Abstract
Purpose
To evaluate the efficacy of fovea-sparing internal limiting membrane (ILM) peeling combined with ILM plug placement in patients with optic disc pit maculopathy (ODP-M).
Patients and Methods
This retrospective study included seven eyes from seven patients diagnosed with ODP-M, treated with fovea-sparing ILM peeling and ILM plug placement. All patients underwent pars plana vitrectomy (PPV), with either SF6 gas or silicone oil used as tamponade. Outcome measures included best-corrected visual acuity (BCVA), central macular thickness (CMT), retinal reattachment, and resolution of retinoschitic lesions (RL). Data were collected at baseline and during follow-ups at 1, 3, 6, and 12 months.
Results
The mean age of the study patients was 31 (± 13.14) years, with a marginal male preponderance (M: F = 4:3). All patients achieved complete retinal reattachment, with a significant reduction in CMT from 503.57 (± 154.74) µm preoperatively to 286.29 (± 22.43) µm at 12 months (P=0.02). BCVA improved in 85.7% of patients, from a mean of 0.77 (± 0.19) logMAR to 0.5 (± 0.25) logMAR by 12 months, though this was not statistically significant (P=0.27). Complete resolution of RL was observed in 71.4% of eyes, while 2 eyes showed partial resolution. One patient developed retinal detachment postoperatively, which was successfully managed with additional surgery.
Conclusion
Fovea-sparing ILM peeling combined with ILM plug placement resulted in favorable anatomical and functional outcomes for ODP-M patients, with a 100% retinal reattachment rate and significant CMT reduction. This technique offers a viable alternative to conventional approaches, preserving foveal architecture while providing a mechanical barrier to fluid accumulation.
Keywords: optic disc pit, optic disc pit maculopathy, fovea-sparing, internal limiting membrane peeling, vitrectomy
Introduction
Optic Disc pit (ODP) is a rare but potentially sight-threatening condition that presents a significant challenge in ophthalmology. First described by Wiethe in 1882,1 ODP are congenital cavitary anomalies of the optic nerve head, resulting from incomplete closure of the embryonic fissure. Though often asymptomatic, these pits can lead to serous macular detachment (SMD), known as optic disc pit maculopathy (ODP-M), which may result in progressive vision loss.2,3 ODP occur with an estimated prevalence of 1 in 10,000 in the general population.2,3 The prevalence of ODP-M, however, is less frequent, affecting around 25–75% of individuals with an ODP.2,3 While the condition can present in both children and adults, it often manifests in young adults and is not typically associated with gender or ethnic predispositions.2,3
The pathogenesis of ODP-M is complex and not entirely understood. ODP are congenital anomalies resulting from incomplete closure of the embryonic fissure during ocular development.1–3 These pits can lead to maculopathy due to the abnormal communication between the subarachnoid space, vitreous cavity, and subretinal space, which allows fluid to accumulate under the macula.2,3
Treatment of ODP-M has evolved over the years, with various strategies documented in the literature, ranging from conservative observation to more invasive surgical interventions. Traditionally, observation was recommended for asymptomatic cases or those with stable visual acuity, as spontaneous resolution has been reported in a minority of cases.2–4 However, given the risk of progressive vision loss, more proactive approaches are often favored for symptomatic patients or those with progressive maculopathy. One of the earliest interventional approaches involved laser photocoagulation, aimed at creating a chorioretinal scar to seal the communication between the ODP and the subretinal space.2,3,5 While this method has shown some efficacy, it carries the risk of damaging the retinal layers, particularly the fovea, which is critical for central vision. The advent of pars plana vitrectomy (PPV) has revolutionized the management of ODP-M. Techniques such as posterior hyaloid detachment, gas tamponade, and internal limiting membrane (ILM) peeling have been employed to facilitate the reattachment of the macula.6,7 Although standard ILM peeling has shown effectiveness in promoting macular reattachment, it also presents significant risks, including mechanical disruption of the retinal architecture. A common complication associated with ILM peeling is the development of dissociated optic nerve fiber layer (DONFL), which can compromise retinal function and lead to suboptimal visual recovery.6,7 Furthermore, conventional ILM peeling over the fovea may result in structural damage that adversely affects visual outcomes.
In light of these limitations, a modified approach using fovea-sparing ILM peeling has been proposed. This technique involves peeling the ILM around the macula while sparing the central foveal region, thereby reducing the likelihood of foveal damage.8,9 To enhance the structural integrity of the optic disc pit, the peeled ILM is then repurposed as an autologous plug, placed within the optic pit to physically seal the abnormal communication and act as a barrier to fluid leakage. This dual approach—fovea-sparing ILM peeling combined with ILM plug placement—not only preserves foveal integrity but also addresses the core anatomical defect in ODP-M.
This article aims to evaluate the efficacy of fovea-sparing ILM peeling combined with ILM plug placement in patients with ODP-M, contributing to the ongoing evolution of treatment strategies for this challenging condition.
Materials and Methods
This retrospective study was conducted at the Retina Institute of Bengal in Siliguri, India, following approval from the Institutional Review Board. All procedures adhered to the ethical standards of the Declaration of Helsinki. Written informed consent was obtained from all patients for both the surgical intervention and the use of their clinical data for research purposes.
Study Population
The study included patients diagnosed with ODP-M with macular detachment who underwent fovea-sparing ILM peeling combined with ILM plug placement between March 2016 to October 2023. Inclusion criteria were the presence of SMD and retinoschiatic lesions (RL) associated with ODP, confirmed by clinical examination and multimodal imaging. Exclusion criteria included previous ocular surgery, other retinal pathologies, and insufficient follow-up data.
Surgical Procedure
All surgeries were performed by a single experienced vitreoretinal surgeon with over 15 years of experience in managing complex retinal conditions. The procedure was conducted under local or general anesthesia, depending on the patient’s preference and medical condition.
The surgical approach involved a standard 25-gauge pars plana vitrectomy (PPV). After core vitrectomy, posterior hyaloid detachment was achieved if not already present. The ILM was stained with Brilliant Blue G dye to facilitate visualization. Fovea-sparing ILM peeling was performed by carefully peeling the ILM in a circumferential pattern around the macula, ensuring that the foveal ILM was left intact to preserve foveal architecture.
Following ILM peeling, the excised ILM was folded and used as an autologous plug. The ILM plug was meticulously placed over the optic disc pit to seal the abnormal communication between the subarachnoid space, vitreous cavity, and subretinal space. This step aimed to provide a mechanical barrier to prevent further fluid accumulation under the macula.
At the end of the procedure, either 18% Sulphur hexafluoride gas or silicone oil was injected into the vitreous cavity as a tamponade. The choice between gas and silicone oil was based on the surgeon’s discretion. Supplementary Video S1 demonstrates the surgical technique.
Following surgery, patients were instructed to maintain a face-down position for at least 5 to 7 days to facilitate the proper placement of the tamponade agent and promote macular reattachment. Postoperative follow-up visits were scheduled at 1, 3, 6, and 12 months. During these visits, a comprehensive ophthalmic examination was performed, including best-corrected visual acuity (BCVA) assessment, anterior and posterior segment examination, and spectral-domain optical coherence tomography (SD-OCT).
Data Collection
Data were extracted from electronic medical records and included demographic information, baseline characteristics, BCVA), central macular thickness (CMT), and SD-OCT analysis. These data were collected at baseline and during follow-up visits at 1, 3, 6, and 12 months postoperatively.
Outcome Measures
The primary outcomes evaluated were the anatomical success of retinal reattachment and improvement in BCVA. Secondary outcomes included changes in CMT and the resolution of retinoschitic lesions (RL). These parameters were assessed at 1, 3, 6, and 12 months postoperatively.
Retinal Reattachment: Defined as the resolution of subretinal fluid on SD-OCT
BCVA: Measured using a Snellen chart and converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis
CMT: Automated measurements using spectral-domain OCT.
Resolution of RL: Evaluated based on the presence or absence of intraretinal fluid on SD-OCT images
Statistical Analysis
Data were analyzed using SPSS software (version 23.0, SPSS Inc., Chicago, IL, USA). Continuous variables, such as BCVA and CMT, were expressed as mean ± standard deviation (SD). Categorical variables, including the rate of retinal reattachment and resolution of RL, were expressed as frequencies and percentages. Changes in BCVA and CMT at different time points (1, 3, 6, and 12 months) were analyzed using a repeated-measures analysis of variance (ANOVA) to assess the statistical significance of the observed changes over time. Pairwise comparisons with Bonferroni correction were performed to identify specific time points at which significant changes occurred. A P-value of less than 0.05 was considered statistically significant for all analyses.
Results
Study Cohort
Seven eyes from seven patients diagnosed with ODP-M were included in the study. The mean age of the patients was 31 (± 13.14) years, with a gender distribution of 4 males and 3 females. The mean duration of decreased vision was 15.43 (± 6.19) months. All patients underwent fovea-sparing ILM peeling combined with ILM plug placement, with either SF6 gas (6 patients) or silicone oil (1 patient) used as a tamponade. Table 1 provides the demographic details of the study population.
Table 1.
Demographic Characteristics of the Study Population
| Characteristic | Study Population |
|---|---|
| Age (years) | |
| Mean (±SD) | 31 (± 13.14) |
| Gender | |
| Male | 4 (57.14%) |
| Females | 3 (42.86%) |
| Duration of DOV Mean (± SD) |
15.43 (± 6.19) |
Abbreviations: SD, Standard deviation; DOV, Decease of vision.
Best-Corrected Visual Acuity
The mean BCVA improved at all follow-up visits, starting from 0.77 (± 0.19) logMAR at baseline to 0.5 (± 0.25) logMAR at 12 months. Although this improvement was not statistically significant (P=0.27), a trend towards improvement was noted over the 12 months. Notably, 6 out of 7 eyes (85.7%) showed improvement by the 12-month follow-up, with one eye maintaining stable vision throughout the study. The BCVA changes in the study population are presented in Table 2.
Table 2.
Changes in the Best-Corrected Visual Acuity (BCVA) in the Study Population Through 12 Months
| BCVA (logMAR) (Mean ± SD) | P value | |
|---|---|---|
| Baseline | 0.77 ± 0.19 | |
| 1 month | 0.67 ± 0.19 | 0.93 |
| 3 months | 0.59 ± 0.3 | 0.63 |
| 6 months | 0.56 ± 0.26 | 0.48 |
| 12 months | 0.5 ± 0.25 | 0.27 |
Abbreviations: BCVA, Best-corrected visual acuity; logMAR, Logarithm of the Minimum Angle of Resolution; SD, Standard deviation.
Central Macular Thickness
The mean CMT decreased at all follow-up visits, with significant reductions observed by month 6 (P=0.02). The mean CMT decreased from 503.57 (± 154.74) µm preoperatively to 283.86 (± 19.41) µm at 6 months and stabilized to 286.29 (± 22.43) µm at 12 months (P=0.02). The CMT changes in the study population are presented in Table 3.
Table 3.
Changes in the Central Macular Thickness (CMT) in the Study Population Through 12 Months
| CMT (µm) (Mean ± SD) | P value | |
|---|---|---|
| Baseline | 503.57 ± 154.74 | |
| 1 month | 370.43 ± 107.07 | 0.28 |
| 3 months | 397.29 ± 200.6 | 0.5 |
| 6 months | 283.86 ± 19.41 | 0.02 |
| 12 months | 286.29 ± 22.43 | 0.02 |
Note: Bold Values: Statistically Significant.
Abbreviations: CMT, Central macular thickness; SD, Standard deviation.
Retinal Reattachment
All 7 eyes (100%) achieved successful retinal reattachment, as evidenced by the resolution of subretinal fluid on spectral-domain optical coherence tomography (SD-OCT).
Retinoschiatic Lesions (RL)
Complete resolution of RL was observed in 5 out of 7 eyes (71.4%), while the remaining 2 eyes exhibited partial resolution of RL by the end of the follow-up period.
Complications
One patient developed a retinal detachment (RD) 3 months after the initial surgery, which was successfully managed with further surgical intervention. No other intraoperative or postoperative complications were noted. All patients complied with postoperative face-down positioning, and no adverse events related to the tamponade were reported.
Figures 1 and 2 are representative cases illustrating the SD-OCT changes over 12 months. The surgical technique is demonstrated in Supplementary Video S1.
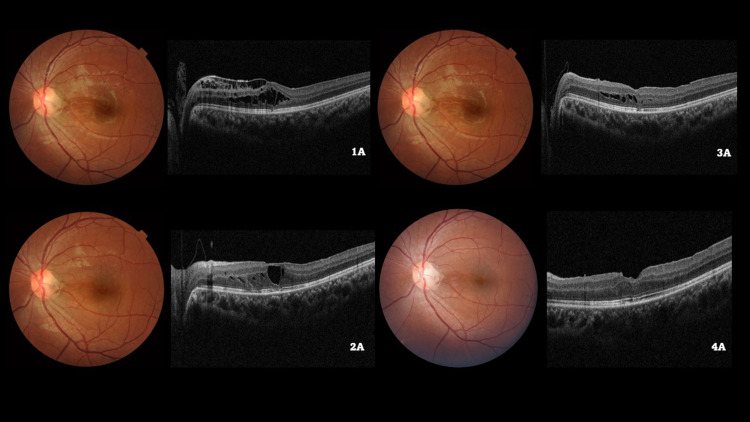
Figure 1.
Color fundus photograph of the left eye of a 19/M illustrating the optic disc pit at baseline (1A) and subsequent visits (2A, 3A, 4A). Baseline spectral domain optical coherence tomography (1B) demonstrates the retinoschiatic lesions (RL) with an epiretinal membrane. Following a pars plana vitrectomy with internal limiting membrane (ILM) peeling and ILM plug and SF6 gas injection, a gradual reduction of RL can be seen on the SD-OCT at months 3 (2B) and 6 (3B), followed by complete resolution by 12 months (4B).
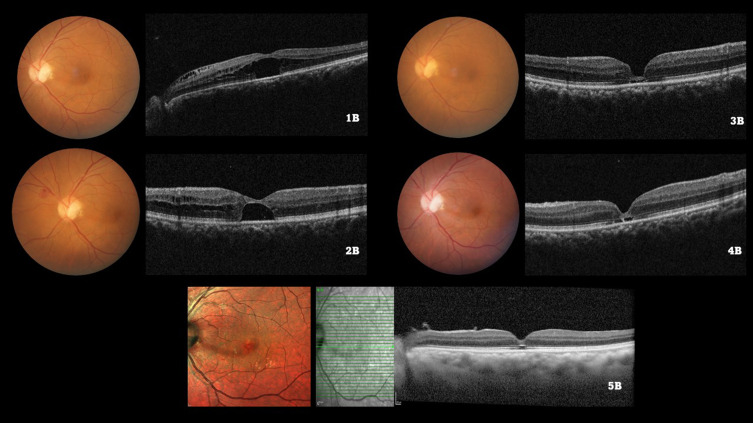
Figure 2.
Color fundus photograph of the left eye of a 55/M illustrating the optic disc pit at baseline (1A) and subsequent visits (2A, 3A, 4A). The baseline spectral domain optical coherence tomography (1B) illustrates the serous macular detachment (SMD) along with the retinoschiatic lesions (RL). After undergoing a pars plana vitrectomy with internal limiting membrane (ILM) peeling and ILM plug and SF6 gas injection, the SMD and RL reduced by 3 months (2B). Subsequently, complete resolution of both the SMD and RL was observed by 6 months (3B), which was maintained upto months 12 (4B) and 24 (5B) respectively.
Discussion
In the current study, we explored the efficacy of fovea-sparing ILM peeling combined with ILM plug placement in patients diagnosed with ODP-M. Our results demonstrated promising anatomical and functional outcomes, with 100% of eyes achieving retinal reattachment, significant reductions in CMT, and improvement in BCVA in 85.7% of cases. Additionally, the majority (71.4%) of eyes showed complete resolution of RL, and no major intraoperative complications were observed, except for one case of post-operative RD that was managed surgically.
The pathogenesis of ODP-M is complex, involving multiple mechanisms for fluid accumulation at the macula. A widely accepted theory suggests that the CSF from the subarachnoid space enters the eye via the optic pit, facilitated by its anatomical connection to the subarachnoid space.1–3,10,11 This allows CSF to move into the subretinal and intraretinal space, where it accumulates, causing SMD and RL formation, progressively impairing vision.10–12 Another theory suggests that vitreous traction on the optic pit and adjacent retina contributes to fluid accumulation.2 Regardless of the origin, the abnormal communication between the subretinal space, vitreous, and optic disc pit leads to fluid accumulation and subsequent macular detachment, necessitating prompt intervention.
Management of ODP-M has evolved significantly over the years. Historically, observation was the initial approach for asymptomatic or stable cases, given reports of spontaneous resolution. However, given the risk of progressive vision loss, conservative approaches are often inadequate for symptomatic cases. Early recommendations for treating ODP-M with oral corticosteroids are now outdated.2 Laser photocoagulation was introduced as an alternative, aiming to create a barrier between the ODP and subretinal space.2,5 Initial xenon lasers were ineffective, leading to the use of argon lasers, though results have been inconsistent.2,5 While some small studies reported fluid absorption and retinal reattachment, others showed poor success rates and severe visual field defects.2,3 The variable outcomes are likely due to the absorption of laser energy by the choroid and retinal pigment epithelium, which may leave the macular schisis unaffected.
The role of PPV in treating ODP-M is well established, with studies showing long-term anatomical success rates between 50–95% and VA improvement in nearly half of the cases.2 In line with these studies, our findings reflect a high anatomical success rate of 100%, with visual improvement noted in 85.7% of cases. We also noted 100% resolution of RL lesion which is similar to that reported in the literature.2 However, as Hirakata et al7 have highlighted, conventional ILM peeling can sometimes lead to postoperative foveal damage, potentially affecting visual recovery. Alternative techniques, such as inserting a temporal ILM flap into the optic pit, have shown promise, achieving a 55.6% anatomical success rate over 10 months, with evidence of faster fluid resolution than with ILM peeling alone. Additionally, autologous scleral plugs have reported an 85.7% success rate at one year, suggesting a comparable yet potentially faster fluid resolution approach. Our fovea-sparing ILM peeling aligns with these findings, demonstrating favorable anatomical and visual outcomes. However, despite a reduction in CMT, we observed that visual improvement, while trending positively, was not statistically significant. This highlights the distinction between anatomical success and functional gains, supporting the hypothesis that chronic retinal microstructural damage in longstanding disease may limit visual recovery. The observed trend towards improved BCVA, despite reduced CMT, underscores the need to evaluate the clinical significance of these anatomical changes, as reductions in CMT alone do not guarantee meaningful visual improvement. Further research is warranted to clarify the relationship between anatomical success and functional outcomes.
Utilizing an autologous ILM flap successfully inhibits the flow of vitreous fluid into the subretinal space.13 This method entails the application of staining and peeling ILM within the temporal arcades while retaining a portion connected to the optic disc in order to form a pedicle-like structure.13 By enveloping the optic disc pit, this flap facilitates fast absorption of fluid under the retina, hence possibly enabling early reattachment of the macular and improvement of visual acuity.14 An alternative technique is removing the ILM from the temporal side of the disc and fitting it into the pit using a membrane scraper coated with a diamond.15 This technique has shown a 55.6% anatomical success rate and a mean reattachment time of 6.5 months, with a notable improvement in best-corrected visual acuity.15 Comparative studies indicate that filling the optic pit with the ILM results in faster fluid resolution when compared to peeling alone, though both methods yield similar anatomical and visual outcomes.16
The fovea-sparing ILM peeling technique represents a significant advancement in the surgical management of macular pathologies. Originally developed for macular hole repair, this technique has been shown to preserve foveal architecture while maintaining the benefits of ILM peeling. In macular hole surgeries, studies have demonstrated that fovea-sparing ILM peeling leads to faster visual recovery and better preservation of central vision compared to traditional ILM peeling, which often risks damaging the delicate foveal structure.17,18 A meta-analysis demonstrated that vitrectomy combined with fovea-sparing ILM peeling resulted in improved visual outcomes for vitreomacular interface disorders and reduced the incidence of FTMH in cases without pre-existing MH.8
The exact mechanism of fovea-sparing ILM peeling remains unclear, but a plausible hypothesis suggests that it preserves the foveal cone cells, which are densely arranged for optimal light reception by minimizing interference from surrounding retinal cells.8,19,20 The ILM, acting as the basement membrane of Müller cells, connects tightly with photoreceptor cells.19–21 Peeling the ILM disrupts Müller cell connections, triggering postoperative macular changes, including retinal nerve fiber swelling.22 Fovea-sparing ILM peeling interrupts the continuity of the ILM around the fovea, altering traction forces and preserving the foveal Müller cells and macular structure integrity.23,24 The current study is the first to demonstrate that this technique can also be successfully applied to ODP-M, yielding good anatomical and functional outcomes. By preserving the foveal structure and using the peeled ILM as an autologous plug, this technique minimizes the risk of foveal damage while effectively addressing fluid accumulation in ODP-M. All patients in this study achieved retinal reattachment, with a majority also demonstrating visual improvement, reinforcing the potential of this novel approach in treating ODP-M.
One patient experienced a RD three months after the initial surgery, which was successfully managed with a secondary surgical intervention. Although rare, RD is a recognized complication in retinal surgeries. In our study, all patients adhered to the recommended postoperative face-down positioning, which has been shown to support the efficacy of tamponade and reduce certain risks. Importantly, there were no intraoperative or immediate postoperative adverse events, and no complications were observed in relation to the tamponade itself. Given the absence of a control group, it is difficult to definitively attribute this case of RD to the procedure or to any individual risk factor. However, RD incidence following retinal surgeries can result from various predisposing conditions, including pre-existing vitreoretinal traction and individual patient anatomy. As such, we believe the single instance of RD in our study does not indicate a heightened procedural risk, although it underscores the importance of vigilant postoperative monitoring.
Despite the promising results, our study has several limitations. The primary limitation is its retrospective design, which introduces inherent biases, including selection bias and variability in follow-up. While retrospective studies are the most feasible approach for studying rare diseases like ODP-M, we acknowledge that this design limits the generalizability of our findings. Additionally, due to the rarity of ODP-M, planning a prospective study is extremely challenging, as demonstrated in the existing literature, where the majority of studies on this condition have also relied on retrospective analyses. This rarity makes it difficult to recruit a sufficient number of patients to conduct a well-powered prospective trial. Future studies with larger sample sizes and longer follow-up periods are needed to validate these findings and confirm the long-term efficacy of this surgical technique. Finally, the absence of a control group in this study makes it difficult to directly compare the outcomes of fovea-sparing ILM peeling combined with ILM plug placement to other surgical approaches. Although this is a limitation, we believe the novelty of the technique and the promising results warrant further investigation.
One of the key strengths of this study is the use of a novel surgical approach, fovea-sparing ILM peeling combined with ILM plug placement, which has shown promising results in preserving foveal structure and improving visual function. Additionally, the study’s comprehensive follow-up and detailed outcome measures, including BCVA, CMT, and the resolution of RL, provide valuable insights into the efficacy of this technique. Our findings contribute to the growing body of literature on the surgical management of ODP-M and suggest that this approach may offer a safer and more effective alternative to traditional methods.
Conclusion
In conclusion, fovea-sparing ILM peeling combined with ILM plug placement represents a promising surgical approach for managing ODP-M. Our study demonstrated that this technique leads to successful macular reattachment, significant reductions in CMT, resolution of RL lesions, and visual improvement in most cases, with minimal complications. However, given the study’s limitations, including its small sample size and relatively short follow-up period, these findings should be interpreted with caution. Future research with larger cohorts and extended follow-up is critical to fully assess the long-term efficacy, safety, and potential risks of this approach. Further validation is needed to determine whether fovea-sparing ILM peeling can offer a reliable alternative to conventional ILM peeling, particularly in cases where foveal preservation is critical.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wiethe T. A case of optic nerve deformity. Arch Augenheilkd. 1882;11:14–19. [Google Scholar]
- 2.Kalogeropoulos D, Ch’ng SW, Lee R, et al. Optic disc pit maculopathy: a review. Asia Pac J Ophthalmol. 2019;8(3):247–255. doi: 10.22608/APO.2018473 [DOI] [PubMed] [Google Scholar]
- 3.Esmaeil A, Ali A, Almutairi S, Alkandari K, Behbehani R, Alali A. Congenital optic disc pits and optic disc pit maculopathy: a review. Front Ophthalmol. 2023;3:1222979. doi: 10.3389/fopht.2023.1222979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikakis EA, Chatziralli IP, Peponis VG, et al. Spontaneous resolution of long-standing macular detachment due to optic disc pit with significant visual improvement. Case Rep Ophthalmol. 2014;5(1):104–110. doi: 10.1159/000362263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gass JD. Serous detachment of the macula. Secondary to congenital pit of the optic nervehead. Am J Ophthalmol. 1969;67(6):821–841. doi: 10.1016/0002-9394(69)90075-0 [DOI] [PubMed] [Google Scholar]
- 6.Shukla D, Kalliath J, Tandon M, Vijayakumar B. Vitrectomy for optic disk pit with macular schisis and outer retinal dehiscence. Retina. 2012;32(7):1337–1342. doi: 10.1097/IAE.0b013e318235d8fc [DOI] [PubMed] [Google Scholar]
- 7.Hirakata A, Inoue M, Hiraoka T, McCuen BW. Vitrectomy without laser treatment or gas tamponade for macular detachment associated with an optic disc pit. Ophthalmology. 2012;119(4):810–818. doi: 10.1016/j.ophtha.2011.09.026 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Zhao X, Zhang W, Yang J, Chen Y. Fovea-sparing versus complete internal limiting membrane peeling in vitrectomy for vitreomacular interface diseases: a systematic review and meta-analysis. Retina. 2021;41(6):1143–1152. doi: 10.1097/IAE.0000000000003140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Xu Q, Luan J. Vitrectomy with fovea-sparing ILM peeling versus total ILM peeling for myopic traction maculopathy: a meta-analysis. Eur J Ophthalmol. 2021;31(5):2596–2605. doi: 10.1177/1120672120970111 [DOI] [PubMed] [Google Scholar]
- 10.Jain N, Johnson MW. Pathogenesis and treatment of maculopathy associated with cavitary optic disc anomalies. Am J Ophthalmol. 2014;158(3):423–435. doi: 10.1016/j.ajo.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 11.Irvine AR, Crawford JB, Sullivan JH. The pathogenesis of retinal detachment with morning glory disc and optic pit. Trans Am Ophthalmol Soc. 1986;84:280–292. [PMC free article] [PubMed] [Google Scholar]
- 12.Imamura Y, Zweifel SA, Fujiwara T, Freund KB, Spaide RF. High-resolution optical coherence tomography findings in optic pit maculopathy. Retina. 2010;30(7):1104–1112. doi: 10.1097/IAE.0b013e3181d87ecb [DOI] [PubMed] [Google Scholar]
- 13.Mohammed OA, Pai A. Inverted autologous internal limiting membrane for management of optic disc pit with macular detachment. Middle East Afr J Ophthalmol. 2013;20(4):357–359. doi: 10.4103/0974-9233.120008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara R, Tsukahara Y, Simoyama T, Mori S. Refined internal limiting membrane inverted flap technique for intractable macular detachment with optic disc pit. Case Rep Ophthalmol. 2017;8(1):208–213. doi: 10.1159/000462956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastor-Idoate S, Gómez-Resa M, Karam S, et al. Efficacy of internal limiting membrane flap techniques with vitrectomy for macular detachment associated with an optic disc pit. Ophthalmologica. 2019;242(1):38–48. doi: 10.1159/000495621 [DOI] [PubMed] [Google Scholar]
- 16.Ravani R, Kumar A, Karthikeya R, et al. Comparison of inverted ILM-stuffing Technique and ILM peeling alone for optic disc pit-associated maculopathy: long-term results. Ophthalmic Surg Lasers Imaging Retina. 2018;49(12):e226–e232. doi: 10.3928/23258160-20181203-12 [DOI] [PubMed] [Google Scholar]
- 17.Murphy DC, Fostier W, Rees J, Steel DH. Foveal sparing internal limiting membrane peeling for idiopathic macular holes: effects on anatomical restoration of the fovea and visual function. Retina. 2020;40(11):2127–2133. doi: 10.1097/IAE.0000000000002724 [DOI] [PubMed] [Google Scholar]
- 18.Ho TC, Yang CM, Huang JS, Yang CH, Chen MS. Foveola nonpeeling internal limiting membrane surgery to prevent inner retinal damages in early stage 2 idiopathic macula hole [published correction appears in Graefes Arch Clin Exp Ophthalmol 2014 Jun;252(6):1025–1026]. Graefes Arch Clin Exp Ophthalmol. 2014;252(10):1553–1560. doi: 10.1007/s00417-014-2613-7 [DOI] [PubMed] [Google Scholar]
- 19.Grossniklaus HE, Geisert EE, Nickerson JM. Introduction to the retina. Prog Mol Biol Transl Sci. 2015;134:383–396. [DOI] [PubMed] [Google Scholar]
- 20.Bringmann A, Syrbe S, Görner K, et al. The primate fovea: structure, function and development. Prog Retin Eye Res. 2018;66:49–84. doi: 10.1016/j.preteyeres.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 21.Bringmann A, Unterlauft JD, Wiedemann R, et al. Morphology of partial-thickness macular defects: presumed roles of Müller cells and tissue layer interfaces of low mechanical stability. Int J Retina Vitreous. 2020;6:28. doi: 10.1186/s40942-020-00232-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilotto E, Midena E, Longhin E, et al. Müller cells and choriocapillaris in the pathogenesis of geographic atrophy secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2019;257:1159–1167. doi: 10.1007/s00417-019-04289-z [DOI] [PubMed] [Google Scholar]
- 23.Morescalchi F, Russo A, Gambicorti E, et al. Peeling of the internal limiting membrane with foveal sparing for treatment of degenerative lamellar macular hole. Retina. 2020;40:1087–1093. doi: 10.1097/IAE.0000000000002559 [DOI] [PubMed] [Google Scholar]
- 24.Seppey C, Wolfensberger TJ. Vitrectomy with fovea-sparing internal limiting membrane peeling for myopic foveoschisis. Klin Monbl Augenheilkd. 2017;234:497–500. doi: 10.1055/s-0043-104429 [DOI] [PubMed] [Google Scholar]