Abstract
Objective
This study evaluates the therapeutic effects of the Yangxin Huoxue Formula in the management of stable angina pectoris associated with Qi deficiency and blood stasis syndrome, co-occurring with anxiety and depressive disorders. The primary objective is to determine its efficacy in enhancing cardiac function and reducing emotional symptoms.
Methods
A randomized, single-blind, placebo-controlled clinical trial was conducted with 94 individuals with stable angina pectoris. Participants were randomly allocated to either a treatment group receiving Yangxin Huoxue Formula granules or a placebo group receiving placebo granules, administered twice daily over a 12-week period. Primary outcome measures included assessments of cardiac function, angina frequency, PHQ-9 and GAD-7 scores, traditional Chinese medicine (TCM) syndrome improvements, and inflammatory markers.
Results
The treatment group exhibited significantly greater improvements in cardiac output and reductions in peripheral vascular resistance compared to the placebo group. Additionally, a significant decrease in the frequency and duration of angina episodes was observed in the treatment group. Improvements in TCM syndrome scores and GAD-7 scores were also notably superior in the treatment group. No significant adverse reactions were recorded during the safety assessment of the treatment group.
Conclusion
The Yangxin Huoxue Formula demonstrates efficacy in improving cardiac function, alleviating symptoms of anxiety and depression, reducing inflammatory mediator release, and enhancing quality of life in individuals with stable angina pectoris. The treatment was well-tolerated, confirming both its safety and therapeutic effectiveness.
Keywords: Anxiety and depression disorders, qi deficiency and blood stasis, stable angina pectoris, traditional Chinese medicine treatment, Yangxin Huoxue Formula
Introduction
The increasing demands of society and the accelerated pace of modern life have positioned coronary artery disease (CAD) as a prominent threat to public health. According to the 2022 China Cardiovascular Health and Disease Report, CAD is responsible for 48% of deaths in rural regions and 45.86% in urban areas. A hospital-based meta-analysis reports that 51% of hospitalized patients with CAD in China exhibit symptoms of depression, with 0.5% to 25.44% experiencing severe depressive symptoms.1 Studies have demonstrated that psychological disorders, particularly depression, increase the incidence and mortality rates of acute coronary syndrome.2–6 Consequently, the field of “psychocardiology” has emerged, encouraging clinicians to integrate the assessment of psychological health into the care of individuals with CAD and to consider personalized treatment approaches.
The pathogenesis of CAD in the presence of psychological disorders involves a complex interaction of multiple factors, including sympathetic nervous system overactivation, dysregulation of the hypothalamic-pituitary-adrenal axis, inflammatory responses, oxidative stress, vascular endothelial injury, abnormal platelet function, and behavioral factors.7–13 Despite well-established diagnostic criteria for both CAD and related psychological conditions, therapeutic efficacy often remains inconsistent. This variability can be attributed to an incomplete understanding of the underlying disease mechanisms, reluctance among patients to use psychiatric medications, and the potential adverse effects of these treatments.14 Consequently, the interplay between psychological states and cardiac symptoms is often insufficiently emphasized, impacting overall treatment outcomes.
This study aims to evaluate the efficacy of a holistic treatment approach—specifically, the Yangxin Huoxue Formula—in managing stable angina pectoris, characterized by “Qi deficiency and blood stasis syndrome”, along with coexisting anxiety and depressive disorders.
Materials and Methods
Collection of Medical Cases and Group Assignment
This clinical research trial was conducted at the Cardiology Department of Shanghai Municipal Hospital of Traditional Chinese Medicine, involving both outpatients and inpatients with a diagnosis of stable angina pectoris classified as “qi deficiency and blood stasis syndrome” according to traditional Chinese medicine (TCM). Patients meeting the inclusion criteria between April 2022 and January 2024 were enrolled. Based on previous clinical findings and literature, a minimum of 104 cases was established as the required sample size for this study.15 Qualified individuals presenting with stable angina pectoris and coexisting anxiety and depression were selected through a randomized, single-blind, placebo-controlled method and subsequently underwent a 12-week treatment period. Patients were assigned to one of two groups: the treatment group, receiving Yangxin Huoxue Formula granules, or the placebo group, receiving placebo granules. Randomization was conducted using SAS software to generate random numbers, ensuring a balanced assignment to each group following comprehensive screening based on the diagnostic, inclusion, and exclusion criteria.
Diagnostic Criteria
Diagnostic criteria for stable angina pectoris:
The diagnostic criteria for stable angina pectoris were based on “Internal Medicine”, published by People’s Medical Publishing House,16 which defines stable angina pectoris as exertional angina, and on the “2018 Guidelines for the Diagnosis and Treatment of Stable Coronary Artery Disease”, by the Chinese Medical Association.17
Criteria for TCM syndromic classification were adapted from the “Guidelines for Clinical Research on the Treatment of Coronary Heart Disease Angina Pectoris with Chinese Medicine and Natural Medicine”18 and the “Clinical Terminology of Traditional Chinese Medicine—Syndromes Section”.19 For this study, “qi deficiency and blood stasis syndrome” were selected as the TCM syndrome type of interest.
Inclusion and Exclusion Criteria
Inclusion Criteria
1. Individuals meeting the diagnostic criteria for CAD according to Western medical standards, including at least one of the following: (1) Having undergone coronary artery revascularization procedures, including percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG); (2) A diagnosis via coronary angiography or coronary CT angiography indicating stenosis of ≥ 50% in at least one coronary artery; (3) Documented myocardial ischemia through nuclear myocardial perfusion imaging at rest or under stress conditions; (4) History of myocardial infarction.
2. Individuals Satisfying Diagnostic Criteria for Stable Angina Pectoris Based on Western Medicine
3. Individuals Fulfilling TCM Syndrome Differentiation Criteria for Qi Deficiency and Blood Stasis Syndrome
4. Individuals with a Patient Health Questionnaire-9 (PHQ-9) score between 10 and 19, or a Generalized Anxiety Disorder 7-item (GAD-7) score between 5 and 14, without prior use of anti-anxiety or antidepressant medications.20,21
5. Outpatients or Inpatients Aged 18 to 80 Years, Inclusive).
6. Individuals of childbearing age who agree to use a non-pharmacological contraceptive method or practice abstinence from the time of informed consent until three months following the last administration of the study drug.
7. Individuals able to understand and adhere to study procedures, complete required assessments, communicate effectively with researchers, and voluntarily sign informed consent.
Exclusion Criteria
Exclusion criteria included individuals with severe cardiac, cerebrovascular, renal, or pulmonary diseases; those with malignant diseases; individuals with known allergies to Chinese medicine; lactating women; and those who had participated in other clinical trials within the previous month.
Dropout Criteria
Cases were designated as dropouts if participants voluntarily chose to withdraw, experienced severe adverse reactions necessitating study discontinuation, or withdrew due to accidental death.
Treatment Protocols
Treatment Group: Participants in the treatment group continued conventional Western medical treatment, with no adjustments to their medications for the two weeks preceding enrollment. In addition to standard Western medical therapy, participants received Yangxin Huoxue Formula granules, which included 15 g of Salviae Miltiorrhizae Radix et Rhizoma, 9 g of Astragali Radix, 2 g of Notoginseng Radix et Rhizoma, 9 g of Angelicae Sinensis Radix, 12 g of Ziziphi Spinosae Semen, 18 g of Albiziae Cortex, 9 g of Bupleuri Radix, and 6 g of Aucklandiae Radix. The dosage was set at one bag per dose, administered twice daily. The formula was supplied by the pharmacy at Shanghai Municipal Hospital of Traditional Chinese Medicine from a verified, consistent source. The treatment duration was 12 weeks.
Placebo Group: Participants in the placebo group received conventional Western medical treatment along with placebo granules formulated to resemble the Yangxin Huoxue Formula. The placebo dosage was similarly set at one bag per dose, taken twice daily, and prepared uniformly by the Chinese medicine preparation room at Shanghai Municipal Hospital of Traditional Chinese Medicine. The treatment duration was also 12 weeks.
Observational Indicators
General Information: Collected demographic and baseline information included sex, age, height, weight; respiratory rate, blood pressure, heart rate, disease duration, treatment history, allergy history, comorbidities, and medication history.
Primary Efficacy Indicators: Cardiac function assessment, specifically non-invasive hemodynamic measurements, was conducted pre- and post-treatment.
Secondary Efficacy Indicators: Secondary assessments included TCM syndrome efficacy evaluation, GAD-7 score for anxiety, and PHQ-9 score for depressive symptoms.
Safety Indicators: Comprehensive safety assessments were performed before and after the intervention. These included routine blood, urine, and stool examinations, as well as measurements of blood pressure, heart rate, and liver and kidney function tests.
Collection and Recording of Adverse Events: Adverse events were monitored continuously throughout the study period. Investigators documented all adverse events observed during the trial, irrespective of severity or potential association with the study drug, within the study’s medical records.
Informed Consent and Ethics
Prior to enrollment, participants provided informed consent to ensure the protection of their legal rights and interests. The study received approval from the Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine, under the clinical research ethics review number 2022SHL-KYYS-66.
Statistical Methods
Data from participants who completed the trial were entered into a database and analyzed using SPSS software, version 25.0. Continuous data following a normal distribution are expressed as mean ± standard deviation. Within-group comparisons were conducted using the paired sample t-test, while between-group comparisons used the independent sample t-test. For data that did not follow a normal distribution, the median (quartiles) was used for expression. Non-parametric tests, such as the Wilcoxon signed-rank test for within-group comparisons and the Mann–Whitney U-test for between-group comparisons, were used. Categorical data are presented as counts and percentages (%), with between-group comparisons performed using the chi-squared (Pearson’s X²) test or Fisher’s exact test. A significance level of P < 0.05 was considered statistically significant.
Results
Enrollment Status and General Information of Cases
A total of 104 patients with stable angina pectoris secondary to CAD, all diagnosed with qi deficiency and blood stasis syndrome according to TCM syndrome differentiation, were enrolled in the study. Among these, 8 participants withdrew due to personal or logistical reasons after 2 weeks of treatment, and 2 were classified as dropouts after developing pulmonary infection and acute exacerbation of chronic cholecystitis, necessitating further medical intervention. Consequently, 10 patients were classified as dropouts, leaving 94 participants who completed the study: 48 in the treatment group and 46 in the placebo group. The dropout rate was 9.61%, remaining below the pre-determined threshold of 10%. Comparative statistical analysis of gender, age, height, weight, disease duration, and past medical history between the treatment and placebo groups showed no statistically significant differences (Table 1, P > 0.05), thus confirming baseline comparability.
Table 1.
The General Information of Patients in Treatment and Placebo Groups Before Treatment (
| Parameters | Treatment group (n = 48) |
Placebo group (n = 46) |
P |
|---|---|---|---|
| Age (year) | 64.56 ± 5.62 | 62.54 ± 4.38 | 0.0565 |
| Gender | |||
| Male | 30 (31.9) | 24 (25.5) | 0.422 |
| Female | 18 (19.1) | 22 (23.4) | |
| Height (cm) | 167.5 ± 7.80 | 169.9 ± 5.31 | 0.0833 |
| Weight (kg) | 69.56 ± 11.34 | 67.67 ± 9.76 | 0.390 |
| Course of disease (month) | 57.6 (9.5,86.5) | 60.07 (15,79.75) | 0.239 |
| Allergic history (n[%]) | 5 (10.42) | 4 (8.70) | 0.073 |
| Past medical history (n[%]) | |||
| Hypertension | 32 (66.67) | 27 (58.7) | 0.558 |
| Diabetes mellitus | 17 (35.42) | 15 (32.61) | 0.945 |
| Hyperlipidaemia | 23 (48.94) | 26 (56.52) | 0.600 |
| Cerebral infarction | 14 (29.17) | 17 (36.96) | 0.559 |
Comparison of Cardiac Function Before and After Treatment
As shown in Table 2, both groups demonstrated improvements in peripheral vascular resistance post-treatment. However, the treatment group showed significantly greater increases in cardiac output and stroke volume compared to the placebo group (P < 0.05). Further analysis in Table 2 revealed that, compared to baseline, the treatment group exhibited significantly greater improvements in heart rate, cardiac output, stroke volume, pleural fluid levels, and peripheral vascular resistance than the placebo group (P < 0.05).
Table 2.
Comparison of Impedance Cardiography Between the Two Groups Before and After Treatment (
| Parameter | Before or after treatment | Treatment group (n = 48) |
Placebo group (n = 46) |
P |
|---|---|---|---|---|
| Heart rate (bpm) | Before | 71.08 ± 11.59 | 73.34 ± 7.93 | 0.463 |
| After | 69.27 ± 9.53 | 72.89 ± 7.49 | ||
| Cardiac output (L/min) | Before | 4.95 ± 0.93 | 5.16 ± 0.71 | 0.0075 |
| After | 5.22 ± 0.87 | 5.27 ± 0.64a | ||
| Stroke volume (mL) | Before | 71.02 ± 16.66 | 73.45 ± 10.31 | 0.0026 |
| After | 74.14 ± 16.09a | 74.06 ± 10.09 | ||
| Pleural fluid level (1/kΩ) | Before | 34.70 ± 8.29 | 33.04 ± 5.89 | 0.0226 |
| After | 33.33 ± 7.11 | 32.90 ± 5.48 | ||
| Peripheral vascular resistance (dynes/sec/cm−5/m2) | Before | 1420.08 ± 318.11 | 1418.96 ± 334.23 | 0.0069 |
| After | 1146.29 ± 293.24a | 1228.74 ± 305.29a |
Note: aP < 0.05 vs before treatment.
Comparison of Angina Attack Frequency Between the Two Groups Post-Treatment
At baseline, no significant difference was observed in the frequency of angina attacks between the treatment and placebo groups, ensuring comparability (P > 0.05). After treatment, both groups demonstrated a significant reduction in angina attack frequency compared to baseline. While no significant difference in attack frequency was noted between the two groups at 4 and 8 weeks of treatment, a statistically significant difference emerged by the 12-week mark. At this point, the treatment group showed a substantially greater reduction in angina attack frequency than the placebo group (Table 3, P < 0.05).
Table 3.
Angina Pectoris Attack Frequency in Patients in the Two Groups Before and After Treatment (Times/Week) [Median (25, 75)]
| Group | n | Before treatment | 4 weeks after treatment | 8 weeks after treatment | 12 weeks after treatment | P |
|---|---|---|---|---|---|---|
| Treatment group | 48 | 3 (2,5) | 2 (2,3) | 2 (1,3) | 1 (1,2) | <0.001 |
| Placebo group | 46 | 3 (2,5) | 2 (2,4) | 2 (2,3) | 2 (1,3) | <0.001 |
| P | 0.969 | 0.441 | 0.284 | 0.0034 |
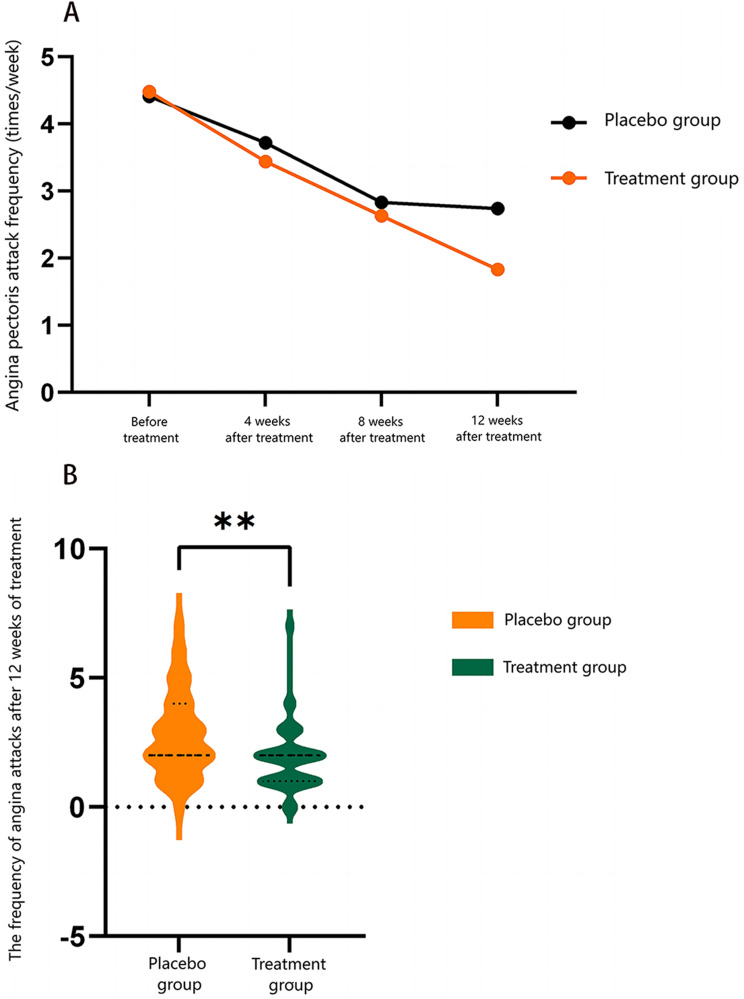
As illustrated in Figure 1, the distribution of angina attack frequency at 12 weeks indicated a markedly lower frequency in the treatment group compared to the placebo group. This further substantiates the treatment group’s significantly improved reduction in angina attack frequency (P < 0.05).
Figure 1.
Comparison of the frequency of angina pectoris attacks (A) and attack frequency distribution (B) before and after treatment with Yangxin Huoxue Formula granules. Participants were divided into placebo and treatment groups. **p < 0.01 vs placebo group.
Comparison of Average Duration of Angina Attacks Between the Two Groups Post-Treatment
The average duration of angina attacks before and after treatment for both groups is shown in Table 4. Statistical analysis indicated no significant difference between the groups in the duration of angina attacks at baseline, ensuring comparability (P > 0.05). Post-treatment, both groups exhibited a significant reduction in the average duration of angina attacks compared to baseline. While no significant difference in attack duration was observed between the groups at 4 and 8 weeks of treatment, by the 12-week endpoint, the treatment group demonstrated a significantly greater reduction in attack duration compared to the placebo group (P < 0.05). These findings suggest that the treatment group achieved a progressively greater reduction in attack duration with prolonged treatment duration.
Table 4.
Duration of Angina Pectoris in Patients Before and After Treatment in the Two Groups (Min/Time) [Median (25, 75)]
| Group | n | Before treatment | 4 weeks after treatment | 8 weeks after treatment | 12 weeks after treatment | P |
|---|---|---|---|---|---|---|
| Treatment group | 48 | 4 (3, 7) | 3 (2, 5) | 2 (2, 3) | 2 (1, 2) | <0.001 |
| Placebo group | 46 | 4 (3, 6) | 3 (3, 5) | 2 (2, 3) | 2 (2, 3) | <0.001 |
| U | 1110.5 | 976 | 1029.5 | 710.5 | ||
| P | 0.962 | 0.318 | 0.542 | 0.0021 |
Comparison of Angina Treatment Efficacy Between the Two Groups Post-Treatment
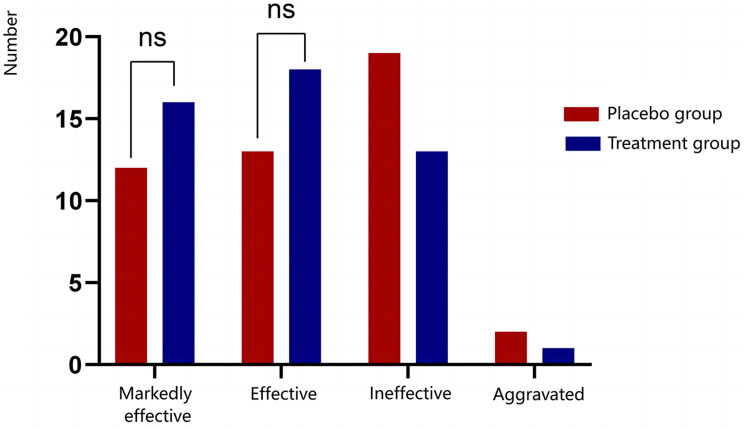
Efficacy was assessed based on categories of “markedly effective”, “effective”, “ineffective”, and “aggravated”. The total effective rate was 52.17% in the placebo group and 70.83% in the treatment group; however, this difference was not statistically significant (P > 0.05). As illustrated in Figure 2, the treatment group showed higher rates of markedly effective and effective responses compared to the placebo group, with a lower rate of aggravated cases. Although these differences did not reach statistical significance, the trend indicates a favorable efficacy profile for the treatment group.
Figure 2.
Comparison of therapeutic efficacy for angina pectoris between the placebo and treatment groups following treatment. The notation ‘ns’ denotes no statistically significant difference.
Comparison of TCM Syndrome Scores Between the Two Groups Post-Treatment
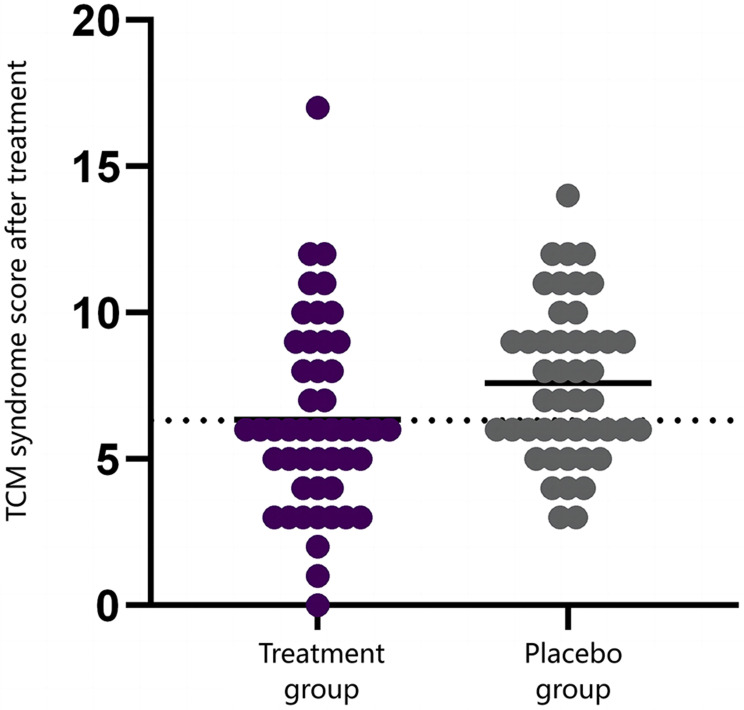
Changes in TCM syndrome scores for both groups before and after the 12-week treatment period are presented in Table 5. Baseline comparisons showed no significant differences in TCM syndrome scores between the groups (P > 0.05), affirming initial comparability. Following 12 weeks of treatment, both groups showed significant improvements in TCM syndrome scores from baseline, with the treatment group demonstrating a significantly greater improvement than the placebo group (P < 0.05). Figure 3 illustrates the distribution of TCM syndrome scores post-treatment, with the treatment group exhibiting significantly lower scores compared to the placebo group, further supporting the superior improvement in TCM syndrome outcomes in the treatment group.
Table 5.
Comparison of TCM Syndrome Scores of Patients in the Two Groups Before and After Treatment
| Group | n | Before treatment | After treatment | T | P |
|---|---|---|---|---|---|
| Treatment group | 48 | 12.04 ± 3.13 | 6.33 ± 3.24 | 10.46 | <0.001 |
| Placebo group | 46 | 12.09 ± 3.14 | 7.59 ± 2.65 | 16.63 | <0.001 |
| P | 0.944 | 0.043 |
Figure 3.
Distribution of TCM syndrome scores in the placebo and treatment groups following treatment.
Comparison of TCM Symptom Efficacy Between the Two Groups Post-Treatment
Assessment using the TCM syndrome score scale indicated an overall effective rate of 67.39% in the placebo group and 79.17% in the treatment group following treatment. However, this difference was not statistically significant (P > 0.05). These results suggest that, while TCM treatment demonstrated a higher effective rate, the difference lacked statistical significance, indicating a potential, though not definitive, impact of TCM treatment on improving TCM syndrome scores.
Comparison of GAD-7 and PHQ-9 Scores Between the Two Groups Post-Treatment
The pre- and post-treatment GAD-7 and PHQ-9 scores for both groups over the 12-week treatment period are shown in Table 6. Initial analysis revealed no significant difference between the groups in GAD-7 and PHQ-9 scores before treatment, ensuring baseline comparability (P > 0.05). After treatment, a statistically significant improvement in GAD-7 scores was observed within both groups, indicating reduced anxiety symptoms compared to baseline. Additionally, the treatment group demonstrated a significantly greater improvement in GAD-7 scores than the placebo group after 12 weeks (P < 0.05).
Table 6.
Comparison of the GAD7 and PHQ9 Scores of the Patients in the Two Groups Before and After Treatment
| Parameters | Before or after treatment | Treatment group (n = 48) |
Placebo group (n = 46) |
P |
|---|---|---|---|---|
| GAD7 score | Before | 6.60 ± 3.05 | 6.61 ± 3.12 | 0.043b |
| After | 2.44 ± 1.87a | 3.59 ± 2.11a | ||
| PHQ9 score | Before | 5.85 ± 2.57 | 5.76 ± 2.42 | 0.006b |
| After | 3.10 ± 2.10a | 3.02 ± 1.57a |
Note: aP < 0.05 vs before treatment; bP < 0.05 for treatment group vs placebo group after treatment.
Both groups also exhibited significant improvements in PHQ-9 scores post-treatment, reflecting reductions in depressive symptoms. However, no statistically significant difference was noted between the two groups in PHQ-9 scores after the 12-week treatment period (P > 0.05).
Comparison of Interleukin Levels Between the Two Groups Post-Treatment
Changes in levels of interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-10 (IL-10) in both groups before and after treatment are detailed in Table 7.
Table 7.
Comparison of IL-1β, IL-6, and IL-10 Levels of Patients in the Two Groups Before and After Treatment (Pg/Ml)
| Parameters | Before or after treatment | Treatment group (n = 48) |
Placebo group (n = 46) |
|---|---|---|---|
| IL-1β (pg/mL) | Before | 4.97 ± 7.75 | 3.41 ± 3.47 |
| After | 1.92 ± 1.55a | 2.46 ± 2.23a | |
| IL-6 (pg/mL) | Before | 5.30 ± 5.68 | 5.21 ± 5.80 |
| After | 3.11 ± 1.83a | 4.03 ± 3.54 | |
| IL-10 (pg/mL) | Before | 3.89 ± 1.87 | 2.74 ± 1.30b |
| After | 2.37 ± 1.37a | 2.62 ± 1.36 |
Note: aP < 0.05 vs before treatment; bP < 0.05 vs treatment group before treatment, without comparability.
Before treatment, IL-1β levels did not differ significantly between the groups, confirming baseline comparability (P > 0.05). After treatment, both groups exhibited significant reductions in IL-1β levels (P < 0.05), suggesting an anti-inflammatory response.
For IL-6 levels, no significant difference was observed between the groups at baseline, maintaining comparability (P > 0.05). Post-treatment, the treatment group showed significant reductions in IL-6 levels (P < 0.05), whereas the placebo group showed no significant change.
A baseline difference in IL-10 levels was noted between the groups (P < 0.05), indicating heterogeneity; therefore, further analysis of IL-10 changes post-treatment is not provided due to the lack of baseline comparability.
Safety Comparison
Statistical analysis revealed no significant differences between the treatment and placebo groups in blood routine parameters, including red blood cell count, white blood cell count, hemoglobin levels, and C-reactive protein (CRP), nor in liver and kidney function parameters such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, gamma-glutamyl transferase (γ-GT), urea, and creatinine levels, before and after treatment (all P > 0.05). These findings indicate that the treatment group did not experience any adverse reactions attributable to the medication, supporting a favorable safety profile for the Yangxin Huoxue Formula.
Discussion
This clinical trial demonstrated that administration of Yangxin Huoxue Formula produced significant improvements in cardiac output, stroke volume, and peripheral vascular resistance, along with a reduction in both the frequency and duration of angina attacks. These cardiovascular enhancements were accompanied by greater improvements in TCM syndrome scores compared to those achieved with conventional Western medical treatment. Additionally, Yangxin Huoxue Formula exhibited significant efficacy in reducing anxiety symptoms and decreasing inflammatory markers IL-1β and IL-6. In terms of safety, oral administration of Yangxin Huoxue Formula showed no significant differences from the placebo group, confirming its good safety profile.
Previous research has highlighted the link between psychological disorders and cardiovascular disease. For example, Lichtman et al found an association between depression and increased cardiovascular risk.22 In the VIRGO study, Smolderen et al demonstrated that treating depression in young patients with myocardial infarction correlated with improved health outcomes.4 However, mechanisms through which Yangxin Huoxue Formula may benefit patients with stable angina, anxiety, and depression require further elucidation. The results of this study suggest that one possible mechanism may involve the direct alleviation of angina symptoms through enhanced cardiovascular function, including improved cardiac pumping capacity and reduced myocardial ischemia.
In TCM, emotional disturbances are recognized as significant contributors to angina symptoms. Thus, Yangxin Huoxue Formula may indirectly alleviate angina by modulating the neuroendocrine system, potentially reducing symptoms of anxiety, depression, and related emotional disturbances.
Additionally, inflammation is a critical factor in the progression of coronary atherosclerosis. Certain components of Yangxin Huoxue Formula, such as Salviae Miltiorrhizae, Radix et Rhizoma, and Bupleuri Radix, are known for their anti-inflammatory properties, which may improve stable angina by mitigating vascular endothelial inflammation and lowering inflammatory mediator release.23–25
Saikosaponins (SSs), the p bioactive compounds in Bupleuri Radix, exhibit significant anti-inflammatory effects in both in vitro and in vivo studies. These compounds also demonstrate antidepressant, neuroprotective, and renal-protective properties, which merit further investigation. Inflammatory factors play a critical role in the progression of angina,9 with Wang et al identifying S100A8/A9 as an inflammatory mediator potentially involved in triggering angina episodes during inflammatory states.26 Additionally, Najjar et al proposed that neuroinflammation following myocardial infarction may contribute to depressive symptoms.10 Depression itself may impair vascular repair capacity, as noted by other researchers, suggesting possible biological links between emotional disorders and angina.21 Collectively, these findings emphasize the biological connections between cardiovascular disease and psychological disorders, supporting the hypothesis that Yangxin Huoxue Formula may exert modulatory effects on both cardiovascular and emotional symptoms through complex, multifactorial mechanisms.
This study opens new directions for future research, including detailed exploration of the mechanisms underlying individual components of Yangxin Huoxue Formula and assessing their therapeutic potential across various angina presentations and psychological disorders. Future studies should seek to confirm these mechanisms and evaluate the long-term efficacy and safety of Yangxin Huoxue Formula for patients with cardiovascular diseases.
Several limitations of this study should be acknowledged. As a single-center clinical trial with a limited sample size, the findings require further validation through larger, multi-center clinical trials, extended follow-up periods, and analysis of long-term outcomes for individuals with CAD and concurrent anxiety and depression. Despite the demonstrated clinical efficacy of TCM, its mechanisms of action and bioactive constituents remain unclear, warranting comprehensive mechanistic studies to clarify these aspects. This study aims to contribute to a solid evidence base for integrating TCM into contemporary clinical practices.
Conclusion
The Yangxin Huoxue Formula demonstrates significant efficacy in improving cardiac function in individuals with stable angina, evidenced by reductions in both the frequency and duration of angina attacks. It also effectively alleviates symptoms of anxiety and depression and reduces inflammatory mediator release, all while maintaining a favorable safety profile.
Funding Statement
This research was supported by Shanghai Science and Technology Commission, Science and Technology Innovation Action Plan, Medical Innovation Research Project, The clinical effect of Yangxin prescription on stable Angina pectoris with anxiety and depression symptoms (No.22Y11920900).
Clinical Registration Details
Trial registration: Chinese Clinical Trial Registry, ChiCTR2000036185.
Register URL link: https://www.chictr.org.cn/
Register date: 2020/08/12
Abbreviations
PHQ9, Patient Health Questionnaire-9; GAD7, Generalized Anxiety Disorder 7-item; CAD, Coronary artery disease; SSs, Saikosaponins.
Data Sharing Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Shanghai municipal Hospital of Traditional Chinese Medicine (No.2022SHL-KYYS-66). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Disclosure
The authors declare that they have no conflicts of interest regarding this work.
References
- 1.Ma LY, Wang ZW, Fan J, Hu SS. Interpretation of Report on Cardiovascular Health and Diseases in China 2022. Chinese General Practice. 2023;26(32):3975. [Google Scholar]
- 2.Su XT, Wang CX, Zhong L. Research Progress on the Correlation between Acute Myocardial Infarction (AMI) and Depression. Adva Clini Medi. 2023;13:12571. [Google Scholar]
- 3.Case SM, Sawhney M, Stewart JC. Atypical depression and double depression predict new-onset cardiovascular disease in U.S. adults. Depress Anxi. 2018;35(1):10–17. doi: 10.1002/da.22666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smolderen KG, Spertus JA, Gosch K, et al. Depression Treatment and Health Status Outcomes in Young Patients With Acute Myocardial Infarction: insights From the VIRGO Study (Variation in Recovery: role of Gender on Outcomes of Young AMI Patients). Circulation.;135(18):1762–1764. PMID: 28461419; PMCID: PMC5755692. doi: 10.1161/CIRCULATIONAHA.116.027042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May HT, Horne BD, Knight S, et al. The association of depression at any time to the risk of death following coronary artery disease diagnosis. Eur Heart J Qual Care Clin Outc.;3(4):296–302. PMID: 28950317. doi: 10.1093/ehjqcco/qcx017 [DOI] [PubMed] [Google Scholar]
- 6.De Luca L, Temporelli PL, Amico AF, et al. Impact of history of depression on 1-year outcomes in patients with chronic coronary syndromes: an analysis of a contemporary, prospective, nationwide registry. Int J Cardiol.;331:273–280. PMID: 33422564. doi: 10.1016/j.ijcard.2020.12.086 [DOI] [PubMed] [Google Scholar]
- 7.Najjar F, Ahmad M, Lagace D, Leenen FHH. Role of Myocardial Infarction-Induced Neuroinflammation for Depression-Like Behavior and Heart Failure in Ovariectomized Female Rats. Neuroscience.;415:201–214. PMID: 31351141. doi: 10.1016/j.neuroscience.2019.07.017 [DOI] [PubMed] [Google Scholar]
- 8.Wilkowska A, Pikuła M, Rynkiewicz A, Wdowczyk-Szulc J, Trzonkowski P, Landowski J. Increased plasma pro-inflammatory cytokine concentrations after myocardial infarction and the presence of depression during next 6-months. Psychiatr Pol. 2015;49(3):455–464. PMID: 26276914. doi: 10.12740/PP/33179 [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in Inflammation. Front Immunol.;9:1298. PMID: 29942307; PMCID: PMC6004386. doi: 10.3389/fimmu.2018.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Stefano R, Felice F, Pini S, et al. Impact of depression on circulating endothelial progenitor cells in patients with acute coronary syndromes: a pilot study. J Cardiovasc Med. 2014;15(4):353–359. PMID: 24685963. doi: 10.2459/JCM.0b013e328365c195 [DOI] [PubMed] [Google Scholar]
- 11.Shi S, Liang J, Liu T, et al. Depression increases sympathetic activity and exacerbates myocardial remodeling after myocardial infarction: evidence from an animal experiment. PLoS One.;9(7):e101734. PMID: 25036781; PMCID: PMC4103791. doi: 10.1371/journal.pone.0101734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu MY, Ren YP, Wei WL, Tian GX, Li G. Changes of Serotonin (5-HT), 5-HT2A Receptor, and 5-HT Transporter in the Sprague-Dawley Rats of Depression, Myocardial Infarction and Myocardial Infarction Co-exist with Depression. Chin Med J.;128(14):1905–1909. PMID: 26168831; PMCID: PMC4717933. doi: 10.4103/0366-6999.160526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amadio P, Colombo GI, Tarantino E, et al. BDNFVal66met polymorphism: a potential bridge between depression and thrombosis. Eur Heart J.;38(18):1426–1435. PMID: 26705390; PMCID: PMC6251610. doi: 10.1093/eurheartj/ehv655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Känel R. Psychosocial stress and cardiovascular risk–current opinion. Swiss Med Weekly. 2012;142(0304):w13502–w13502. [DOI] [PubMed] [Google Scholar]
- 15.Weng JH, Zhou M. Observation on the curative effect of Yangxin Prescription on coronary heart disease with anxiety and depression. Chinese J Integrative Med Cardio-Cerebrovr Dis. 2019;17(9):1436–1438. [Google Scholar]
- 16.Guo-Wei L, Ji-Yao W. Ge Jun-Bo. Jf Pra Internal Med 1-15 Science-Techn Public. 2017(12):2. doi: 10.16510/j.cnki.kjycb.2017.12.001 [DOI] [Google Scholar]
- 17.Section of Interventional Cardiology of Chinese Society of Cardiology. Section of Atherosclerosis and Coronary Artery Disease of Chinese Society of Cardiology, Specialty Committee on Prevention and Treatment of Thrombosis of Chinese College of Cardiovascular Physicians. Guideline on the diagnosis and treatment of stable coronary artery disease. Chin J Cardiol. 2018;46(9):680–694. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L. Interpretation of Guiding Principles of Clinical Research on the treatment of Angina pectoris with Traditional Chinese. Medi Natural Med Chin J Rat Drug Use. 2012;9(03):3–6. [Google Scholar]
- 19.Zhu WF. “TCM clinical diagnosis and treatment terms”established disease, syndrome system. China J Tradi Chin Med Pharm. 1999. [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. PMID: 11556941; PMCID: PMC1495268. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med.;166(10):1092–1097. PMID: 16717171. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 22.Lichtman JH, Froelicher ES, Blumenthal JA, et al. American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation.;129(12):1350–1369. PMID: 24566200. doi: 10.1161/CIR.0000000000000019 [DOI] [PubMed] [Google Scholar]
- 23.Li X, Li X, Huang N, Liu R, Sun R. A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine.;50:73–87. PMID: 30466994; PMCID: PMC7126585. doi: 10.1016/j.phymed.2018.09.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Guo J, Bao J, Lu J, Wang Y. The anticancer properties of Salvia miltiorrhiza Bunge (Danshen): a systematic review. Med Res Rev. 2014;34(4):768–794. PMID: 24123144. doi: 10.1002/med.21304 [DOI] [PubMed] [Google Scholar]
- 25.Niu Q, Xing WL, Wang YT, Zhu Y, Liu HX. Danhong injection improves elective percutaneous coronary intervention in UA patients with blood stasis syndrome revealed by perioperative metabolomics. World J Tradit Chin Med. 2022;8:247–256. [Google Scholar]
- 26.Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflammation.;10:43. PMID: 23547920; PMCID: PMC3626880. doi: 10.1186/1742-2094-10-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.