Abstract
Purpose
Resistance exercise training (RET) effectively increases skeletal muscle mass and strength in healthy postmenopausal women. However, its effects on these parameters in postmenopausal breast cancer survivors are controversial or limited. Therefore, the aim of this study was to compare the effects of a 12-week progressive whole-body RET program on skeletal muscle mass, strength, and physical performance in healthy postmenopausal women versus postmenopausal women who survived breast cancer.
Methods
Thirteen healthy postmenopausal women (HEA, 54 ± 3 years, BMI 26.6 ± 2.7 kg·m2, n = 13) and eleven postmenopausal breast cancer survivors (BCS, 52 ± 5 years, BMI 26.8 ± 2.1 kg·m2, n = 11) participated in the study. Before and after the RET program, evaluations were performed on quadriceps muscle thickness, one-repetition maximum strength (1RM) for various exercises, grip strength, and physical performance.
Results
Both groups showed significant improvements in quadriceps muscle thickness (time effect, P < 0.001); 1RM strength for leg extension, leg press, chest press, horizontal row, and elbow extension (time effect, all P < 0.001); as well as handgrip strength (time effect, P = 0.035) and physical performance (time effect, all P < 0.001) after the 12-week RET program. There were no significant differences between the groups in response to RET for any of the outcomes measured.
Conclusion
Twelve weeks of RET significantly increases skeletal muscle mass, strength, and physical performance in postmenopausal women. No differences were observed between healthy postmenopausal women and postmenopausal breast cancer survivors. These findings point out that this study’s RET promotes skeletal muscle mass, strength, and performance gains regardless of breast cancer.
Pre-Print Platform Research Square: https://doi.org/10.21203/rs.3.rs-4145715/v1; https://www.researchsquare.com/article/rs-4145715/v1
Clinical trial registration: NCT05690295.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-024-08973-7.
Keywords: Cancer, Resistance training, Rehabilitation, Exercise, Atrophy, Muscle mass
Introduction
Breast cancer ranks first in global cancer incidence, with approximately 2.3 million new cases every year as of 2022. Among women, breast cancer constitutes 16% of cancer deaths [1]. The most common age for breast cancer diagnosis is between 51 and 70 years, typically post-menopause [2].
In postmenopausal women, around 80% of breast cancer cases exhibit positive estrogen receptors, frequently resulting in the prescription of endocrine therapy (such as tamoxifen, a selective estrogen-receptor modulator or aromatase inhibitors, which block the production of estrogens) as adjuvant treatment following the primary surgical intervention for localized disease [3]. These medications effectively reduce estrogen exposure in the breast tissue, thus preventing the breast tumor from receiving growth stimuli [4]. Consequently, the probability of cancer recurrence is significantly reduced [4] when these therapies are administered for 5 years or longer [5, 6].
Despite the effectiveness of endocrine therapy, the treatment side effects are common and can include increased adipose tissue, skeletal muscle mass loss, and muscle weakness [7, 8]. The loss of skeletal muscle mass, strength, and physical performance is known as sarcopenia. It is commonly associated with aging and is partially attributed to the decline of estrogen in older women [9]. The use of estrogen-effect suppressors such as tamoxifen and aromatase inhibitors hastens the depletion of skeletal muscle during menopause, diminishes mobility, and impacts the overall quality of life for breast cancer survivors undergoing endocrine therapy [10].
Additionally, diseases such as cancer and aging-related factors promote skeletal muscle atrophy, with growth differentiation factor 15 (GDF-15) identified as a potential biomarker for muscle disease [11]. Tumor-derived exosomes containing GDF-15 promote cancer-induced skeletal muscle atrophy through the Bcl-2/caspase-3 pathway [12, 13]. Moreover, serum GDF-15 has been associated with poor physical function in pre-frail older adults with diabetes [14].
To effectively counteract these adverse effects, supervised resistance exercise training (RET) has been shown to improve muscle strength and reduce plasma levels of lipids, inflammatory cytokines, and oxidative stress markers in breast cancer survivors treated with endocrine therapy such as tamoxifen [15]. Although the effectiveness of this type of training has been explored previously in women with breast cancer in terms of skeletal muscle mass, little information is available on the response magnitude in postmenopausal breast cancer survivors compared with postmenopausal healthy women [16].
Recent systematic reviews analyzing data from cancer survivors of advanced age and patients diagnosed with cancer during adjuvant treatment have shown that RET improves muscle strength but does not induce a substantial increase in skeletal muscle mass [17, 18]. These results differ from meta-analyses conducted in healthy postmenopausal women, where it has been demonstrated that this type of training, with or without nutritional supplementation, improves skeletal muscle mass, strength, and physical performance measures in women aged 45 to 80 years [19].
Based on these backgrounds, this study aimed to compare the effects of a 12-week progressive RET on skeletal muscle mass, muscle strength, and physical performance of healthy postmenopausal women versus postmenopausal women who are breast cancer survivors. The correlation of GDF-15 with markers of sarcopenia was also investigated. We hypothesize that the effects of RET on skeletal muscle mass are lower in postmenopausal breast cancer survivors compared to healthy postmenopausal women.
Methods
Participants
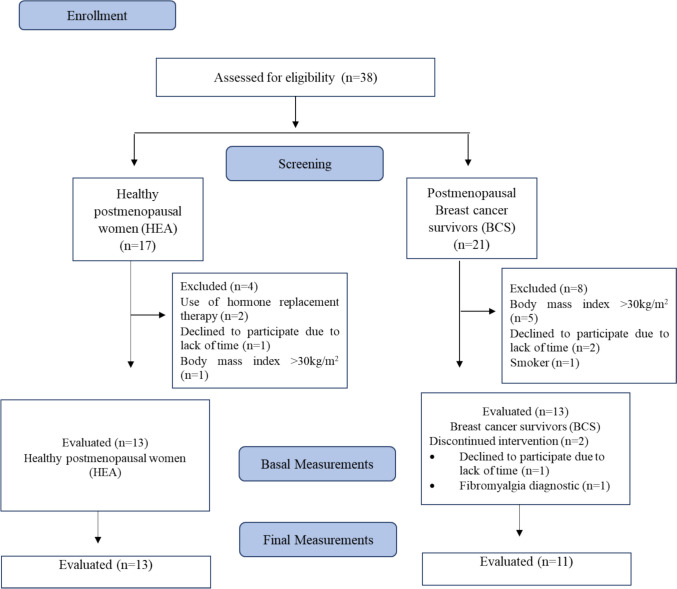
Thirteen healthy postmenopausal women (HEA, 54 ± 3 years, BMI 26.6 ± 2.7 kg·m2, n = 13) and eleven postmenopausal women who are breast cancer survivors (BCS, 52 ± 5 years, BMI 26.8 ± 2.1 kg·m2, n = 11) completed the study (Fig. 1). The study was approved by the scientific ethics committee of Universidad de La Frontera, Temuco, Chile (registration record N°004_23) according to the Declaration of Helsinki and was registered on clinicaltrials.gov as NCT05690295. A signed informed consent was obtained from each participant. One week before the study, the participants completed a routine medical screening and general health questionnaire to ensure their suitability for the study. Inclusion criteria were postmenopausal women between 45 and 59 years of age, healthy and breast cancer survivors who completed primary treatment ≥ 6 months ago with or without endocrine therapy, BMI between 18.5 and 30 kg·m2, and willingness to participate in the study and follow the proposed intervention scheme. The exclusion criteria were performing regular RET in the previous 6 months, cardiovascular diseases incompatible with physical activity, all comorbidities affecting the mobility of the body and muscle metabolism and that do not allow to safely perform the RET program, smoking, use of nutritional supplementation (leucine, glutamine, casein, whey-protein, fatty acids, and creatine), and use of estrogen replacement therapy.
Fig. 1.
Flow diagram of study participants
Study design
All volunteers performed 12 weeks of supervised whole-body RET (three times per week). Before and after 12 weeks of RET, muscle ultrasonography (US) was performed to assess quadriceps muscle thickness as our primary outcome. In addition, fasting blood samples were obtained to determine biochemical and inflammatory markers, and a whole-body bioelectric impedance analysis was performed to determine lean and fat mass. Maximal strength was determined by one-repetition maximum (1RM); functional capacity was assessed through the 6-min walk test and physical performance by the timed up and go (TUG) test and short physical performance battery (SPPB) at the same time points.
Exercise intervention program
Participants in both groups underwent an identical, supervised, progressive whole-body RET program three times a week for 12 weeks, as described previously [20]. During the intervention, 1RM was reassessed to adjust workloads (60–80%) in the sixth week of training. Compliance for the protocol analyses was set at completing at least 80% of the training sessions (i.e., at least 29 of the 36 sessions).
The training regimen began with a 5-min warm-up on a bicycle ergometer followed by global upper limb movements. A series of warm-up exercises were performed followed by four regular sets on the leg press and leg extension machines (TuffStuff Fitness International, California, USA). The upper body exercises comprised three sets for each exercise, including chest press, triceps extension, and horizontal row machines (Fit Tech, Portugal). The cool-down consisted of 5 min of recovery using global muscle stretching exercises.
Dietary intake and physical activity standardization
Participants were instructed to maintain their usual dietary habits and levels of physical activity throughout the exercise program. Before and in week 11 of the RET, participants completed 3-day dietary intake and physical activity records, including two weekdays and one weekend day [20, 21]. These records were reviewed by a blinded nutrition expert and analyzed using FatSecret® software (version 2023, Melbourne, Australia), which has been validated in previous studies for its reliability in estimating macronutrient and caloric intake [22]. Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ), widely validated in the Chilean population [23]. Both methods ensured consistent and reliable assessments of dietary intake and physical activity levels during the study. No other dietary control was implemented.
Skeletal muscle mass
All measurements of the thickness were assessed based on the protocol described by Galvão et al. (2006) [24]. The assessment was conducted by an evaluator experienced in measuring muscle thickness using clinical ultrasonography, who was blinded to participant coding. The muscle thickness assessment was performed before and after 12 weeks of the RET program. Ultrasonography equipment (LOGIQTM F8, GE Healthcare, USA) was used with a convex transducer of 5 MHz with water-soluble gel placed on the skin, perpendicular to the tissue interface. In assessing muscle thickness of the rectus femoris, vastus intermedius, and total quadriceps, reference points were established at the anterosuperior iliac spine and the superior border of the patella. For the evaluation of brachial biceps and brachial triceps muscle thickness, the acromion and olecranon were designated as reference points. Consistency in measurements for both upper and lower limbs was maintained by locating the midpoint between these reference points. Participants stayed in a lying position with the head and ankles in a neutral position and the upper and lower limbs fully extended. The measurements were made using the muscle mode preset specific to the ultrasound. The transducer was placed with minimal pressure over the gel so the muscle thickness would not be reduced due to compression. The image was frozen at the reference point, and the measurements were recorded in cm using the same equipment.
Body composition
Whole-body and regional lean mass and whole-body fat mass were determined by bioelectrical impedance analysis (HBF-514C, Omron©, Japan), with participants in an overnight fast and standing position, ensuring they had not engaged in intense physical activity within the previous 48 h. In addition, weight, height, and waist circumference were determined. Waist circumference was measured on exhalation at the midpoint between the lowest rib and the iliac crest on the right half of the body with a SECA® retractable metric measuring tape with a graduation of centimeters (Madison, WI, USA).
Muscle strength
Maximum strength was measured through 1RM strength tests, where repetition was deemed valid only if the entire lift was executed with control and without assistance. The initial estimation through a familiarization trial was conducted to establish maximum strength; subsequently, in a separate session, 1RM strength was determined for both lower (leg press and leg extension) and upper (chest press, elbow extension, and horizontal row) body exercises using the same equipment employed in the training sessions. Additionally, maximal handgrip strength was assessed using a Jamar electronic handheld dynamometer (model Plus+, Patterson Medical) as previously reported [20].
Physical performance measures and functional capacity
Physical performance was measured by performing the timed up and go test (TUG) and short physical performance battery (SPPB); specifically, the 4-m walk time, walk speed, and chair stand test were considered [25]. Functional capacity was assessed through the 6-min walk test, with the maximum distance covered in 6 min [26].
Plasma measurements
Blood samples were collected from a superficial vein in the cubital fossa after a 12-h fasting period in the morning. The samples were collected 48 h before the initial RET session and 48 h after completing the final RET session. Samples were drawn into tubes without anticoagulant. After centrifugation at 2500 rpm for 15 min, the resulting serum was aliquoted into microtubes and stored at − 80 °C for subsequent analysis. The lipid and glucose profiles were determined using enzymatic-colorimetric methods with an automatic photometer (Metrolab 2300 plus, Wiener lab, Argentina). Insulin and GDF-15 were assessed via ELISA kits (#KAQ1251 and #BMS2258, respectively, Thermo Fisher Scientific Inc., Waltham, MA, USA) by the manufacturer’s guidelines. Insulin sensitivity was evaluated through the HOMA-IS (homeostasis model assessment-insulin sensitivity) calculation, utilizing the formula published by Acosta et al. [27].
Statistics
The results underwent analysis using the statistical software SPSS (IBM SPSS Statistics, v. 21, NY, USA), while the figures were generated utilizing GraphPad Prism 8.2 software (GraphPad Software, San Diego, CA, USA). Data is presented as mean ± standard deviation (SD) and percentage change (from baseline to post-training) to facilitate comparing absolute and relative improvements between the groups. Baseline characteristics between groups were compared using an independent sample t-test. Pre- vs. post-intervention data were analyzed using a repeated-measures analysis of variance (ANOVA) with time (PRE vs. POST) as the within-subject factor and group (HEA vs. BCS) as the between-subject factor. In the case of a significant interaction, separate analyses were performed to determine time effects within groups and independent t-tests for group differences in the PRE and POST values. For the main parameters, partial eta squared was used to estimate effect sizes and represented as η2. Statistical significance was established as P < 0.05.
Results
Participants
Participants’ characteristics are shown in Table 1. Two breast cancer survivor participants withdrew from the study (Fig. 1).
Table 1.
Participant baseline characteristics
| HEA (n = 13) | BCS (n = 11) | P-value | |
|---|---|---|---|
| Age (y) | 54 ± 3 | 52 ± 5 | 0.155 |
| Weight (kg) | 63.8 ± 9.2 | 67.1 ± 7.2 | 0.175 |
| Height (m) | 151 ± 14.4 | 158 ± 7.2 | 0.068 |
| BMI (kg.m2) | 26.6 ± 2.7 | 26.8 ± 2.1 | 0.539 |
| HR (bpm) | 71.2 ± 11.3 | 73.3 ± 6.3 | 0.284 |
| SBP (mm Hg) | 115.5 ± 11.8 | 114.2 ± 9.8 | 0.389 |
| DBP (mm Hg) | 79.8 ± 10.7 | 78.5 ± 7.1 | 0.365 |
| Type of cancer, n (%) | |||
| Invasive carcinoma | 9 (82%) | ||
| Carcinoma in situ | 2 (18%) | ||
| Type of surgery | |||
| Mastectomy | 5 (45%) | ||
| Quadrantectomy | 6 (55%) | ||
| Lymph nodes removed | |||
| Complete axillary dissection | 1(10%) | ||
| Sentinel lymph node biopsy | 10 (90%) | ||
| Cancer stage | |||
| I | 2 (18%) | ||
| II | 5 (46%) | ||
| III | 4 (36%) | ||
| General treatment received. | |||
| Chemotherapy | 6 (55%) | ||
| Radiotherapy | 11 (100%) | ||
| Endocrine Therapy | |||
| Tamoxifen | 3 (27%) | ||
| Aromatase Inhibitor | 3 (27%) | ||
| Without therapy | 5 (46%) | ||
| Time since breast cancer surgery (y) | 4 ± 4 | ||
Data presented as mean ± SD and as percentages. Data were analyzed using independent samples t-tests
HEA healthy group, BCS breast cancer survivors’ group, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate, bpm beats per minute
Skeletal muscle mass
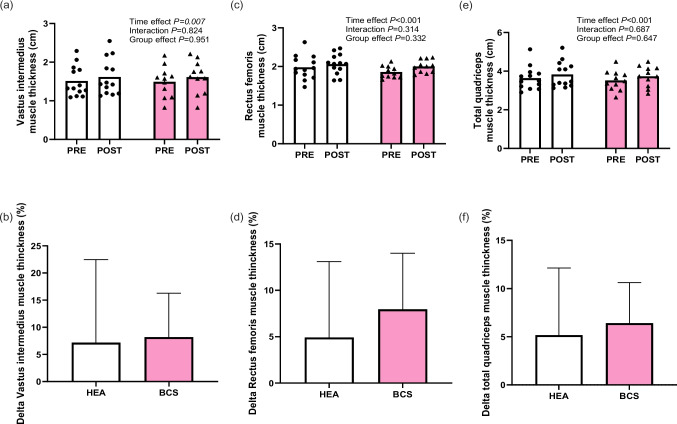
At baseline, upper and lower limb muscle thickness was not different between HEA and BCS participants (P ≥ 0.113). The results depicting muscle thickness of the vastus intermedius, rectus femoris, and quadriceps before and after 12 weeks RET are illustrated in Fig. 2. Whole-body RET increased muscle thickness of dominant lower limbs after 12 weeks (Fig. 2a, c, e), from 1.5 ± 0.4 to 1.6 ± 0.5 cm (7 ± 15%) in vastus intermedius, from 1.9 ± 0.3 to 2.1 ± 0.3 cm (5 ± 8%) in rectus femoris, and from 3.6 ± 0.6 to 3.9 ± 0.7 cm (5 ± 7%) in total quadriceps after training in HEA participants and from 1.5 ± 0.4 to 1.6 ± 0.4 cm (8 ± 8%) in vastus intermedius, from 1.9 ± 0.2 to 2.0 ± 0.2 cm (8 ± 6%) in rectus femoris, and from 3.5 ± 0.5 to 3.7 ± 0.5 cm (7 ± 4%) in total quadriceps after training in BCS (time effect, all P ≤ 0.007; η2 ≥ 0.29) with no differences between groups (time × group, all P ≥ 0.314; η2 ≤ 0.01; Fig. 2b, d, f). Brachial biceps and brachial triceps muscle thicknesses did not differ between groups, and no changes were observed throughout the 12-week intervention period in either group (supplementary Table S1).
Fig. 2.
Thickness of (a) vastus intermedius, (c) rectus femoris, and e total quadriceps muscle of the dominant leg before and after 12 weeks of resistance exercise training. Percentage change in (b) vastus intermedius, (d) rectus femoris, and (f) total quadriceps muscle thicknesses following 12 weeks of training in HEA (healthy group) (n = 13) and BCS (breast cancer survivors group) (n = 11) groups. Data were analyzed using repeated-measures ANOVA (time × group) (a, c, e) and independent t-test (b, d, f), not observing interaction or differences between the groups, respectively
Body composition
Table 2 shows the baseline and post-12-week RET results for body composition. After the intervention, no significant differences over time between groups were observed in terms of body weight, BMI, waist circumference, visceral fat, body fat, and lean body mass percentages.
Table 2.
Anthropometry and body composition parameters before and after 12 weeks of resistance exercise training
| HEA (n = 13) | BCS (n = 11) | Statistics (P-value) | |||||
|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | Time | Time × group | Group | |
| Weight (kg) | 63.9 ± 9.2 | 63.9 ± 8.9 | 67.1 ± 7.2 | 67.6 ± 8.1 | 0.420 | 0.392 | 0.318 |
| BMI (kg/m2) | 26.6 ± 2.7 | 26.6 ± 2.7 | 26.8 ± 2.1 | 26.9 ± 2.0 | 0.392 | 0.594 | 0.779 |
| Waist circumference (cm) | 86.5 ± 9.2 | 86.2 ± 10.4 | 91.9 ± 7.7 | 85.6 ± 8.89 | 0.285 | 0.159 | 0.363 |
| Body fat mass (%) | 40.9 ± 4.1 | 41.0 ± 4.5 | 41.8 ± 2.7 | 40.9 ± 3.4 | 0.172 | 0.073 | 0.828 |
| Lean body mass (%) | 24.1 ± 1.5 | 25.3 ± 4.8 | 23.9 ± 1.3 | 24.7 ± 1.8 | 0.197 | 0.773 | 0.712 |
| Basal metabolic rate (Kcal) | 1301.6 ± 107.3 | 1303.5 ± 103.9 | 1349.5 ± 97.2 | 1356.5 ± 107.9 | 0.145 | 0.400 | 0.250 |
| Visceral fat (kg) | 8.2 ± 1.5 | 8.5 ± 1.4 | 8.3 ± 1.2 | 8.5 ± 0.9 | 0.096 | 0.659 | 0.968 |
Data presented as mean ± SD. Data were analyzed using repeated-measures ANOVA (time × group)
HEA healthy group, BCS breast cancer survivors group, BMI body mass index
Habitual dietary intake and physical activity
The data on dietary intake and physical activity are presented in Supplementary Tables S2 and S3, respectively. No significant differences were observed in the macronutrient composition of the diet. Protein intake averaged 0.8 ± 0.2 and 0.7 ± 0.2 g/kg BW·day−1 in HEA and BCS participants, respectively. No differences were reported between groups, and no changes were found over time for any dietary intake parameters. Similarly, no significant changes were observed after 12 weeks of RET in the moderate physical activity level (time effect P = 0.979) and sedentary behavior (time effect P = 0.271). However, vigorous physical activity levels increase after 12 weeks of RET (time effect P = 0.009).
Strength
At baseline, 1RM of the upper and lower limbs did not significantly differ between the groups (Table 3). After 12 weeks of whole-body RET, 1RM leg extension increased from 57 ± 10 to 80 ± 15 kg (41 ± 12%) in HEA and from 54 ± 13 to 77 ± 18 kg (43 ± 6%) in BCS (time effect, all P < 0.001; η2 = 0.88). Similar improvements were observed for 1RM leg press, elbow extension, chest press, horizontal row (time effect, all P < 0.001; η2 ≥ 0.85), and handgrip strength (time effect, P = 0.035; η2 = 0.19) in the HEA and BCS groups (Table 3). For all strength outcomes, no differences in RET response were observed between groups for both the absolute and the relative (i.e., percentage) improvements (time × group, all P ≥ 0.185; all η2 ≤ 0.09; Table 3).
Table 3.
Strength parameters before and after 12 weeks of resistance exercise training
| HEA (n = 13) | BCS (n = 11) | Statistics (P-value) | |||||
|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | Time | Time × group | Group | |
| 1RM chest press (kg) | 51.2 ± 8.7 | 62.7 ± 9.0 | 46.5 ± 12.0 | 59.0 ± 11.3 | < 0.001 | 0.668 | 0.323 |
| 1RM horizontal row (kg) | 38.1 ± 5.2 | 50.4 ± 6.6 | 39.5 ± 6.9 | 50.7 ± 7.6 | < 0.001 | 0.315 | 0.325 |
| 1RM elbow extension (Kg) | 28.5 ± 3.8 | 36.5 ± 5.8 | 27.0 ± 4.2 | 35.8 ± 5.8 | < 0.001 | 0.185 | 0.855 |
| 1RM leg extension (kg) | 57.1 ± 10.2 | 80.3 ± 15.0 | 53.7 ± 13.0 | 76.8 ± 18.1 | < 0.001 | 0.993 | 0.551 |
| 1RM leg press (kg) | 85.3 ± 27.8 | 123.1 ± 34.4 | 70.7 ± 21.4 | 102.5 ± 20.4 | < 0.001 | 0.314 | 0.106 |
| Dominant handgrip strength (kg) | 27.1 ± 3.2 | 28.5 ± 4.7 | 26.6 ± 5.5 | 27.8 ± 5.9 | 0.035 | 0.878 | 0.774 |
Data presented as mean ± SD. Data were analyzed using repeated-measures ANOVA (time × group)
HEA healthy group, BCS breast cancer survivors group, 1RM 1-repetition maximum
*Bold numbers at the P < 0.05 level
Physical performance and physical capacity
At baseline, physical performance (TUG, 4-m walk time, walk speed, and chair stand test) and physical capacity (6-min walk test) did not show a significant difference between groups (Table 4). Twelve weeks of RET promoted a 10 ± 11% (from 6.6 ± 10.7 to 5.9 ± 0.7 s) and 9 ± 16% (from 6.5 ± 0.7 to 5.9 ± 0.7 s) improvement in TUG in HEA and BCS, respectively (time effect, P < 0.001; η2 = 0.45), with no differences between groups (time × group, P = 0.891). In accordance, performance on the 4-m walk time, walk speed, and chair stand tests was improved by RET in both HEA and BCS participants (time effect, all P ≤ 0.040; η2 ≥ 0.19), with no differences between groups (time × group, all P ≥ 0.471). Moreover, the 12-week RET intervention program effectively increased the distance covered in the 6-min walk test in both groups (time effect, P = 0.001, HEA 10% vs. BCS 6%), also with no significant difference between groups (time × group, P = 0.277).
Table 4.
Functional capacity and physical performance before and after 12 weeks of resistance exercise training
| HEA (n = 13) | BCS (n = 11) | Statistics (P-value) | |||||
|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | Time | Time × group | Group | |
| 6MWT (m) | 588.8 ± 28 | 646.5 ± 35 | 566.6 ± 62 | 603.9 ± 35 | 0.001 | 0.277 | 0.054 |
| TUG (s) | 6.6 ± 0.7 | 5.9 ± 0.7 | 6.5 ± 0.7 | 5.9 ± 0.7 | < 0.001 | 0.891 | 0.905 |
| 4 m walk time (s) | 2.8 ± 0.5 | 2.6 ± 0.5 | 2.9 ± 0.5 | 2.5 ± 0.3 | 0.029 | 0.451 | 0.968 |
| Walk speed (m/s) | 1.5 ± 0.3 | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.6 ± 0.2 | 0.040 | 0.471 | 0.903 |
| Chair stand test (s) | 8.8 ± 1.3 | 7.6 ± 1.5 | 9.4 ± 1.2 | 8.0 ± 1.1 | 0.002 | 0.741 | 0.214 |
Data presented as mean ± SD. Data were analyzed using repeated-measures ANOVA (time × group)
6MWT 6-min walk test, HEA healthy group, BCS breast cancer survivors group, TUG timed up and go
*Bold numbers at the P < 0.05 level
Plasma measurements
No changes over time were observed in both groups’ cholesterol, triglycerides, HDL, and LDL parameters, including fasting plasma glucose, insulin, and homeostasis model assessment (HOMA) (Supplementary Table S4).
Correlation between GDF-15 and sarcopenia markers
At baseline, no significant differences in GDF-15 levels were observed between groups (Supplementary Table S4). After 12 weeks of RET, no significant change in GDF-15 levels was evident in both HEA and BCS groups (time effect, P = 0.568; η2 = 0.02). GDF-15 PRE training was negatively correlated with 1RM leg extension when considering all participants (R = − 0.439, P = 0.046) or the HEA group specifically (R = − 0.652, P = 0.030). Also, GDF-15 POST training was correlated negatively with walk speed when including all participants in the analysis (R = − 0.419, P = 0.05) or the HEA group alone (R = − 0.640, P = 0.034) (Supplementary Tables S5 and S6).
Discussion
In the present study, 12 weeks of progressive whole-body RET was shown to effectively increase muscle thickness in the lower limb muscles and muscle strength in the upper and lower body and improve physical performance (TUG and SPPB) and physical capacity (6-min walk test) in both HEA and BCS groups. Importantly, no differences in the beneficial effects of prolonged RET were observed between the HEA and BCS.
The decrease in estrogen production during menopause and the use of endocrine therapy in female BCS is associated with skeletal muscle mass and strength loss, leading to deterioration in physical performance. RET can effectively increase skeletal muscle mass, strength, and physical performance in the healthy postmenopausal population and in breast cancer survivors [28]. We observed an approximate increase of 5 ± 7% and 7 ± 4% in quadriceps muscle thickness in HEA and BCS participants, respectively.
Our results stand out in terms of skeletal muscle mass increase compared to a previous study published, which included women diagnosed with metastatic breast cancer who underwent 12 weeks of concurrent training. The study conducted by Escriche-Escuder et al. (2021) reports a significant 12% reduction in quadriceps muscle thickness, which could be explained by the more advanced progression of the neoplastic disease in the recruited participants and the training protocol that incorporated both aerobic and strength exercises at a lower intensity by recommendations for patients with bone lesions due to metastasis [29].
Additionally, no differences were found in the responses to RET when the data were stratified to specifically compare patients who received endocrine therapy (tamoxifen or aromatase inhibitors) (n = 6) with those who did not receive such therapy (n = 5).
Scientific evidence has reported difficulties and limitations in increasing skeletal muscle mass through exercise interventions in the oncological population [30–32]. This refers to the heterogeneity of the RET protocols applied, as indicated by the systematic review conducted by Fairman et al. [33]. Therefore, it is crucial to apply a minimal necessary exercise dose to improve skeletal muscle mass. Discrepancies in muscle hypertrophy outcomes have also been described in postmenopausal women who are BCS. Our study hypothesized a lower gain in skeletal muscle mass compared to healthy postmenopausal women.
Lower estrogen concentrations due to menopause and the use of adjuvant endocrine therapy to prevent breast cancer recurrence are currently reported to be associated with a reduction in skeletal muscle mass and strength [30]. Previous studies have shown that estrogen protects skeletal muscle by attenuating muscle damage and inflammation, stimulating muscle repair and regenerative processes, and maintaining satellite cell function [31, 32]. These findings highlight the importance of estrogen for skeletal muscle maintenance in women and suggest that prescribing tamoxifen or aromatase inhibitors could make it even more challenging to preserve muscle health in postmenopausal women [34, 35].
We found no significant differences between the groups after 12 weeks of RET. Schoenfeld et al. (2017) identified that training parameters such as intensity, volume, and frequency of RET are crucial for hypertrophic and strength adaptations in the general population [36, 37]. Therefore, the progressive training regimen with high-intensity resistance exercises applied in the present study effectively improved the skeletal muscle mass of postmenopausal BCS. Our RET protocol and results were comparable to those achieved by Galvão et al. (2006), who showed a 15% increase in quadriceps muscle thickness in men diagnosed with prostate cancer under androgen deprivation treatment after a more extended period of RET compared to our study (20 weeks vs. 12 weeks) [24].
Regarding muscle strength, other studies evaluating the effects of a 24-week and a 16-week RET training protocol in postmenopausal breast cancer survivors showed an average increase of 25% and 26% in 1RM of chest press and an increase of 26% and 30% in 1RM of leg extension [38, 39]. It is important to note that these improvements are smaller than those obtained in our study, which, despite a training period of only 12 weeks, but at high intensity, demonstrated a 29% and 43% 1RM increase in the exercises, respectively. These discrepancies could be attributed to a lower number of weekly sessions and the potentially more challenging initial conditions of the participants in the study by Simonavice et al. (2014), as well as a lower volume in the total exercise load in the study by Serra et al. (2018). On the other hand, Serra et al. (2018) also studied physical performance and functional capacity, reporting significant improvements in the chair stand time test and the 6-min walk test without changes in walking speed. As mentioned above, this research team implemented a lower exercise volume and intensity, which could explain the discrepancies in their results compared to our study [39].
Considering that muscle strength serves as a substantial predictor of mortality among BCS [40], the findings from this study hold crucial clinical significance. An improvement in the plasmatic lipid profile of female BCS under treatment with tamoxifen who undergo RET has already been described and is attributed to adaptations in fat oxidation capacity[15]. de Jesus Leite et al. (2021) demonstrated that 12 weeks of RET promoted a significant reduction in triglycerides (15%), total cholesterol (6.8%), and LDL cholesterol (9.7%) levels in addition to an increase in HDL cholesterol (17%) in the described population. These results differ from those obtained by our study, where we observed a slight decrease in lipid profile parameters without statistically significant differences over time and between groups (HEA vs. BCS) [15]. The scientific literature has documented the beneficial hypocholesterolemic and hypotriglyceridemic effects produced by the oral use of tamoxifen, along with decreased mortality related to coronary heart disease observed in patients receiving treatment with this endocrine therapy [15, 41, 42]. On the contrary, the effects of the prescription of aromatase inhibitors on the plasma profile are not apparent yet. However, most studies have shown that the use of this medication unfavorably alters the lipid profile [43, 44]. The reasons above would explain the lower effects on biochemical markers observed in our study because the sample was heterogeneous and included BCS with and without endocrine therapy (tamoxifen or aromatase inhibitors). Therefore, future studies could investigate these differences in detail by comparing each type of endocrine therapy as separate study groups. Finally, a higher concentration of plasma GDF-15 was negatively correlated with muscle strength and walking speed, mainly in healthy postmenopausal women. A decrease in these physical parameters is related to sarcopenia, as is the loss of muscle mass associated with higher concentrations of GDF-15 in other studies [16]. These findings support the potential of GDF-15 as a possible biomarker for changes associated with age-related sarcopenia.
Among the limitations of this study, it is important to note that the findings cannot be extrapolated to premenopausal women diagnosed with breast cancer or those undergoing active chemotherapy and radiotherapy treatment. This study included an optimal number of participants per group, as determined by post hoc statistical power analysis, which demonstrated a 95% power to detect the pre-established difference in total quadriceps muscle thickness between groups. However, a larger sample size of postmenopausal breast cancer survivors is needed to examine results based on the presence or absence of endocrine therapy and to differentiate between specific types of therapy, such as tamoxifen or aromatase inhibitors. Consequently, this study focuses exclusively on comparing the effects of RET between healthy postmenopausal women and postmenopausal women with BCS.
Despite these limitations, our results clearly indicate that RET is beneficial in mitigating the adverse effects of menopause and breast cancer on skeletal muscle mass, strength, physical performance, and overall physical capacity. The strengths of this study include high participant compliance and adherence to the exercise program, stable diet, and moderate physical activity levels, with expected increases in vigorous activity due to the high-intensity RET program. We strongly recommend the implementation of personalized and supervised RET for both healthy postmenopausal women and postmenopausal breast cancer survivors.
In conclusion, this study indicates that 12 weeks of RET increases skeletal muscle mass, strength, and physical performance in postmenopausal women. No differences were observed between healthy postmenopausal women and postmenopausal BCS, suggesting similar benefits for both groups.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We greatly appreciate the assistance of the following colleagues in the execution of the experiments: Mariel Sánchez, Valentina Barra, and Ignacio Vilar-Bertolotto (all part of the physiotherapy graduation program, Universidad de La Frontera) and the technical expertise from Ignacio Obreque in obtaining and transporting blood samples.
Author contribution
M.A-A and G.N.M-N: Study design, M.A-A, A.A-M, N.V-S, R.M-C, J.C-L, A.S-L, N.H, J.S, L.A-S: Experiments’ organization and performance, M.A-A, K.F-V, R.C, G.N.M-N: Data analysis, M.A-A, K.F-V, R.C, G.N.M-N: Manuscript drafting, M.A-A and G.N.M-N: Editing and All author: revision of manuscript and Approved of final version.
Funding
This research was carried out using financial support from the Dirección de Investigación (DIUFRO) of Universidad de La Frontera, Chile (N°DFP22-0020 and FPP22-0016) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Brazil, N°2022/09341-4), and Universidade Cruzeiro do Sul. M.A-A. and N.V-S. were funded by the National Research and Development Agency (ANID)/Human Capital Sub-directorate/National Doctorate Scholarships 2021–21211236 and 2022–21220848, respectively. R. C. is the recipient of a CNPq scholarship.
Data availability
Some data is provided within the manuscript or supplementary information files. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The protocol and informed consent were approved (registration record N°004_23) by the scientific ethics committee of Universidad de La Frontera, Temuco, Chile.
Consent for publication
A signed informed consent was obtained from each participant. The informed consent is available upon request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kataki AC, Tiwari P, Thilagavthi R, Krishnatreya M (2022) Epidemiology of gynaecological cancers. Fundamentals in gynaecologic malignancy, Springer Nature Singapore, Singapore 1–8. 10.1007/978-981-19-5860-1_1
- 2.Oprean CM, Negru SM, Popovici DI, Saftescu S, Han R-A, Dragomir G-M et al (2020) Postmenopausal breast cancer in women, clinical and epidemiological factors related to the molecular subtype: a retrospective cohort study in a single institution for 13 years. Follow-Up Data. Int J Environ Res Publ Health 17:8722. 10.3390/ijerph17238722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao H, Lei X, Niu J, Zhang N, Duan Z, Chavez-MacGregor M et al (2021) Prescription patterns, initiation, and 5-year adherence to adjuvant hormonal therapy among commercially insured patients with breast cancer. JCO Oncol Pract 17:e794-808. 10.1200/OP.20.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucciniello L, Garufi G, Di Rienzo R, Martinelli C, Pavone G, Giuliano M et al (2023) Estrogen deprivation effects of endocrine therapy in breast cancer patients: incidence, management and outcome. Cancer Treat Rev 120:102624. 10.1016/j.ctrv.2023.102624 [DOI] [PubMed] [Google Scholar]
- 5.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE et al (2010) American Society of Clinical Oncology Clinical Practice Guideline: update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer. J Clin Oncol 28:3784–3796. 10.1200/JCO.2009.26.3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet 365:1687–1717. 10.1016/S0140-6736(05)66544-0 [DOI] [PubMed] [Google Scholar]
- 7.Hyder T, Marino CC, Ahmad S, Nasrazadani A, Brufsky AM (2021) Aromatase inhibitor-associated musculoskeletal syndrome: understanding mechanisms and management. Front Endocrinol 12:713700. 10.3389/fendo.2021.713700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright LE, Harhash AA, Kozlow WM, Waning DL, Regan JN, She Y et al (2017) Aromatase inhibitor-induced bone loss increases the progression of estrogen receptor-negative breast cancer in bone and exacerbates muscle weakness in vivo. Oncotarget 8:8406–8419. 10.18632/oncotarget.14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho EJ, Choi Y, Jung SJ, Kwak HB (2022) Role of exercise in estrogen deficiency-induced sarcopenia. J Exerc Rehab 18:2–9. 10.12965/jer.2244004.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borreani C, Alfieri S, Infante G, Miceli R, Mariani P, Bosisio M et al (2021) Aromatase inhibitors in postmenopausal women with hormone receptor-positive breast cancer: profiles of psychological symptoms and quality of life in different patient clusters. Oncology 99:84–95. 10.1159/000509651 [DOI] [PubMed] [Google Scholar]
- 11.De Paepe B (2022) The cytokine growth differentiation factor-15 and skeletal muscle health: portrait of an emerging widely applicable disease biomarker. Int J Mol Sci 23:13180. 10.3390/ijms232113180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Sun W, Gu X, Miao C, Feng L, Shen Q et al (2022) GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Dis 8:162. 10.1038/s41420-022-00972-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artigas-Arias M, Curi R, Marzuca-Nassr GN (2024) Myogenic microRNAs as therapeutic targets for skeletal muscle mass wasting in breast cancer models. Int J Mol Sci 25:6714. 10.3390/ijms25126714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merchant RA, Chan YH, Duque G (2023) GDF-15 is associated with poor physical function in prefrail older adults with diabetes. J Diabetes Res 2023:1–10. 10.1155/2023/2519128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jesus Leite MAF, Mariano IM, Dechichi JGC, Giolo JS, de Gonçalves ÁC, Puga GM (2021) Exercise training and detraining effects on body composition, muscle strength and lipid, inflammatory and oxidative markers in breast cancer survivors under tamoxifen treatment. Life Sci 284:119924. 10.1016/j.lfs.2021.119924 [DOI] [PubMed] [Google Scholar]
- 16.Strasser B, Steindorf K, Wiskemann J, Ulrich CM (2013) Impact of resistance training in cancer survivors. Med Sci Sports Exerc 45:2080–2090. 10.1249/MSS.0b013e31829a3b63 [DOI] [PubMed] [Google Scholar]
- 17.Stene GB, Helbostad JL, Balstad TR, Riphagen II, Kaasa S, Oldervoll LM (2013) Effect of physical exercise on muscle mass and strength in cancer patients during treatment—a systematic review. Crit Rev Oncol/Hematol 88:573–593. 10.1016/j.critrevonc.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 18.Lee J (2022) The effects of resistance training on muscular strength and hypertrophy in elderly cancer patients: a systematic review and meta-analysis. J Sport Health Sci 11:194–201. 10.1016/j.jshs.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ransdell LB, Wayment HA, Lopez N, Lorts C, Schwartz AL, Pugliesi K et al (2021) The impact of resistance training on body composition, muscle strength, and functional fitness in older women (45–80 years): a systematic review (2010–2020). Women 1:143–168. 10.3390/women1030014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzuca-Nassr GN, Alegría-Molina A, SanMartín-Calísto Y, Artigas-Arias M, Huard N, Sapunar J et al (2024) Muscle mass and strength gains following resistance exercise training in older adults 65–75 years and older adults above 85 years. Int J Sport Nutri Exerc Metab 34:11–19. 10.1123/ijsnem.2023-0087 [DOI] [PubMed] [Google Scholar]
- 21.Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH et al (2009) Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutri 89:608–616. 10.3945/ajcn.2008.26626 [DOI] [PubMed] [Google Scholar]
- 22.Ji Y, Plourde H, Bouzo V, Kilgour RD, Cohen TR (2020) Validity and usability of a smartphone image-based dietary assessment app compared to 3-day food diaries in assessing dietary intake among canadian adults: randomized controlled trial. JMIR MHealth UHealth 8:e16953. 10.2196/16953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serón P, Muñoz S, Lanas F (2010) Nivel de actividad física medida a través del cuestionario internacional de actividad física en población Chilena. Rev Med Chil 138(10):1232–1239. 10.4067/S0034-98872010001100004 [PubMed] [Google Scholar]
- 24.Galvão DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, Mcguigan MR et al (2006) Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc 38:2045–2052. 10.1249/01.mss.0000233803.48691.8b [DOI] [PubMed] [Google Scholar]
- 25.Podsiadlo D, Richardson S (1991) The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatri Soc 39:142–148. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 26.Galiano-Castillo N, Arroyo-Morales M, Ariza-Garcia A, Sánchez-Salado C, Fernández-Lao C, Cantarero-Villanueva I et al (2016) The six-minute walk test as a measure of health in breast cancer patients. J Aging Phys Act 24:508–515. 10.1123/japa.2015-0056 [DOI] [PubMed] [Google Scholar]
- 27.Acosta AM, Escalona M, Maiz A, Pollak F, Leighton F (2002) Determinación del índice de resistencia insulínica mediante HOMA en una población de la Región Metropolitana de Chile. Revista Médica de Chile 130(11):1227–31. 10.4067/S0034-98872002001100004 [PubMed] [Google Scholar]
- 28.Dam TV, Dalgaard LB, Thomsen CB, Hjortebjerg R, Ringgaard S, Johansen FT et al (2021) Estrogen modulates metabolic risk profile after resistance training in early postmenopausal women: a randomized controlled trial. Menopause 28:1214–1224. 10.1097/GME.0000000000001841 [DOI] [PubMed] [Google Scholar]
- 29.Escriche-Escuder A, Trinidad-Fernández M, Pajares B, Iglesias-Campos M, Alba E, Cuesta-Vargas AI et al (2021) Ultrasound use in metastatic breast cancer to measure body composition changes following an exercise intervention. Sci Rep 11:8858. 10.1038/s41598-021-88375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung Y, Sato A, Takino Y, Ishigami A, Machida S (2022) Influence of oestrogen on satellite cells and myonuclear domain size in skeletal muscles following resistance exercise. J Cachexia Sarcopenia Muscle 13:2525–2536. 10.1002/jcsm.13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enns DL, Tiidus PM (2010) The influence of estrogen on skeletal muscle. Sports Med 40:41–58. 10.2165/11319760-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 32.Coyle-Asbil B, Ogilvie LM, Simpson JA (2023) Emerging roles for estrogen in regulating skeletal muscle physiology. Physiol Genomics 55:75–78. 10.1152/physiolgenomics.00158.2022 [DOI] [PubMed] [Google Scholar]
- 33.Fairman CM, Zourdos MC, Helms ER, Focht BC (2017) A scientific rationale to improve resistance training prescription in exercise oncology. Sports Med 47:1457–1465. 10.1007/s40279-017-0673-7 [DOI] [PubMed] [Google Scholar]
- 34.Bär PDR, Koot RW, Amelink G, Hans J (1995) Muscle damage revisited: does tamoxifen protect by membrane stabilisation or radical scavenging, rather then via the E2-receptor? Biochem Soc Trans 23:236S-236S. 10.1042/bst023236s [DOI] [PubMed] [Google Scholar]
- 35.Roberts K, Rickett K, Greer R, Woodward N (2017) Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early breast cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 111:66–80. 10.1016/j.critrevonc.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 36.Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW (2017) Strength and hypertrophy adaptations between low- vs. high-load resistance training: a systematic review and meta-analysis. J Strength Cond Res 31:3508–3523. 10.1519/JSC.0000000000002200 [DOI] [PubMed] [Google Scholar]
- 37.Grgic J, Schoenfeld BJ, Davies TB, Lazinica B, Krieger JW, Pedisic Z (2018) Effect of resistance training frequency on gains in muscular strength: a systematic review and meta-analysis. Sports Med 48:1207–1220. 10.1007/s40279-018-0872-x [DOI] [PubMed] [Google Scholar]
- 38.Simonavice E, Liu P-Y, Ilich JZ, Kim J-S, Arjmandi B, Panton LB (2014) The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl Physiol Nutr Metab 39:730–739. 10.1139/apnm-2013-0281 [DOI] [PubMed] [Google Scholar]
- 39.Serra MC, Ryan AS, Ortmeyer HK, Addison O, Goldberg AP (2018) Resistance training reduces inflammation and fatigue and improves physical function in older breast cancer survivors. Menopause 25:211–216. 10.1097/GME.0000000000000969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardee JP, Porter RR, Sui X, Archer E, Lee I-M, Lavie CJ et al (2014) The effect of resistance exercise on all-cause mortality in cancer survivors. Mayo Clin Proc 89:1108–1115. 10.1016/j.mayocp.2014.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owoade AO, Adetutu A, Ogundipe OO, Owoade AW, Kehinde EO (2022) Effects of tamoxifen administration on lipid profile in female albino rats. Asian J Res Biochem 10(3):10–22. 10.9734/ajrb/2022/v10i330224 [Google Scholar]
- 42.He T, Li X, Li J, Wang Z, Fan Y, Li X et al (2022) Lipid changes during endocrine therapy in breast cancer patients: the results of a 5-year real-world retrospective analysis. Front Oncol 11:670897. 10.3389/fonc.2021.670897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo J-J, Jung E-A, Kim Z, Kim B-Y (2023) Risk of cardiovascular events and lipid profile change in patients with breast cancer taking aromatase inhibitor: a systematic review and meta-analysis. Curr Oncol 30:1831–1843. 10.3390/curroncol30020142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell LN, Nguyen ATP, Li L, Desta Z, Henry NL, Hayes DF et al (2012) Comparison of changes in the lipid profile of postmenopausal women with early stage breast cancer treated with exemestane or letrozole. J Clin Pharmacol 52:1852–1860. 10.1177/0091270011424153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Some data is provided within the manuscript or supplementary information files. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.