, constipation, and vomiting, as well as nervous system disorders, such as headaches and dizziness, were commonly reported, similar to the findings of other RCTs.11–13,18,19 In our study, the population experienced fewer side effects than those in an RCT of COR-II11 except for headache (Table S1). In the COR-BMOD study, which reported weight loss effects of naltrexone-bupropion 32/360 mg in patients undergoing intensive lifestyle modification, the incidence of adverse effects was higher than in our study (nausea 34.1%, headache 23.8%, constipation 24.1%, vomiting 11.0%).18 This difference may be attributed to the combination dosage of naltrexone and bupropion, as our study group adjusted the drug dosage according to the individual conditions, whereas in the previous RCT, the majority of the population received the full dosage. In the COR-I study, side effects were reduced with naltrexone-bupropion 16/180 mg compared to 32/360 mg, highlighting the need for dose adjustments based on individual patient tolerance. Notably, our study demonstrated that the incidence of adverse events was elevated in populations with comorbidities, such as hypertension, diabetes, and dyslipidemia. This finding aligns with those of other studies,20,21 which also showed that an increased incidence of adverse events is common among populations with underlying chronic diseases. Also, particular caution is necessary for patients with diabetes and obesity because the use of metformin is known to increase the gastrointestinal side effects of naltrexone-bupropion.13
In our study, naltrexone-bupropion 32/360 mg showed promising weight reduction, achieving a weight reduction of −7.21±5.00% at 12 weeks (p < 0.0001) and −7.69±5.60% at 24 weeks (p < 0.0001), demonstrating an effect comparable to that seen in other clinical trials.11 Notably, the naltrexone-bupropion 16/180 mg group showed a similar weight reduction effect to that of 32/360 mg, with a reduction of −5.99±4.75% at 12 weeks (p<0.0001) and −9.18±4.58% at 24 weeks (p=0.0008). A significant number of participants drop out due to adverse effects during RCTs of anti-obesity medication: 9%,11 16.9%,22 and 11.6%23 in naltrexone-bupropion, phentermine and topiramate combination-, and semaglutide trials, respectively. Therefore, increasing to the highest dose of naltrexone-bupropion was considered individually for each participant. For high-risk patients expected to experience adverse effects, maintenance treatment with naltrexone-bupropion 16/180 mg could be an effective alternative that balances effectiveness while minimizing adverse events.
Our study demonstrated that significant weight loss was associated with a younger age, and the absence of underlying health conditions, history of cancer, and prior use of obesity medications. These findings are comparable to those observed with other anti-obesity medications in which weight loss effectiveness is relatively prominent in populations without underlying health conditions.24 This finding suggests that increased insulin resistance in patients with comorbidities can diminish the effectiveness of these medications. A persistent increase in adiposity signals, such as leptin and insulin, can lead to desensitization, ultimately impairing the responsiveness of the body’s homeostatic mechanisms for weight regulation.25 A significantly disrupted microbiome in individuals with obesity and diabetes, along with a genetic predisposition to weight gain in this population, may also be seen as contributing factors.26
In this study, a clear dose-response relationship was not established. Regarding effectiveness, the 12-week data showed that a weight reduction of −3.59 kg for the naltrexone-bupropion 24/270 mg dosage was lower than that of the lower dose (18/190 or 9/90 mg) (Table S2). This outcome should be interpreted in the context of this study as a post-marketing surveillance. In this study, drug administration followed a titration method, beginning with the lowest dosage. The dosage was increased weekly until the maximum tolerable dose was reached and maintained. As a result, there may be differences in maintenance doses among individuals. Data from individuals administered the lowest dose, naltrexone-bupropion 8/90 mg, could indicate greater susceptibility to adverse effects, whereas data from those taking naltrexone-bupropion 32/360 mg may reflect greater tolerance to adverse events. Therefore, it is crucial to consider these factors when interpreting our results. Future research should focus on conducting RCTs to evaluate the safety and effectiveness of different naltrexone-bupropion doses.
Our study has several strengths. First, this study used a comprehensive post-marketing surveillance approach, which provided a robust evaluation of the real-world effectiveness and safety of naltrexone-bupropion in Korean populations with obesity. Although comprehensive real-world data on the safety and effectiveness of other FDA-approved obesity treatments, such as the phentermine-topiramate combination7 and liraglutide,27 are available, research on naltrexone-bupropion remains limited, especially in Asian populations. Therefore, this study is important in bridging the gap between the controlled environment of RCTs and the diverse conditions of real-world applications and in improving the understanding of how this treatment is performed in clinical settings in Asian populations. Second, the rigorous methodology of the study, including the inclusion of a large and diverse participant pool, allowed for a detailed analysis of the treatment effects across various subgroups, enhancing the reliability of the findings.
Notably, our study had some key limitations. First, this study was a post-marketing surveillance with a prospective observational design that lacked a control group, leading to potential issues with controlling confounding factors that could affect the accuracy of the effectiveness and safety outcomes. Second, the focus of the study on weight loss outcomes over 12 and 24 weeks provided insights into short-term effectiveness, but did not fully capture long-term safety concerns or the sustainability of the weight reduction. Third, the distribution of participants across the different naltrexone-bupropion dosage groups was uneven, with fewer participants in the 8/90 and 24/270 mg groups than in the 16/180 and 32/360 mg groups. This discrepancy introduces statistical challenges in comparing the effectiveness and safety profiles across dosages, as there is a risk of overrepresentation of more heavily represented dosages. Notably, this analysis was based on real-world evidence, not RCTs, which present their own set of limitations. Owing to these factors, our description of effectiveness outcomes primarily focused on data from the 16/180 and 32/360 mg dosage groups. Fourth, adverse events were self-reported, which could have led to underreporting or recall bias, and the multi-center nature of the study may have introduced variations in reporting standards that could have impacted the quality of the adverse event data. Finally, this study was conducted in Korea, which limits its generalizability to populations with different demographics, genetic backgrounds, and healthcare practices.
Conclusions
This post-marketing surveillance study demonstrated that naltrexone-bupropion is both effective and safe for inducing significant weight loss in individuals with obesity, with the greatest benefits observed in young individuals without comorbidities. The adverse events were predominantly mild, confirming the need for personalized titration to maximize therapeutic outcomes. These findings highlight the potential of pharmacotherapy as an adjunct to comprehensive obesity management strategies.
Funding Statement
No funding was used for the design and conduction of this study.
Abbreviations
BMI, Body Mass Index; FDA, Food and Drug Administration; GLP-1, Glucagon-Like Peptide-1; MFDS, Ministry of Food and Drug Safety (Korea); NBSR, naltrexone SR/bupropion SR combination; RCT, Randomized Controlled Trial.
Data Sharing Statement
The dataset supporting the findings of this study is available from Cheol-Young Park, but the availability of this data is limited. This data is used under license for current research and is not publicly available. However, the authors can provide the data upon reasonable request and with the permission of Cheol-Young Park.
Ethics Approval and Consent to Participate
This post-marketing survey was carried out in compliance with the principles of the Helsinki declaration and Good Clinical Practice guidelines. It received approval from both the Ministry of Food and Drug Safety (MFDS) in Korea and the Institutional Review Boards of the participating hospitals. This study was conducted in compliance with ethical standards and local regulatory requirements. Informed consent was obtained from all participants before enrollment in the study. All subjects’ data were handled with strict confidentiality and used solely for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no conflicts of interest.
References
- 1.Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. DOI: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeong S-M, Jung J-H, Yang YS, et al. Obesity fact sheet: prevalence of obesity and abdominal obesity in adults, adolescents, and children in Korea from 2012 to 2021. J ObesiMetab Syndr. 2024;33(1):27. doi: 10.7570/jomes24012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garvey WT, Mechanick JI, Brett EM, et al. American Association Of Clinical Endocrinologists And American College Of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care Of Patients With Obesity. Endocr Pract. 2016;22(3):1–203. doi: 10.4158/ep161365.Gl [DOI] [PubMed] [Google Scholar]
- 4.Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237–248. doi: 10.1016/s2213-8587(17)30236-x [DOI] [PubMed] [Google Scholar]
- 5.Kim KK, Haam JH, Kim BT, et al. Evaluation and treatment of obesity and its comorbidities: 2022 update of clinical practice guidelines for obesity by the Korean society for the study of obesity. J Obes Metab Syndr. 2023;32(1):1–24. doi: 10.7570/jomes23016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816 [DOI] [PubMed] [Google Scholar]
- 7.Tak YJ, Lee SY. Long-term efficacy and safety of anti-obesity treatment: where do we stand? Curr Obes Rep. 2021;10(1):14–30. doi: 10.1007/s13679-020-00422-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenway FL, Whitehouse MJ, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity. 2009;17(1):30–39. doi: 10.1038/oby.2008.461 [DOI] [PubMed] [Google Scholar]
- 9.Greenway FL, Dunayevich E, Tollefson G, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab. 2009;94(12):4898–4906. doi: 10.1210/jc.2009-1350 [DOI] [PubMed] [Google Scholar]
- 10.Jeon E, Lee KY, Kim -K-K. Approved anti-obesity medications in 2022 KSSO guidelines and the promise of Phase 3 clinical trials: anti-obesity drugs in the sky and on the horizon. J ObesiMetab Syndr. 2023;32(2):106. doi: 10.7570/jomes23032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity. 2013;21(5):935–943. doi: 10.1002/oby.20309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nissen SE, Wolski KE, Prcela L, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315(10):990–1004. doi: 10.1001/jama.2016.1558 [DOI] [PubMed] [Google Scholar]
- 13.Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. doi: 10.2337/dc13-0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sposito AC, Bonilha I, Luchiari B, et al. Cardiovascular safety of naltrexone and bupropion therapy: systematic review and meta‐analyses. Obesity Rev. 2021;22(6):e13224. doi: 10.1111/obr.13224 [DOI] [PubMed] [Google Scholar]
- 15.Weiss T, Yang L, Carr RD, et al. Real-world weight change, adherence, and discontinuation among patients with type 2 diabetes initiating glucagon-like peptide-1 receptor agonists in the UK. BMJ Open Diabetes Res Care. 2022;10(1):e002517. doi: 10.1136/bmjdrc-2021-002517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaccardi F, Davies MJ, Khunti K. The present and future scope of real-world evidence research in diabetes: what questions can and cannot be answered and what might be possible in the future? Diabetes Obes Metab. 2020;22(3):21–34. doi: 10.1111/dom.13929 [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Food and Drug Safety (MFDS). Provision of safety information management regulation of drugs. MFDS Notice No. 2015-79. 2015
- 18.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity. 2011;19(1):110–120. doi: 10.1038/oby.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JG, Park C-Y. Anti-obesity drugs: a review about their effects and safety. Diabet Metabol J. 2012;36(1):13–25. doi: 10.4093/dmj.2012.36.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Lee WJ, Hur KY, Cho JH, Lee BW, Park CY. Safety and effectiveness of dulaglutide in the treatment of Type 2 diabetes mellitus: a Korean real-world post-marketing study. Diabetes Metab J. 48:418–428. 2024. doi: 10.4093/dmj.2023.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechman K, Clarke BD, Rutherford AI, et al. Polypharmacy is associated with treatment response and serious adverse events: results from the British society for rheumatology biologics register for rheumatoid arthritis. Rheumatology. 2019;58(10):1767–1776. doi: 10.1093/rheumatology/kez037 [DOI] [PubMed] [Google Scholar]
- 22.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. doi: 10.3945/ajcn.111.024927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984. doi: 10.1016/s0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 24.Drucker DJ. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metab. 2022;57:101351. doi: 10.1016/j.molmet.2021.101351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21(3):201–223. doi: 10.1038/s41573-021-00337-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilding JP. Medication use for the treatment of diabetes in obese individuals. Diabetologia. 2018;61(2):265–272. doi: 10.1007/s00125-017-4288-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wharton S, Liu A, Pakseresht A, et al. Real-world clinical effectiveness of liraglutide 3.0 mg for weight management in Canada. Obesity. 2019;27(6):917–924. doi: 10.1002/oby.22462 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the findings of this study is available from Cheol-Young Park, but the availability of this data is limited. This data is used under license for current research and is not publicly available. However, the authors can provide the data upon reasonable request and with the permission of Cheol-Young Park.