Abstract
The neuropeptide S receptor (NPSR) has been identified as a potential therapeutic target for anxiety and post-traumatic stress disorder. Central administration of neuropeptide S (NPS) in male mice produces anxiolytic-like effects, hyperlocomotion, and memory enhancement. Currently, the literature is limited in the number of studies investigating the effects of NPS in female test subjects despite females facing a higher prevalence of anxiety-related pathology, as well as greater risk for adverse effects while taking psychoactive drugs. Moreover, no previous studies have considered the influence of estrous cycle on the effects of NPS. The present study investigates whether NPS-mediated behavioral phenotypes seen in males translate to females, and whether they are affected by estrous cycle stage. Female C57BL/6NCr mice were intracerebroventricularly cannulated and underwent behavioral paradigms to test locomotion, anxiety, and memory. Estrous cycle stage was determined through examination of vaginal cytology. Our results provide evidence that NPS-mediated behaviors are influenced by the estrous cycle. Administration of NPS decreased anxiety-like behaviors more robustly when the female mice were in high estrogen stages of the estrous cycle. Therefore, the desired anxiolytic-like effects of targeting the NPSR are intact in female mice. However, these effects may to be influenced by the stage of the estrous cycle. The NPSR remains a strong potential drug target for new anxiolytic compounds and based on our initial observations further studies exploring the interaction of estrous cycle and the NPS system are warranted.
SIGNIFICANCE STATEMENT
The neuropeptide S (NPS) receptor has been identified as a potential target for treating anxiety, a condition that is most prevalent in females. Therefore, the potential interaction of estrous cycle with the NPS system described in the present study is an important first step in understanding the function of the NPS system in females.
Introduction
From 2001 to 2003, an estimated 19.1% of adults in the United States suffered from an anxiety disorder (NIMH, https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder), and the situation may have worsened due to the COVID-19 pandemic (Salari et al., 2020; Nochaiwong et al., 2021). Included among these are generalized anxiety disorder, panic disorder, obsessive compulsive disorder, and post-traumatic stress disorder. Women show a higher prevalence for all of these disorders and are up to two times more likely than men to meet the criteria for a life-long anxiety disorder (Kessler et al., 1994). In preclinical investigation of potential anxiolytic compounds, high male subject bias during in vivo animal testing has been common (Beery and Zucker, 2011). Thus, female subjects are often underrepresented in these antianxiety drug development studies, which may contribute to the fact that females are more likely to have adverse reactions to psychoactive drugs (Ekhart et al., 2018). These facts lead to two important areas of unmet need: necessity for new antianxiety treatments and for the inclusion of female animals in in vivo drug development. Research on novel neuropeptide systems may provide insight into potential drug targets.
Neuropeptide S (NPS) is an endogenous peptide that activates the G protein-couple receptor neuropeptide S receptor (NPSR). Both genetic and preclinical studies support a role for this system in sleep disorders, anxiety-related pathologies, and obesity (Xu et al., 2004; Niimi, 2006; Gottlieb et al., 2007; Okamura et al., 2007; Cline et al., 2008; Leonard et al., 2008; Donner et al., 2010; Raczka et al., 2010; Dannlowski et al., 2011; Domschke et al., 2011; Lennertz et al., 2012; Glotzbach-Schoon et al., 2013; Kumsta et al., 2013; Tupak et al., 2013; Klauke et al., 2014; Laas et al., 2014a, 2014b, 2015; Spada et al., 2014; Streit et al., 2014, 2017; Xing et al., 2019). For example, a common (allelic frequency 50%) gain-of-function single nucleotide polymorphism in the NPSR is associated with anxiety/stress phenotypes in humans (Okamura et al., 2007; Dannlowski et al., 2011; Kumsta et al., 2013). Centrally administered NPS has previously been shown to have anxiolytic-like effects, hyperlocomotion, and enhanced memory phenotypes in rodents. This is in line with high expression of the NPSR in the basolateral amygdala, parasbuiculum, hypothalamus, and a number of cortical and subcortical areas (Clark et al., 2011). However, research into the pharmacological effects of NPS has been conducted almost exclusively in male rodents, despite females showing a higher prevalence in anxiety disorders (Kessler et al., 1994). Moreover, research has shown that alternating levels of ovarian hormones modulate anxiety-like behaviors, especially around estrus (Inoue, 2022). In regard to the NPS system, other groups have failed to show the hyperlocomotive effects of NPS in rats, although these groups did not habituate the rats before NPS administration (Badia-Elder et al., 2008; Cannella et al., 2016). Most NPS-sex studies have been done using NPSR knockout (KO) mice, where littermates are compared. Unlike males, female NPSR-KO mice did not exhibit depression-like behavior in forced swim test (Zhu et al., 2010), there were no changes in safety learning (Kreutzmann et al., 2020), and corticosterone treatment did not produce weight increases (Kolodziejczyk and Fendt, 2020). In addition, the effects of NPS in rats was both sex- and strain-dependent (Wegener et al., 2012). Taken together, these data suggest that there is an interaction between biological sex and the NPS system. However, it remains to be determined whether this is organizational or due to circulating hormones that may have differing effects depending on the stage of estrous.
In the present study, we investigated the effects of NPS administration in female mice and the influence of the estrous cycle on a series of behavioral paradigms to assess locomotion, anxiety, and memory. The results of the studies suggest interplay between the estrous cycle on acute and long-term effects of NPS administration.
Materials and Methods
Animals.
Female C57BL/6NCr mice (#556; Charles River Laboratories) were housed with free access to water and food under a 12-hour light/dark cycle. Mice were cannulated intracerebroventricularly at 8–9 weeks of age. After surgery, mice were single-housed in cages with corncob bedding. All experiments were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo (PMY09073N) and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Stereotaxic Surgery.
Mice were given an intraperitoneal injection of ketamine (100 mg/kg) and xylazine mixture (10 mg/kg) as presurgical anesthetic, and then secured into the stereotaxic surgery equipment. Coordinates for intraventricular cannulation were determined relative to bregma using a mouse brain atlas (AP, 0.4 mm from bregma; ML, 1.0 mm from midline; DV, 2.0 mm from dura) (Paxinos and Franklin, 2004). Dental cement was used to secure cannula in place (Metabond, Parkell, Brentwood, NY). Cannula caps were used to keep the cannula free of debris. For postoperative care, mice were given 1 ml of saline and 5 mg/kg Carprofen (Zoetis Inc., MI). Carprofen was given subcutaneously for 3 days following surgery at 5 mg/kg. Animals were given a subcutaneous injection of antibiotic Baytril (2.27%) (Norbrook, Northern Ireland, UK), at a dose of 5 mg/kg every day after surgery as preventative treatment until the animals were euthanized.
Microinjections.
Neuropeptide S (mouse form, AnaSpec, Freemont, CA) was dissolved in artificial cerebrospinal fluid (aCSF) with 0.1% BSA to create a 0.5 mM solution. Other concentrations were made from these stocks by diluting in aCSF/0.1% BSA. Before treatment, individual syringe pumps were set up for injections for each of the concentrations of compounds (e.g., 1 nmol NPS). Each mouse was assigned a treatment group, and when treated always received the same treatment (e.g., NPS-treated in light–dark box and NPS-treated in marble burying). Each pump contained one Hamilton syringe connected to polyethylene tubing with a sterilized intracerebroventricular injector at the end. Sterile deionized distilled water was drawn into the syringe to fill both the syringe and the tubing. After this, air was drawn into the tubing to create an air bubble before drawing up either aCSF/0.1% BSA or NPS. The pumps dispensed 2 μl/min for 1 minute so that the appropriate dose was delivered (e.g., 0.5 mM NPS, 2 μl, for a total of 1 nmol of NPS). For drug administration, cannula caps were gently removed and the injector was placed securely inside the cannula. Mice were previously habituated to the handling required to administer treatment. After the treatment was administered, the injector was removed and the cannula cap was replaced. At the end of the study, angiotensin II was used to verify cannula patency. A microinjection of 1 μl of angiotensin II (AnaSpec, Freemont, CA, AS-20633, 10 μM) was used to induce drinking behavior (Epstein et al., 1970). Mice who failed to drink robustly after injection were considered to have incorrect intracerebroventricular cannula placement and were excluded from analysis.
Estrous Staging.
After each behavioral test in experiments 2, 3, and 4, samples were taken by a female experimenter to determine the estrous stage at the time of testing not necessarily the day of treatment (e.g., for acoustic startle and inhibitory avoidance this was test day). Before the vaginal lavage was performed, two pictures, one with flash and one without, were taken of the vaginal opening of each mouse. Pictures were saved for future reference when determining estrous stage from cytology. To perform vaginal cytology, mice were held with ventral side facing upward. Using a pipette, the vaginal opening was flushed with 10 μl of distilled water. The vaginal opening was then flushed with 10 μl of PBS four times, with samples saved from the third and fourth flushes. Cytology samples were kept on glass microscope slides and allowed to fully dry. Once dry, slides were stained using Cresyl violet for 30 minutes. Coverslips were not placed on the slides. Slides were viewed under a microscope by blinded experimenters to determine the estrous phase of each mouse. Estrous phase was determined by the ratio of cells present in the sample as described by Byers et al. (2012), and those that were ambiguous were not used in the analysis for that particular behavioral assay. Briefly, estrus is dominated by the presence of cornified epithelial cells. As the cycle moves to metestrus, leukocytes begin to appear. The longest phase, diestrus, has abundant polymorphonuclear leukocytes. This will wane as nucleated epithelial cells will dominate during proestrus, which will cycle back to predominately cornified epithelial cells once again back in estrus. Proestrus and estrus are considered high estrogen phases (Hi-E), whereas metestrus and diestrus are low estrogen phases (Lo-E). The use of the terms Hi-E and Lo-E are a method of classification and should not be interpreted as estrogen causing the effects observed in our results. There are many organizational differences between males and females, as well, there are many circulating factors whose levels fluctuate over the estrous cycle. All of which should be equally considered in future mechanistic studies.
Behavioral Testing.
Experiment 1 was performed with 40 mice and estrous staging was not performed. Experiment 2 established dose–response of NPS (55 animals and estrous stage was assessed). The purpose of experiment 3 was to directly compare the effects of the biased NPSR agonist RTI-263 (Clark et al., 2017) to the effects of NPS (53 mice). Experiment 4 established the estrous cycle–NPS system interactions more concretely by comparing a single dose of NPS to vehicle (79 animals and estrous stage samples were collected). All experiments were conducted by female experimenters (as per recommendations from colleagues and Sorge et al., [2014]). All experiments were conducted during the light phase of the 12-hour light/dark cycle. Animals were habituated to the testing room for 30 minutes prior to behavioral testing.
Experiment 1—Locomotor Activity.
Locomotor activity was performed as a surrogate measure for NPS-mediated arousal that has been previously established by electroencephalogram (Xu et al., 2004). Transparent Plexiglas boxes (L 51 × W 39 × H 35 cm) were used as an open-field, in which infrared beams connected to a computer system tracked and quantified the movements of the mice (Omnitech Instruments, Columbus, OH). Mice were habituated to the locomotor box for 90 minutes. The mice were then administered a test compound and placed back into the apparatus immediately for an additional 60 minutes. Estrous stage was not assessed during this experiment because its purpose was to simply establish the starting dose of NPS that is efficacious in males. The data are collected by a computer and the subjects are identified as to the treatment group during the analysis phase.
Experiment 2—Dose–Response Trial.
Behavioral paradigms that administered NPS were done 1 week apart, whereas the acoustic startle reflex (ASR) was assessed without treatment between the inhibitory avoidance and locomotor activity testing (Fig. 1). One week between testing sessions was used to optimize the workflow and to lessen the stress on the animal, which could be induced by back-to-back testing. The NPS should be degraded within minutes to hours after administration by endogenous peptidases. To control for potential confounding effects of NPS-induced locomotor changes in mice on measures of anxiolysis, we employed two separate tests with opposing reliance on locomotor activity to gauge anxiety-like behavior. In the marble burying task, less movement (less burying) is indicative of an anxiolytic-like effect, whereas in the light–dark box, more movement (exploration of light side) is considered anxiolytic. The acoustic startle paradigm is also used to test anxiety-like states in the mice. In humans, the magnitude of ASR is believed to be an endophenotype for anxiety. We use this test to probe for long-lasting effects of NPS treatment. The exploration of long-lasting effects is warranted because a gain-of-function single nucleotide polymorphism in the NPSR has been found to be associated with anxiety and/or stress phenotypes in humans (Okamura et al., 2007; Dannlowski et al., 2011; Kumsta et al., 2013). Therefore, it was posited that the repeated injection of NPS in a rodent may produce similar effects as a more active NPSR in humans. Last, the inhibitory avoidance paradigm is used to assay for the NPS-mediated effects on memory consolidation. This effect on memory has been well-established in the literature (Okamura et al., 2011; Clark et al., 2017). In experiment 2, locomotor activity was performed last because our previous experience is this is the most robust of the behavioral effects and is impacted little by the order in which the behavioral testing is performed. Therefore, apart from locomotor activity, the least stressful assays were performed first. The inhibitory avoidance was prioritized because there was no treatment during the ASR. Once a mouse was placed into a treatment group, they remained in that group for the duration of the experiment.
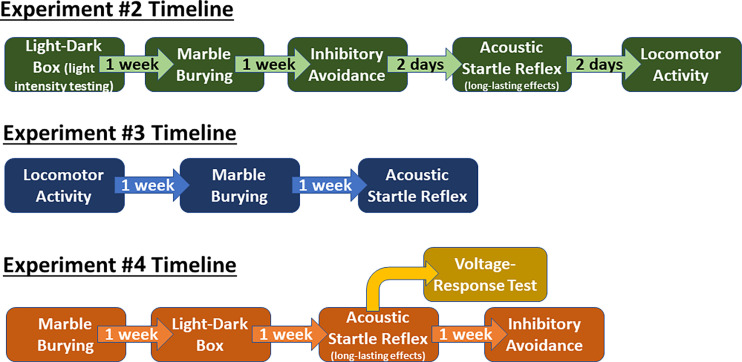
Fig. 1.
Experimental timelines. Experiment 2—establish dose-responsiveness of NPS in female mice (aCSF, NPS – 1, 0.1, 0.01 nmol). Experiment 3—test the efficacy of the biased NPSR agonist RTI-263 (aCSF, NPS 1 nmol, RTI-263 1 nmol). Experiment 4—a larger cohort of female mice were used to test the effects of free estrous cycling on NPS administration (aCSF, NPS 1 nmol). After the completion of the ASR paradigm, 20 mice were removed from the main study to verify the voltage necessary to produce reliable effects in the inhibitory avoidance paradigm. The voltage–response relationship, where we test groups of animals with different intensities of electric shock, is completed to guard against cohort-to-cohort variation in the responsiveness to the shock. The remainder of the mice proceeded into the inhibitory avoidance using the optimal voltage as determined by the voltage–response test (see below). Those mice that are used as part of the voltage–response testing are not used for any subsequent studies.
Experiment 3—NPS versus RTI-263 Efficacy Study.
The biased NPSR ligand RTI-263 (Clark et al., 2017) retains the anxiolytic-like and memory consolidation effects of NPS but has dramatically lower hyperlocomotive effects in male mice and rats (Clark et al., 2017; Huang et al., 2023). The effects of RTI-263 have not been previously assessed in female rodents. Therefore, using the efficacious dose of 1 nmol, NPS and RTI-263 were compared for their effects in habituated locomotor activity, marble burying, and acclimatized acoustic startle. Each paradigm was carried out 1 week apart (as per Fig. 1), with the first paradigm conducted 1 week after surgery. A total of 53 mice were used for this series of experiments (aCSF, n = 18; NPS, n = 18; RTI-263 n = 17).
Experiment 4—Large Cohort for Testing the Influence of Estrous Cycle.
This experiment was done with a large set of animals to solidify the influence of estrous cycle; aCSF compared with one dose of NPS (1 nmol). Each behavioral paradigm was performed 1 week apart in the following order: marble burying, light–dark box, acclimatized acoustic startle, and inhibitory avoidance. The order of the assays was established so that the least stressing paradigms were done first. The initial goal was to have a total of 90 animals in the study. There was significant attrition during and postsurgery (11 mice). A total of 79 animals began the study, with eight being removed from all analyses due to poor cannula placements (angiotensin II or cap dislodgement). Another 15 mice were found at some point during the testing to have signs of illness (e.g., hunching, scruffy fur, scabby eyes, hindlimb reflex deficit) and were removed from the study and any analysis that was performed 2 or less days before removal. Therefore, please refer to individual results sections for the number of animals per experimental group. Power analysis was conducted using male marble burying data from Clark et al. (2017). We calculated for comparing two means [seven buried marbles and three buried marbles; see Fig. 6A from Clark et al., (2017)] with a standard deviation of 2. A standardized effect size of 1 was calculated for a two-way ANOVA, with the following limitations: 1) two levels of the first factor, 2) two levels of the second factor, 3) interaction is paramount, 4) compute sample size for 80% power, and 5) define statistical significance as P < 0.05. The resulting standardized effect size of 1 results in experimental group numbers of nine (18 per treatment, half of which would be for Hi-E or Lo-E), with four groups (N = 36). This number was more than doubled (N = 90). Final sample numbers per experiment fluctuate due to some animals having ambiguous estrous stage or other factors such as flooding their cages, and these are described in the individual results sections. All behavioral experiments were conducted by female experimenters.
Marble Burying.
A novel 45 cm × 22 cm cage containing 5 cm of corncob bedding (same type as home cage) with 18 mm diameter marbles evenly spaced in three rows of six. The ambient light at the point where the chambers were placed was 530 lux. Mice were placed into cages 10 minutes after administration of test compound. Animals were left in the test cages for 45 minutes. Pictures were taken after removing the mice and later used to score the number of buried marbles by a blinded observer. Marbles were considered buried if two-thirds or more of the marble was covered by bedding.
Light–Dark Box.
A custom-built Plexiglas box containing two equal compartments (12 cm × 15 cm × 22 cm) separated by an opening was used. The light section of the chamber consisted of transparent Plexiglas walls with an open top. This side of the light–dark box was exposed to a light source of between 3700 to 4200 lux that was checked between each run. The dark section was made of black infrared transparent acrylic walls with a removable black acrylic top. Mice were administered test compound 10 minutes before being put into the light compartment. Movements of the mice were tracked for 10 minutes using infrared beams (OMNITECH Electronics, Columbus, OH). Data were used to detect the total time spent on the light side and number of transitions between the compartments. The data are collected by a computer, and the subjects are identified as to the treatment group during the analysis phase.
Acclimatized Acoustic Startle.
The acclimatized acoustic startle test consists of mice being placed in the apparatus the day before testing to acclimatize the mice to the testing paradigm. Purpose-built sound-attenuated boxes (Kinder Scientific, Ponway, CA) were used to measure startle responses. On acclimatization day, mice were placed in the startle box for 30 minutes. No sound stimuli were presented. Twenty-four hours later, on test day, mice are placed in the startle chamber, and sound stimuli were presented. No treatments were given before or after this paradigm, however, animals had been treated in the previous paradigms (e.g., light–dark box, see Fig. 1). The program consisted of a 5-minute acclimatization period, four trials presenting a 120 db noise, followed by 64 sound trials randomized between the range of 70 db–125 db. Throughout the course of the program, a background noise of 65 dB was played. Estrous staging was assessed after ASR testing was complete. The startle data are collected by a computer and the subjects’ treatment group is identified during the data analysis phase.
Inhibitory Avoidance.
A custom-made rectangular chamber that was uniformly wider at the top and narrowed toward the bottom was used [modeled after that used by McGaugh et al., (2002)]. The chamber is divided into two sections separated by a manual door. One section was made from black acrylic with a hinged, black acrylic top and had stainless steel plates on the inside walls attached to square-pulse stimulator (Grass model S-48), in series with a constant current unit (Grass model CCU-1). This was connected to a handheld pushbutton switch that administered a shock when pressed. The other section of the chamber was made from white acrylic with a clear, removable top, and was exposed to ambient light (530 lux). A camcorder atop a tripod recorded all trials.
On training day, mice were placed on the light side. Once the mouse crossed over to the dark side, the experimenter closed the door separating the compartments and a shock was administered through the metal floor (0.5 mA, 1 second). Mice were left in the dark side for 30 seconds postshock, then removed and placed in their home cage. Mice were injected with test compound 20 minutes after training. Forty-eight hours later, mice were tested for their latency to enter the dark side in the same manner as training day, except no shock was delivered. Videos were taken and later used to score the latency to enter the dark side by a blinded observer.
Statistical Analysis.
Statistical analysis and graphing were performed using the two-way ANOVA and mixed-effects analysis features in GraphPad Prism Software (v9.0) as well as unpaired t tests and Bonferroni posthoc tests as needed. QQ plots were generated in GraphPad Prism and linearity of this plot was interpreted that the data were normally distributed. All results are expressed as mean ± S.E.M. Group means were considered significantly different when P ≤ 0.05. A single asterisk (*) indicates significant difference at the P ≤ 0.05; double asterisk (**) indicates significance at P ≤ 0.01; triple asterisk (***) indicates significance at P ≤ 0.001; quadruple asterisk (****) indicates significance at P ≤ 0.0001.
Results
Experiment 1
Locomotor Activity.
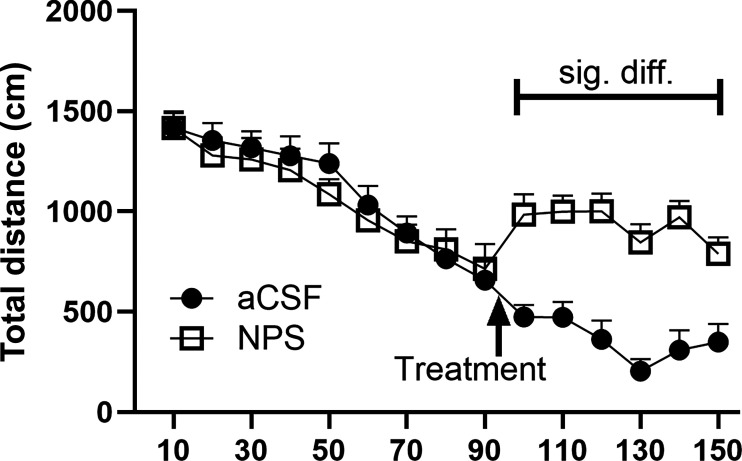
A 1 nmol dose of NPS or vehicle (aCSF) was administered intracerebroventricularly to habituated female mice and assessed for locomotor activity. As previously seen in males (Xu et al., 2004; Clark et al., 2017), NPS produced significant hyperlocomotion in females (Fig. 2) (two-way ANOVA with treatment as the between group and time as within variable; there was a significant treatment effect: P = 0.0397 [F(1,34) = 4.575]; significant time effect: P < 0.0001 [F(5.756,195.7) = 48.26], and a significant interaction time × treatment P < 0.0001 [F(14,476) = 13.59]). Multiple comparisons posthoc analysis revealed that NPS treatment significantly increased distance traveled at each of the 100-, 110-, 120-, 130-, 140-, and 150-minute time points, P = 0.0027, 0.0005, 0.0003, <0.0001, 0.0002, and 0.0152, respectively. Estrous staging was not performed, however, low variability in the data suggest there may be no effects of estrous on this measure. Two mice were excluded from the analysis due to incorrect cannula placement and illness.
Fig. 2.
NPS-mediated hyperlocomotion. Mice were habituated to the apparatus for 90 minutes. They were then administered vehicle or test compound and returned to the apparatus for testing. Their overall movement was recorded for 60 minutes. Mice that received NPS treatment (1 nmol i.c.v.) displayed significantly higher distance traveled than mice aCSF-treated at the 100-, 110-, 120-, 130-, 140-, and 150-minute time points, P = 0.0027, 0.0005, 0.0003, <0.0001, 0.0002, and 0.0152, respectively (see Results section for analysis details). aCSF, n = 18; NPS, n = 18.
Experiment 2—Dose–Response Trial
Light–Dark Box—Light Intensity Trial (No Treatment).
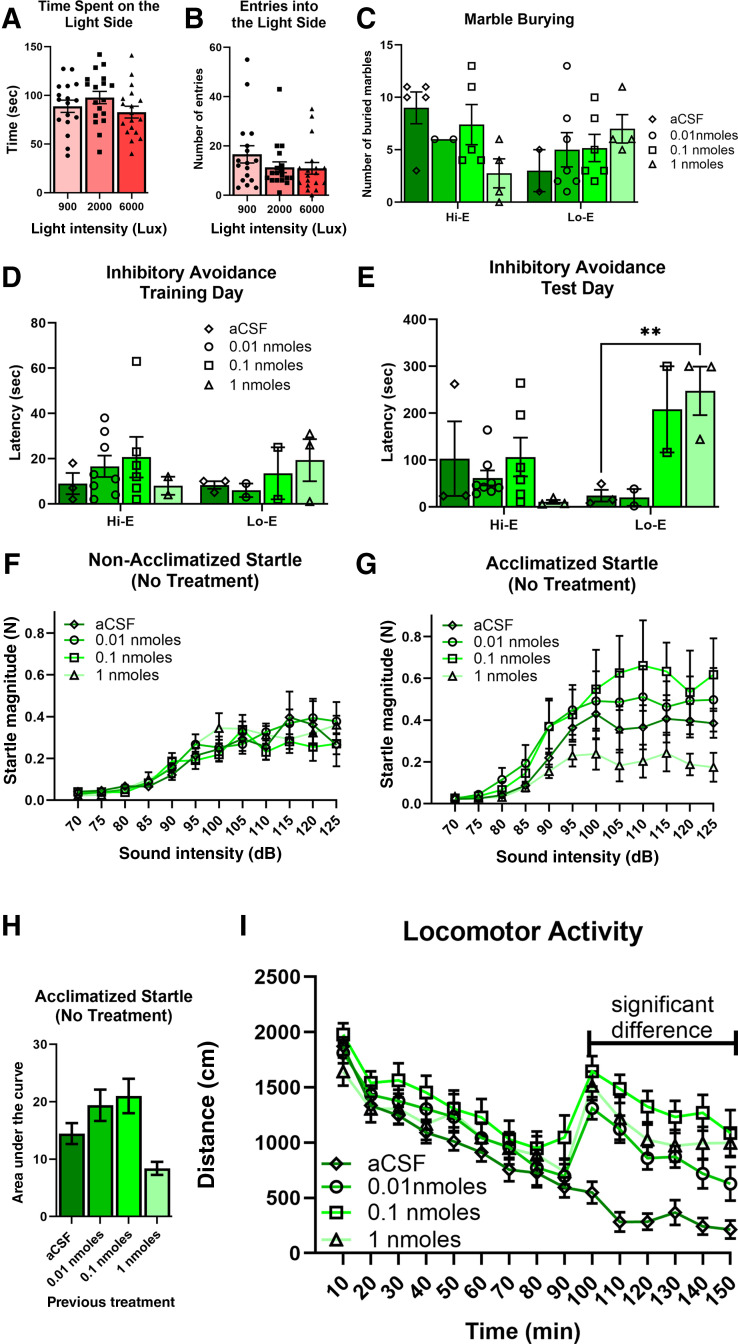
This experiment was performed because in our and others’ experience female mice are seemingly less anxious (more time on the light side) in the light–dark box than males. Therefore, we assessed whether a higher intensity of light was required for the female mice to display an optimal dark side bias (typically 25%–30% of time on the light side). After cannulation, mice were randomly assigned one of three groups: 900, 2000, or 6000 lux. There were no significant differences between the conditions (light intensities) for either the time spent on the light side (Fig. 3A; P = 0.2301) or the number of entries to the light side (Fig. 3B; P = 0.2750). However, there is a numerical decrease in the number of entries for the 2000 and 6000 lux groups compared with the 900 lux group. Therefore, the midpoint between 2000 and 6000 lux was used for our subsequent studies.
Fig. 3.
Dose–response of NPS effects in female mice. (A and B) After cannulation, but before treatments, mice were used to assess conditions for the light–dark box paradigm. (C) The highest dose of NPS produced a nonsignificant decrease in marble burying in the Hi-E stages. The aCSF-treated mice in the Lo-E stages had a numerically lower number of marbles buried (Hi-E: aCSF, n = 5; NPS–1 nmol, n = 2; 0.1 nmol, n = 5; 0.01 nmol, n = 4; Lo-E aCSF, n = 2; NPS – 1 nmol, n = 7; 0.1 nmol, n = 6; 0.01 nmol, n = 4). (D and E) Inhibitory avoidance: there were no numerical or statistically significant effects observed during training day. There was a significant increase in latency during the Lo-E stages with the highest dose of NPS. However, this appears to be driven by the fact that the Lo-E aCSF mice did not learn the association (Hi-E: aCSF, n = 3; NPS – 1 nmol, n = 8; 0.1 nmol, n = 6; 0.01 nmol, n = 2; Lo-E aCSF, n = 3; NPS – 1 nmol, n = 2; 0.1 nmol, n = 2; 0.01 nmol, n = 3). (F and G) ASR: when mice were acclimatized to the behavioral apparatus 24 hours prior to testing, there were numerical differences in the amplitude of the startle. This was not observed in animals that did not have the opportunity to acclimatize to the apparatus (acclimatized: aCSF, n = 4; NPS – 1 nmol, n = 3; 0.1 nmol, n = 4; 0.01 nmol, n = 4; nonacclimatized: aCSF, n = 5; NPS – 1 nmol, n = 5; 0.1 nmol, n = 6; 0.01 nmol, n = 6). (H) After calculating the AUC for responses in acclimatized mice, there is a numerical decrease in startle amplitude in the highest NPS dose group (∼50%). (I) All the doses of NPS used produced robust hyperlocomotion when administered after a 90-minute habituation period (aCSF, n = 11; NPS – 1 nmol, n = 10; 0.1 nmol, n = 12; 0.01 nmol, n = 12).
Initially, we ran experiment 2 hypothesizing there would be no influence of estrous cycle on NPS-mediated behaviors. However, we did collect vaginal cytology samples as described in the Materials and Methods. The data generated were highly variable and were segregated based on estrous cycle stage. This resulted in group sizes that are below the ideal for statistical analysis. Nonetheless, the data are shared as “trial” or “preliminary” data to inform the reader of our process and thinking as we moved through this project.
Marble Burying.
There were no significant effects detected in the marble burying dose–response experiment (mixed-effects model; estrous P = 0.3464, treatment P = 0.8674, estrous × treatment P = 0.1036) (Fig. 3C). This is primarily due to low sample numbers because of the estrous cycle distribution. However, there is one trend of note. Animals that were treated with 1 nmol NPS in the high estrogen stages appear to have lower marble burying than Hi-E stage mice treated with aCSF.
Inhibitory Avoidance.
There are no significant results on training day, as expected (mixed-effects model: estrous, P = 0.7775; treatment, P = 0.7932; estrous × treatment, P = 0.6471) (Fig. 3D). On test day, there is an interaction between estrous stage and treatment, with no main effects (mixed-effects model: estrous, P = 0.1042, F[1,22] = 2.873; treatment: P = 0.0573, F[3,22] = 2.909; estrous × treatment: P = 0.0084, F[3,22] = 5.026) (Fig. 3E). The lack of main effects is likely due to low sample numbers because of the distribution after estrous cycle staging in freely cycling mice. Latencies on training day for Hi-E mice were typical of what is seen in our hands for this assay when experimenting with male mice (Clark et al., 2017). However, on test day, control and 0.01 nmol-treated mice that were in Lo-E stages did not display the typical longer latency indicative of aversive association, whereas the two highest doses of NPS produced expected “NPS-mediated” levels of latency (aCSF vs. 1 nmol, P = 0.0068). Conversely, in the high estrogen stages, vehicle-treated control mice exhibited the typical latency indicative of an associative memory, whereas 1 nmol NPS-treated animals had very low latency. There is one data point missing for a 1 nmol-treated animal on training day, due to file corruption (video). To iterate, we are sharing these data and pointing to trends and numerical differences to allow the reader to follow our process and thinking as we navigated through this study.
ASR.
Mice were run without acute treatment (not treated the week of ASR testing), and so the animals are segregated by what they had been administered twice previously (2 days before for inhibitory avoidance, and 9–10 days before for marble burying). Traditionally ASR in our laboratory has been performed without acclimatization to the box (the day before) and is run simply with 5-minute acclimatization directly before presenting the acoustic stimuli. Due to the number of animals, we split the cohort into two groups to be run on subsequent days. However, due to a technical error the first group was not presented with acoustic stimuli but rather was simply in the boxes for 30 minutes. Therefore, these mice were run with the proper acoustic stimuli the following day. This resulted in the two groups being different in that one group was acclimatized to the boxes the day before data acquisition and the other group was not. There was no statistically significant difference between the groups of the nonacclimatized mice (Fig. 3F). Running a two-way ANOVA for the acclimated mice data did not show significant effect of treatment or an interaction, only the expected effect of sound intensity (Fig. 3G; P < 0.0001). However, when calculating the area under the curve (AUC), the subsequent one-way ANOVA revealed a significant effect (P = 0.0211). Although the post hoc analysis (Dunnett’s multiple comparisons) did not identify the point of significance. This is despite the NPS 1 nmol group had having a startle magnitude less than 50% of the aCSF-treated animals (Fig. 3H).
Locomotor Activity.
All doses of NPS produced significant hyperlocomotion, as compared with the aCSF-treated animals (two-way ANOVA, time × treatment: P < 0.0001, F[42,574] = 4.650; time: P < 0.0001, F[6.634,272.0] = 48.82; treatment: P = 0.0021, F[3,41] = 5.811) (Fig. 3I). Post hoc analysis revealed that the point of significance was each of the dose groups was significantly different from aCSF from the time of injection to the end of the experiment, with the one exception the lowest dose of NPS (0.001 nmol) was significant postinjection just not during the last time bin. There was no difference between the different doses and so a clear dose–response relationship was not observed.
Experiment 3—RTI-263 Study
Using the efficacious dose of 1 nmol (as per Figs. 2 and 3) a series of experiments were conducted to compare NPS effects to that of RTI-263 (a biased NPSR agonist) (Clark et al., 2017) in female mice. The workflow is depicted in Fig. 1, with one week separating each of the behavioral tests; locomotor activity (treated), marble burying (treated), and ASR (no acute treatment; long-lasting effects test). Once a mouse was placed into a treatment group, they remained in that group for the duration of the experiment. Any discrepancy in the number of mice per group from one assay to another are solely due to some animals having ambiguous estrous stage and so were excluded from the data analysis for that specific test.
Locomotor Activity.
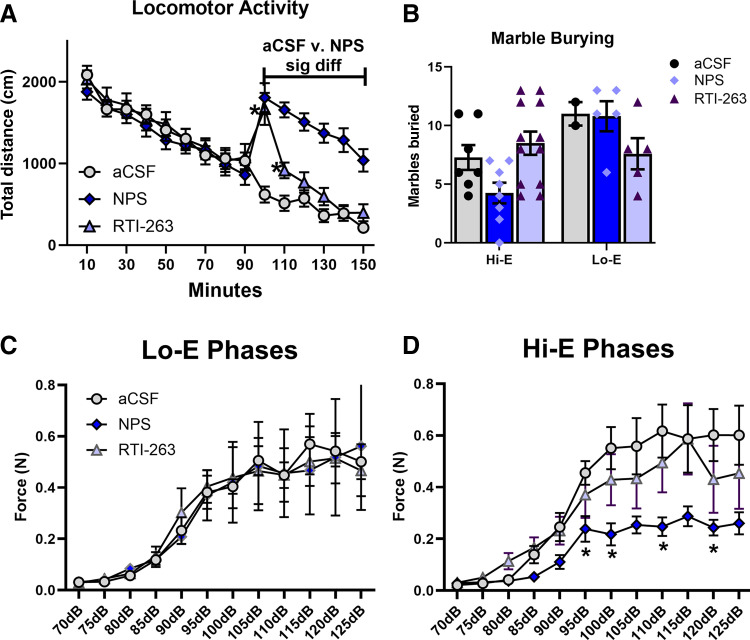
After a 90-minute habituation phase, animals were injected intracerebroventricularly with either NPS (1 nmol), RTI-263 (1 nmol), or aCSF. Two-way ANOVA revealed a significant effect of time (P < 0.0001, F[6.163,308.2] = 77.67), a significant effect of treatment (P = 0.0319, F[2,50] = 3.694), and a significant time × treatment interaction (P < 0.0001, F[28,700] = 15.08). Post hoc analysis identified that NPS (1 nmol) produced a significant effect from 100 minutes to the end of the session (after injection) (Fig. 4A). RTI-263 (1 nmol) produced significant locomotor activity only during the 100- and 110-minute bins (Fig. 4A). The determination of estrous phase during this paradigm was not done.
Fig. 4.
Comparison of the effects of NPS and RTI-263 in female mice. Comparison of the effect of NPS to that of RTI-263 (aCSF, NPS, 1 nmol; RTI-263, 1 nmol). (A) Hyperlocomotion: female mice were injected intracerebroventricularly after a 90-minute habituation phase. NPS produced the expect hyperlocomotion, and RTI-263 produced an unexpected increase in locomotor activity during the first 20 minutes after injection as compared with aCSF-injected mice (aCSF, n = 18; NPS, n = 18; RTI-263, n = 17). (B) Marble burying: there were no significant effects. However, the expected NPS-mediated decrease in marble burying appeared to be only present in the Hi-E mice. Hi-E: aCSF, n = 7; NPS, n = 8; RTI-263, n = 12; Lo-E: aCSF, n = 2; NPS, n = 5; RTI-263, n = 5. (C and D) ASR: mice previously treated with NPS had lower startle amplitudes during Hi-E stages of estrous. There were no effects of prior treatment in those animals in Lo-E stages of estrous during the testing. Hi-E: aCSF, n = 5; NPS, n = 11; RTI-263, n = 6; Lo-E: aCSF, n = 5; NPS, n = 4; RTI-263, n = 5.
Marble Burying.
Due to the groups having an uneven number of animals caused by the randomness of the estrous cycle, these data were analyzed by fitting a mixed model. There was a significant effect of estrous stage (P = 0.0157, F[1,9] = 8.821) (Fig. 4B), with treatment having no significant effect (P = 0.6205, F[2,24] = 0.4868). However, there was a significant estrous stage × treatment interaction (P = 0.0180, F[2,9] = 6.492). The only notable post hoc result was the comparison of aCSF versus NPS in the Hi-E stage (post hoc, P = 0.0979). No effects of RTI-263 (1 nmol) were noted. Due to segregation into estrous, this study was underpowered.
ASR.
ASR was assessed 7 days after the last NPS or RTI-263 treatment, without treatment on the acclimation day or the testing day. There was no significant effect of RTI-263 nor any other statistically significant effects during Lo-E phase (Fig. 4C). In the Hi-E phases (Fig. 4D), two-way ANOVA revealed an expected significant effect of dB (P < 0.0001, F[2.525,47.98] = 49.46), a significant effect of treatment (P = 0.0089, F[2,19] = 6.116), and a significant dB × treatment interaction (P < 0.0001, F[22,209] = 3.482). Post hoc analysis revealed specific points of significant difference between aCSF- and NPS-treated mice during Hi-E stages at 95 dB (P = 0.0132), 100 dB (P = 0.0176), 110 dB (P = 0.0324), and 120 dB (P = 0.0345).
Experiment 4—Large Cohort
Marble Burying.
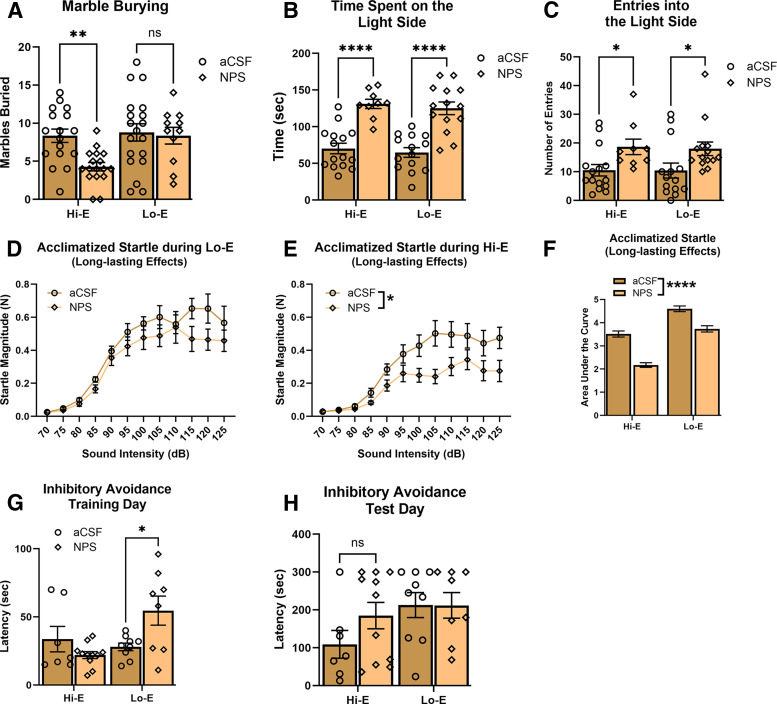
In the marble burying paradigm, treatment of 1 nmol NPS caused a significant decrease in the number of marbles buried compared with control animals in mice in estrous stages defined by Hi-E (Fig. 5A). Because groups had uneven number of animals due to the randomness of the estrous cycle, these data were analyzed by fitting a mixed model (estrous stage significant effect [P = 0.0229, F[1,26] = 5.852]) with treatment having a significant effect (P = 0.0302, F[1,34] = 5.115), and there was a significant estrous stage × treatment interaction (P = 0.0497, F[1,26] = 4.235). Post hoc multiple comparisons detected a significant difference between aCSF- and NPS-treated animals during the Hi-E stages (P = 0.0021) and between NPS-treated animals between estrous stages (P = 0.0067). From these results, and consistent with our smaller scale studies (Fig. 3C; Fig. 4B), it appears that NPS produced an anxiolytic-like effect in animals in estrous stages characterized by high estrogen. Five mice were excluded from data analysis due to ambiguous estrous stage.
Fig. 5.
Estrous cycle interacts with short-term and long-term effects of NPS administration. (A) Marble burying: mice administered vehicle or test compound 10 minutes before testing. The number of marbles buried was recorded after 45 minutes. Hi-E mice that received NPS treatment buried significantly less marbles than mice treated with aCSF (**P = 0.0021) (aCSF Hi-E, n = 17; NPS Hi-E, n = 18; aCSF Lo-E, n = 17; NPS Lo-E, n = 12). (B and C) Light–dark box: mice were administered vehicle or test compound ten minutes before testing. The time spent on the light side and the number of entries into the light side was measured. (B) NPS-treated mice spent significantly more time on the light side, regardless of estrous stage. (****<0.00001). (C) NPS-treated mice showed a significant increase in the number of entries into the light side, regardless of estrous stage (*P = 0.0024) (aCSF Hi-E, n = 15; NPS Hi-E, n = 9; aCSF Lo-E, n = 14; NPS Lo-E, n = 14). (D–F) ASR: magnitude of startle elicited from 64 sound trials between 70 dB and 125 dB in (D) Lo-E mice and (E) Hi-E mice that had been microinjected with NPS or aCSF twice prior (1 and 2 weeks prior). (F) Previous NPS treatment reduced startle response in female mice, and the mice in Lo-E stages appear to have higher startle magnitudes (aCSF Hi-E, n = 11; NPS Hi-E, n = 10; aCSF Lo-E, n = 5; NPS Lo-E, n = 7). (G and H) Inhibitory avoidance: on training day, mice were trained to associate an electrical shock with the dark compartment and were then administered vehicle or test compound 20 minutes later. Before that day’s treatment, those mice which were in Lo-E age and previously treated with NPS had a significantly higher latency to enter the dark compartment. After 48 hours, on test day, the latency to enter the dark compartment was measured again (aCSF Hi-E, n = 7; NPS Hi-E, n = 11; aCSF Lo-E, n = 9; NPS Lo-E, n = 8).
Light–Dark Box.
Mice were given a microinjection treatment of either aCSF or NPS 10 minutes prior to testing. Mice that were treated with 1 nmol NPS spent significantly more time in the light side of the light–dark box compared with aCSF animals regardless of estrous phase (Fig. 5B). Intracerebroventricular microinjection of NPS also significantly increased the total number of entries into the light side (a measure of locomotor activity) of the light–dark box with no interaction between estrous stage and treatment (Fig. 5C). For the total time spent on the light side, because groups had uneven number of animals due to the randomness of the estrous cycle, these data were analyzed by fitting a mixed model (estrous stage no significant effect: P = 0.4540, F[1,48] = 0.5699) with treatment having a significant effect (P = <0.0001, F[1,48] = 62.51), and there was no significant estrous stage × treatment interaction (P = 0.9753, F[1,48] = 0.0009700). Similarly, for the number of entries to the light side, because groups had uneven number of animals due to the randomness of the estrous cycle, these data were analyzed by fitting a mixed model (estrous stage no significant effect: P = 0.8758, F[1,48] = 0.02468) with treatment having a significant effect (P = 0.0024, F[1,48] = 10.23), and there was no significant estrous stage × treatment interaction (P = 0.9094, F[1,48] = 0.01310). The number of entries into the light side is often considered a measure of locomotive activity. Therefore, the increase in entries in NPS-treated mice, regardless of estrous stage, may be indicative of a hyperlocomotive effect (Fig. 2; Fig. 3I; Fig. 4A) rather than an anxiolytic-like effect in females. Ten mice were excluded from data analysis due to ambiguous estrous stage.
Acclimatized Acoustic Startle.
At the time of acclimatized acoustic startle testing, animals had been previously treated with either aCSF or NPS during the marble burying (2 weeks prior) and light–dark box (1 week prior) tests. Mice were not treated at any point during the ASR testing. Twenty-four hours before testing, mice were habituated to the acoustic startle chambers. In Lo-E stages, a two-way ANOVA showed that there was no effect of treatment but also no interaction between dB and treatment (two-way ANOVA treatment: P = 0.2393, F[1,10] = 1.566; dB: P < 0.0001, F[2.212,22.12] = 60.87; and dB × treatment: P = 0.2451, F[11,110] = 1.280) (Fig. 5D). In Lo-E stages of the estrous cycle, it does not appear that NPS is producing an anxiolytic-like effect.
In mice that were in Hi-E stages of estrous on the day of ASR testing, previous treatment (1 and 2 weeks prior, as above) produced a significant effect and an interaction (two-way ANOVA dB: P < 0.0001, F[1.736,32.98] = 40.64; treatment: P = 0.0388, F[1,19] = 4.925; and dB × treatment: P = 0.0004, F[11,209] = 3.225) (Fig. 5E). Post hoc analysis did not reveal a specific point of significant difference between aCSF- and NPS-treated. Despite the significant effect of NPS only in Hi-E stages (above), we performed a more extensive analysis. When calculating the AUC, the subsequent two-way ANOVA revealed a significant effect of treatment (P < 0.0001) and estrous stage (P < 0.0001), but there was no interaction (P = 0.0854) (Fig. 5F). It is interesting, but not surprising that there is an effect of estrous stage on ASR. Despite the analysis of AUC not revealing a specific effect in the post hoc analysis, the analysis of the raw data (Fig. 5, D and E) did reveal a significant effect of NPS during Hi-E stages.
Inhibitory Avoidance.
For inhibitory avoidance behavioral testing, the latency for mice to enter the dark side of the chamber on a training day was recorded and compared with the latency recorded on test day 48 hours later. On training day, mice were treated 20 minutes after entering the dark side of the chamber. When training day latencies were grouped by estrous phase, a mixed-effects analysis showed that there was an effect of estrous stage on latency time, as well as a significant interaction between estrous stage and treatment (two-way ANOVA estrous stage: P = 0.0483, F[1,31] = 4.226; treatment: P = 0.2634, F[1,31] = 1.297; and estrous stage × treatment: P = 0.0063, F[1,31] = 8.578) (Fig. 5G). Post hoc analysis revealed a significant difference between aCSF- and NPS-treated in the animals in Lo-E on training day (P = 0.0073). When looking at test day latencies grouped by estrous phase, there were no significant differences (mixed-effects analysis estrous stage: P = 0.0801, F[1,13] = 3.604; treatment: P = 0.3295, F[1,18] = 1.005; and interaction estrous stage × treatment: P = 0.2977, F[1,13] = 1.177) (Fig. 5H). Twenty mice were not run in the present experiment because they were used for validation of the voltage-response prior to the experiment. Five mice were excluded from analysis due to ambiguous estrous stage and one mouse was excluded due to “timing out” in training day (>300 seconds to enter the dark compartment).
Discussion
The purpose of this study was to determine if NPS-mediated phenotypes translate across sexes. Our results suggest that NPSR activation produces the same behavioral phenotypes in females as compared with males (Xu et al., 2004; Clark et al., 2017), however, these may be influenced by the phase of the estrous cycle. Specifically, central administration of NPS resulted in hyperlocomotion likely independent of estrous (Fig. 2; Fig. 3I; Fig. 4A), whereas the anxiolytic-like effect (as initially measured in marble burying) (Fig. 5A) was expressed only in animals during Hi-E stages (proestrus and estrus). In addition, it was revealed that when tested in Hi-E stages in ASR, NPS treatment in the weeks prior to testing produces lowered startle magnitudes, also indicative of an anxiolytic-like effect. (Fig. 4D; Fig. 5E). Whether this is due to treatment alone, or is an interaction between treatment, estrous stage, and the context (behavioral paradigm at time of treatment) remains to be determined.
The hyperlocomotive effect produced by NPS treatment in females appears to be as efficacious as previously determined in males (Fig. 2; Fig. 3I; Fig. 4A). If estrous phase was modulating the hyperlocomotion effect of NPS, we would expect significant variance in the NPS-treated group, which was not the case. This low variation was seen in all of the locomotor data we collected. The biased NPSR agonist RTI-263, which does not produce appreciable hyperlocomotion in male mice (1 nmol) (Clark et al., 2017), produced hyperlocomotion in female mice (Fig. 4A). This effect was to a degree that was far less than the NPS-mediated effects in female mice but far more than previously seen in male mice (Clark et al., 2017). Additionally, it was determined that NPS-treated female mice had significant hyperlocomotion in the light–dark box test regardless of estrous phase (Fig. 5C), as seen by the number of crossings between compartments. Together, the light–dark box entries and locomotor activity data, at least within the testing context of our studies, suggests that the hyperlocomotive effects of NPS does not appear to be impacted by the estrous cycle. However, estrous effects on NPS-mediated hyperlocomotion could be masked by the hypersensitivity of this effect in females. In our hands, all of the doses of NPS tested were efficacious in the locomotor testing (Fig. 3I). The lowest dose used (0.01 nmol) does not produce hyperlocomotion in male mice, and the second lowest dose used in our studies when administered to males produces only half the maximal effect (Xu et al., 2004). In addition, that RTI-263 produces a hyperlocomotor effect in females that reaches significance (Fig. 4A), suggesting that female mice are more susceptible to the arousal effects of NPS. More comprehensive future investigations should be completed in female mice, with a lower dose range and in parallel with males. In addition, more direct measures of arousal (e.g., electroencephalogram) (Xu et al., 2004) should be included in those future studies.
In the marble burying paradigm, NPS-treated females buried significantly less marbles only if they were in a high estrogen phase at the time of testing (Fig. 5A). This effect was significant in experiment 4 where there were a large number of animals, but was seen as a numerical change in the other two experiments (Fig. 3C; Fig. 4B) (experiment 3, P = 0.097). In low estrogen phases (metestrus or diestrus), there was no statistically significant difference in the number of marbles buried (Fig. 5A). It needs to be highlighted that marble burying was the first test completed in which NPS was administered in experiments 2 and 4 (Fig. 1) and so would not be impacted by the long-term effects we saw in acoustic startle (Fig. 3G; Fig. 4D; Fig. 5E). Therefore, there appear to be biological changes during estrous that influences the anxiolytic-like effects of the NPS system as assayed by marble burying. Alternatively, marble burying has been considered a measure of compulsive behavior. However, this interpretation of marble burying is contentious. Therefore, the view that marble burying is a bioassay for clinically efficacious anxiolytics may be the most straightforward and for which there is the most evidence.
In the light–dark box test, NPS treatment caused mice to spend significantly more time in the light side of the box regardless of estrous stage (Fig. 5B). Due to the repeated dosing study design that was used, it is possible that effects seen in the light–dark box are long-lasting effects from the treatment during the marble burying. However, the long-lasting effects that were seen in the present study (e.g., acoustic startle Fig. 4D [post hoc effects] and Fig. 5E [treatment effect, P = 0.0388]) were only significant during Hi-E stages. The effects in ASR are indicative of an anxiolytic-like effect, but only during Hi-E stages, which is counter to what was observed in the light–dark box test. Moreover, in our RTI-263 study (experiment 3), mice had been treated in the locomotor activity assay a week before the marble burying assay, and the anxiolytic-like effects of NPS seem to be only during Hi-E stages (nonsignificant posthoc effect, P = 0.097) (Fig. 4B). If the effects seen in light–dark box are viewed as not having been affected by the previous NPS treatment, the light–dark box results support the notion that NPS treatment produced a significant anxiolytic-like effect regardless of estrous stage. However, caution must be taken because this result may be attributed to NPS producing hyperlocomotion and not an anxiolytic-like effect. Mice treated with NPS had significantly more entries into the light side of the light–dark box than aCSF-treated mice regardless of estrous stage (Fig. 5C). Again, there was no significant effect of estrous stage or interaction between estrous stage and treatment. This significant increase in number of entries was also seen in male mice that were treated with NPS and could be attributed to hyperlocomotion (Xu et al., 2004). However, in male mice, the biased NPSR agonist RTI-263 shows very little arousal effects but still produces significant anxiolytic-like effects (Clark et al., 2017). The confounding effect of NPS-mediated hyperlocomotion is particularly relevant for female mice because they appear to be more sensitive to the hyperlocomotor effects of NPSR agonists (Fig. 3I; discussed above). In summary, single and acute treatment with NPS produced anxiolytic-like effects in marble burying that were dependent on estrous stage, and although anxiolytic-like effects are seen in light–dark box test, there may be confounding factors (hyperlocomotion) that make interpretation of these results less than clear.
Perhaps the strongest evidence from our studies that the NPS system can produce anxiolytic-like effects in female mice is from data when the mice did not have recent acute treatment. Mice previously treated with NPS had lower startle magnitudes, again when in Hi-E phases of the estrous cycle (Fig. 4D; Fig. 5E). In contrast, within the group of mice that were in low estrogen stages at the time of testing, there were no differences seen (Fig. 5D). This same finding was observed in our RTI-263 study (Fig. 4C). Because a decrease in the force of startle is associated with decreased anxiety levels, this again suggests that NPS is producing an anxiolytic-like effect in mice during Hi-E stages of the estrous cycle. Therefore, due to the anxiolytic-like effects of NPS being only observed during Hi-E stages in marble burying (acute treatment) (Fig. 5A) and ASR (long-lasting effects) (Fig. 4D; Fig. 5E), we speculate that there is an interaction between the NPS system and the estrous cycle. Because anxiolytic-like effects were seen in both stages in the light–dark box, more studies are required to disentangle these issues.
Our results in the inhibitory avoidance paradigm are inconsistent and likely suggest that more thorough and in-depth study of female subjects in this paradigm is warranted. After only one prior exposure to NPS, female mice showed a significant memory enhancement effect of NPS only in Lo-E stages (experiment 2) (Fig. 3E). This effect was not seen after two prior exposures to NPS (experiment 4) (Fig. 5, G and H). Experiment 4 showed that mice in Lo-E stages that were previously treated with NPS had a longer latency to enter the dark compartment of the apparatus during training (Fig. 5G). This is prior to the postshock injection of aCSF or NPS, and the effect appears to be due to the long-lasting effects of NPS (the mice had not been injected for 2 weeks). How exactly this should be interpreted is difficult. The increases in latency could mean that these mice have a lower imperative to enter the dark compartment (e.g., less risk avoidance). The increased latency on “training day” is seen during the Lo-E phases and so may be a different phenomenon than what is seen in the acoustic startle. However, it should be emphasized that the estrous stage for inhibitory avoidance was determined on “test day” which is 2 days after “training day.” Therefore, even though we accounted for this in our estrous staging, it is conceivable that the “training day” data are indeed showing an effect during high estrogen estrous phases (estrous cycle is only 3–4 days in mice). Alternatively, the increased latency on training day could be the result of freezing and a sign of anxiety. Taken together, our data suggest that there are long-lasting effects of NPS that manifest during Lo-E stages (Fig. 5G; Fig. 3E, 2 weeks [during training] and 2 days post-treatment [during testing], respectively). However, there appears to be a lack of literature that addresses the effects of estrous cycle on the inhibitory avoidance or related-paradigms. Therefore, to overlay the effects of NPS in female mice onto this paradigm is likely premature at this point. Moreover, because the paradigm uses a noxious stimulus, possible explanations for the complications with the inhibitory avoidance paradigm could be due to pain sensitivity differences over the estrous cycle. One study has provided evidence for this cause, showing that patients exhibit a worsening of panic and/or anxiety symptoms in premenstrual phases that would correlate with high estrogen phases in mice (Perna et al., 1995). Another study has shown that female rats in metestrus and diestrus, which are both low estrogen phases, had increased pain sensitivity during a hot plate test (Ibironke and Aji, 2011). If these mice are, in fact, experiencing increased pain sensitivity during low estrogen phases, the voltage of the shock used on “training day” may be too high and cause these animals to have stronger associative learning, hiding any NPS-mediated effects. Unfortunately, it seems that there is not enough research on pain sensitivity during the rodent estrous cycle. The studies that do exist have differing results, and pain sensitivity seems to be test-dependent in rodents (Vinogradova et al., 2001; Ibironke and Aji, 2011). However, to properly test this, foundational studies on the influence of the estrous cycle on this paradigm need to be completed. The results would then allow for the effects of NPS to be tested in relation to estrous cycle.
For the purposes of this discussion and data analysis, the animals were segregated into stages of estrous that are known to exhibit high or low levels of estrogen. However, this should not be interpreted as estrogen levels per se, are regulating the effects that are described in the present study. The estrous cycle is a multidimensional physiological phenomenon with many circulating factors fluctuating over the course of the cycle. In addition, due to organizational differences between the sexes, it should also be appreciated that low estrogen stages in a female, just as an ovariectomized adult female mouse, is not equivalent to a male mouse. Therefore, that low estrogen stages in female mice seem to be resistant to the anxiolytic-like effects of NPS should not be interpreted as being inconsistent with NPS-mediated anxiolytic-like effects being observed in males (also low estrogen). The interplay between the NPS system and the estrous cycle, in light of the current observations, should be thoroughly investigated. Paramount would be to determine whether the NPS administration or even the lack of the NPSR (NPSR-KO mice) alters aspects of the estrous cycle. In the present study, the estrous cycle was shown to influence NPS-mediated anxiolytic-like effects, but had no apparent effect in locomotion. This suggests that these NPS-mediated behavioral phenotypes are mechanistically distinct. The same conclusion has been put forth with the use of biased NPSR agonists that separate hyperlocomotor effects from the anxiolytic-like in male mice; RTI-263 (Clark et al., 2017). The mediation of these behaviors has even been suggested to be due to the activation of distinct signaling pathways (Clark et al., 2017). How the estrous cycle modulates these signaling pathways within specific NPSR expressing neurons will need to be investigated.
Conclusion
Our study found that the main NPS-mediated phenotypes are translatable across sexes and they may be influenced by the estrous cycle in female mice. We observed anxiolytic-like effects in marble burying (Fig. 5A) and ASR (Fig. 4D; Fig. 5E) only during Hi-E stages. In addition, NPS treatment produced effects in inhibitory avoidance only during Lo-E stages (Fig. 3E; Fig. 5G), but further research is warranted. Additional work on the long-lasting effects of NPS, especially how it relates to anxiety and stress, will need to be completed. Specifically, light–dark box and marble burying should be run on mice that have been previously treated with NPS but are run with “no treatment.” The potential long-lasting anxiolytic effects could elevate NPSR as a strong drug target. The NPS-mediated hyperlocomotive effects, however, appear to be independent of estrous cycle influence [low variability in locomotor assays (Fig. 2; Fig. 3I; Fig. 4) and increased entries in light–dark box (Fig. 5B)]. Overall, these results support the concept of the NPSR as being a target to treat anxiety disorders (Table 1). However, in light of 1) the apparent higher sensitivity to the hyperlocomotor effects in females, and 2) the potential that the repeated administration of NPS itself could influence the estrous cycle, further research into these interesting effects in females is needed.
TABLE 1.
NPS effects across the sexes in mice
The NPS-mediated effects seen previously after central administration in males, was largely recapitulated in female mice. Although hyperlocomotion effects were seen in female mice, both a low dose of NPS and a bias ligand known not to produce hyperlocomotion effects in males, produced robust hyperlocomotion in female mice. The anxiolysis effects, as measured in marble burying and ASR appear to be dependent on the stage of estrous when tested. In addition, there was a long-term effect of NPS in inhibitory avoidance. However, the effects appear to be complicated by other factors inherent to the paradigm (e.g., noxious stimuli) and will require more in-depth study of female subjects in this assay before more concrete conclusions of the effects of NPS can be made.
| Males | Females | |
|---|---|---|
| Hyperlocomotion | Yes | Hypersensitive |
| Anxiolysis | Yes | Yes; estrous-dependent |
| Memory consolidation | Yes | Possible estrous-dependent effects (requiring foundational studies) |
Acknowledgments
S.D.C. and S.R. were supported by a grant from the National Institutes of Health (R01 MH122196). P.C. was supported by The Honors College (UB) and CSTEP (NY State) and Summer Undergraduate Research Fellow (ASPET) through the CLIMB UP at the University at Buffalo. In addition, the authors would like to thank Dr. Ashley Russell for their insights and recommendations regarding the use of female subjects and study design. The authors thank the following people for technical contributions to this study: Herika Fernandez, Yuanli Huang, Gabriel Jewula, and Sarah LoCurto. The authors greatly appreciate the efforts and support of the staff and veterinarians of the University at Buffalo Laboratory Animal Facilities.
Data Availability
On publication, the data that support the findings of this study will be archived at Figshare (figshare.com). To negate the need for third party specialized software, our data will be presented as minimally processed Microsoft Excel spreadsheets. In this format, the data are accessible to perform independent statistical analysis. Data will be searchable by a simple author name search (e.g., “Stewart Clark”). In addition, publications will have the principal investigator’s Figshare user and ORCID (e.g., 0000-0001-8841-2728) numbers, which are linked to the data sets within Figshare. Each data set will be clearly linked to the publications using PMID. All data are contained within the manuscript data.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ASR
acoustic startle reflex
- AUC
area under the curve
- Hi-E
high estrogen stage
- KO
knockout
- Lo-E
low estrogen stage
- NPS
neuropeptide S
- NPSR
neuropeptide S receptor
Authorship Contributions
Participated in research design: Wood, Runyon, Clark.
Conducted experiments: Costa, Salinas, Wojciechowski, Clark.
Performed data analysis: Costa, Salinas, Wojciechowski, Clark.
Contributed new reagents or analytic tools: Runyon.
Wrote or contributed to the writing of the manuscript: Costa, Salinas, Wood, Clark.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Badia-Elder NE, Henderson AN, Bertholomey ML, Dodge NC, Stewart RB (2008) The effects of neuropeptide S on ethanol drinking and other related behaviors in alcohol-preferring and -nonpreferring rats. Alcohol Clin Exp Res 32:1380–1387. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I (2011) Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA (2012) Mouse estrous cycle identification tool and images. PLOS ONE 7:e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Kallupi M, Li HW, Stopponi S, Cifani C, Ciccocioppo R, Ubaldi M (2016) Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats. Psychopharmacology (Berl) 233:2915–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SD, Duangdao DM, Schulz S, Zhang L, Liu X, Xu Y-L, Reinscheid RK (2011) Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J Comp Neurol 519:1867–1893. [DOI] [PubMed] [Google Scholar]
- Clark SD, Kenakin TP, Gertz S, Hassler C, Gay EA, Langston TL, Reinscheid RK, Runyon SP (2017) Identification of the first biased NPS receptor agonist that retains anxiolytic and memory promoting effects with reduced levels of locomotor stimulation. Neuropharmacology 118:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MA, Prall BC, Smith ML, Calchary WA, Siegel PB (2008) Differential appetite-related responses to central neuropeptide S in lines of chickens divergently selected for low or high body weight. J Neuroendocrinol 20:904–908. [DOI] [PubMed] [Google Scholar]
- Dannlowski UKugel HFranke FStuhrmann AHohoff CZwanzger PLenzen TGrotegerd DSuslow TArolt V, et al. (2011) Neuropeptide-S (NPS) receptor genotype modulates basolateral amygdala responsiveness to aversive stimuli. Neuropsychopharmacology 36:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke KReif AWeber HRichter JHohoff COhrmann PPedersen ABauer JSuslow TKugel H, et al. (2011) Neuropeptide S receptor gene – converging evidence for a role in panic disorder. Mol Psychiatry 16:938–948. [DOI] [PubMed] [Google Scholar]
- Donner JHaapakoski REzer SMelén EPirkola SGratacòs MZucchelli MAnedda FJohansson LESöderhäll C, et al. (2010) Assessment of the neuropeptide S system in anxiety disorders. Biol Psychiatry 68:474–483. [DOI] [PubMed] [Google Scholar]
- Ekhart C, van Hunsel F, Scholl J, de Vries S, van Puijenbroek E (2018) Sex differences in reported adverse drug reactions of selective serotonin reuptake inhibitors. Drug Saf 41:677–683. [DOI] [PubMed] [Google Scholar]
- Epstein AN, Fitzsimons JT, Rolls BJ (1970) Drinking induced by injection of angiotensin into the rain of the rat. J Physiol 210:457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzbach-Schoon E, Andreatta M, Reif A, Ewald H, Tröger C, Baumann C, Deckert J, Mühlberger A, Pauli P (2013) Contextual fear conditioning in virtual reality is affected by 5HTTLPR and NPSR1 polymorphisms: effects on fear-potentiated startle. Front Behav Neurosci 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DJ, O’Connor GT, Wilk JB (2007a) Genome-wide association of sleep and circadian phenotypes. BMC Med Genet 8 (Suppl 1):S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YWojciechowski AFeldman KEttaro RVeros KRitter MCarvalho Costa PDiStasio JPeirick JJReissner KJ, et al. (2023) RTI-263, a biased neuropeptide S receptor agonist that retains an anxiolytic effect, attenuates cocaine-seeking behavior in rats. Neuropharmacology 241:109743. [DOI] [PubMed] [Google Scholar]
- Ibironke GF, Aji KE (2011) Pain threshold variations in female rats as a function of the estrus cycle, Niger. Niger J Physiol Sci 26:67–70. [PubMed] [Google Scholar]
- Inoue S (2022) Neural basis for estrous cycle-dependent control of female behaviors. Neurosci Res 176:1–8. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS (1994) Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 51:8–19. [DOI] [PubMed] [Google Scholar]
- Klauke B, Deckert J, Zwanzger P, Baumann C, Arolt V, Pauli P, Reif A, Domschke K (2014) Neuropeptide S receptor gene (NPSR) and life events: G x E effects on anxiety sensitivity and its subdimensions. World J Biol Psychiatry 15:17–25. [DOI] [PubMed] [Google Scholar]
- Kolodziejczyk MH, Fendt M (2020) Corticosterone treatment and incubation time after contextual fear conditioning synergistically induce fear memory generalization in neuropeptide S receptor-deficient mice. Front Neurosci 14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzmann JC, Khalil R, Kohler JC, Mayer D, Florido A, Nadal R, Andero R, Fendt M (2020) Neuropeptide-S-receptor deficiency affects sex-specific modulation of safety learning by pre-exposure to electric stimuli. Genes Brain Behav 19:e12621. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Chen FS, Pape H-C, Heinrichs M (2013) Neuropeptide S receptor gene is associated with cortisol responses to social stress in humans. Biol Psychol 93:304–307. [DOI] [PubMed] [Google Scholar]
- Laas K, Eensoo D, Paaver M, Lesch K-P, Reif A, Harro J (2015) Further evidence for the association of the NPSR1 gene A/T polymorphism (Asn107Ile) with impulsivity and hyperactivity. J Psychopharmacol 29:878–883. [DOI] [PubMed] [Google Scholar]
- Laas K, Reif A, Akkermann K, Kiive E, Domschke K, Lesch K-P, Veidebaum T, Harro J (2014a) Interaction of the neuropeptide S receptor gene Asn(1)(0)(7)Ile variant and environment: contribution to affective and anxiety disorders, and suicidal behaviour. Int J Neuropsychopharm 17:541–552. [DOI] [PubMed] [Google Scholar]
- Laas K, Reif A, Kiive E, Domschke K, Lesch K-P, Veidebaum T, Harro J (2014b) A functional NPSR1 gene variant and environment shape personality and impulsive action: a longitudinal study. J Psychopharmacol 28:227–236. [DOI] [PubMed] [Google Scholar]
- Lennertz LQuednow BBSchuhmacher APetrovsky NFrommann ISchulze-Rauschenbach SLandsberg MWSteinbrecher AHöfels SPukrop R, et al. (2012) The functional coding variant Asn107Ile of the neuropeptide S receptor gene (NPSR1) is associated with schizophrenia and modulates verbal memory and the acoustic startle response. Int J Neuropsychopharmacol 15:1205–1215. [DOI] [PubMed] [Google Scholar]
- Leonard SKDwyer JMSukoff Rizzo SJPlatt BLogue SFNeal SJMalberg JEBeyer CESchechter LERosenzweig-Lipson S, et al. (2008) Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 197:601–611. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE (2002) ‘Amygdala modulation of memory consolidation: interaction with other brain systems’. Neurobiol Learn Mem 78:539–552. [DOI] [PubMed] [Google Scholar]
- Niimi M (2006) Centrally administered neuropeptide S activates orexin-containing neurons in the hypothalamus and stimulates feeding in rats. Endocrine 30:75–79. [DOI] [PubMed] [Google Scholar]
- Nochaiwong S, Ruengorn C, Thavorn K, Hutton B, Awiphan R, Phosuya C, Ruanta Y, Wongpakaran N, Wongpakaran T (2021) Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: a systematic review and meta-analysis. Sci Rep 11:10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Garau C, Duangdao DM, Clark SD, Jüngling K, Pape H-C, Reinscheid RK (2011) Neuropeptide S enhances memory during the consolidation phase and interacts with noradrenergic systems in the brain. Neuropsychopharmacology 36:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Hashimoto K, Iyo M, Shimizu E, Dempfle A, Friedel S, Reinscheid RK (2007) Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry 31:1444–1448. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2004) The Mouse Brain in Stereotaxic Coordinates, Elsevier Academic Press, Amsterdam; Boston. [Google Scholar]
- Perna G, Brambilla F, Arancio C, Bellodi L (1995) Menstrual cycle-related sensitivity to 35% CO2 in panic patients. Biol Psychiatry 37:528–532. [DOI] [PubMed] [Google Scholar]
- Raczka KA, Gartmann N, Mechias M-L, Reif A, Büchel C, Deckert J, Kalisch R (2010) A neuropeptide S receptor variant associated with overinterpretation of fear reactions: a potential neurogenetic basis for catastrophizing. Mol Psychiatry 15:1067–1074. [DOI] [PubMed] [Google Scholar]
- Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, Rasoulpoor S, Khaledi-Paveh B (2020) Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health 16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge REMartin LJIsbester KASotocinal SGRosen STuttle AHWieskopf JSAcland ELDokova AKadoura B, et al. (2014) Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 11:629–632. [DOI] [PubMed] [Google Scholar]
- Spada J, Sander C, Burkhardt R, Häntzsch M, Mergl R, Scholz M, Hegerl U, Hensch T (2014) Genetic association of objective sleep phenotypes with a functional polymorphism in the neuropeptide S receptor gene. PLOS ONE 9:e98789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit FAkdeniz CHaddad LKumsta REntringer SFrank JYim ISZänkert SWitt SHKirsch P, et al. (2017) Sex-specific association between functional neuropeptide S receptor gene (NPSR1) variants and cortisol and central stress responses. Psychoneuroendocrinology 76:49–56. [DOI] [PubMed] [Google Scholar]
- Streit FHaddad LPaul TFrank JSchäfer ANikitopoulos JAkdeniz CLederbogen FTreutlein JWitt S, et al. (2014) A functional variant in the neuropeptide S receptor 1 gene moderates the influence of urban upbringing on stress processing in the amygdala. Stress 17:352–361. [DOI] [PubMed] [Google Scholar]
- Tupak SVReif APauli PDresler THerrmann MJDomschke KJochum CHaas EBaumann CWeber H, et al. (2013) Neuropeptide S receptor gene: fear-specific modulations of prefrontal activation. Neuroimage 66:353–360. [DOI] [PubMed] [Google Scholar]
- Vinogradova EP, Zhukov DA, Batuev AS (2001) [Effect of estrous cycle phase on pain threshold in female white rats]. Ross Fiziol Zh Im I M Sechenova 87:1244–1249. [PubMed] [Google Scholar]
- Wegener G, Finger BC, Elfving B, Keller K, Liebenberg N, Fischer CW, Singewald N, Slattery DA, Neumann ID, Mathé AA (2012) Neuropeptide S alters anxiety, but not depression-like behaviour in Flinders sensitive line rats: a genetic animal model of depression. Int J Neuropsychopharmacol 15:375–387. [DOI] [PubMed] [Google Scholar]
- Xing L, Shi G, Mostovoy Y, Gentry NW, Fan Z, McMahon TB, Kwok P-Y, Jones CR, Ptáček LJ, Fu Y-H (2019) Mutant neuropeptide S receptor reduces sleep duration with preserved memory consolidation. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YLReinscheid RKHuitron-Resendiz SClark SDWang ZWLin SHBrucher FAZeng JALy NKHenriksen SJ, et al. (2004) Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron 43:487–497. [DOI] [PubMed] [Google Scholar]
- Zhu H, Mingler MK, McBride ML, Murphy AJ, Valenzuela DM, Yancopoulos GD, Williams MT, Vorhees CV, Rothenberg ME (2010) Abnormal response to stress and impaired NPS-induced hyperlocomotion, anxiolytic effect and corticosterone increase in mice lacking NPSR1. Psychoneuroendocrinology 35:1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]