Abstract
Introduction
In this study, we tested the hypothesis that pre-osteoclast signaling is key in triggering post-traumatic angiogenesis in alveolar bone via the SDF-1/CXCR4 pathway. Interruption of osteoclast differentiation through zoledronate (Zol) disrupts the crosstalk between pre-osteoclasts and endothelial cells, hindering the initial angiogenic reaction following dental trauma. This disruption could therefore play a role in the pathogenesis of medication-related osteonecrosis of the jaw (MRONJ).
Methods
The effect of zoledronate on the expression of SDF1 was tested in pre-osteoclasts (POC) in vitro. Then, we tested the effect of pre-osteoclast conditioned medium on HUVEC cell differentiation, migration, tube-formation, and CXCR4 expression and activity in-vitro. Lastly, we quantified the effect of zoledronate treatment on post-traumatic vascular perfusion of alveolar bone, using microCT-angiography and immunohistochemistry.
Results
SDF-1 mRNA expression decreased in Zol-treated POCs (p = 0.02). Flow-Cytometry analysis showed a decrease in CXCL-12+ (SDF-1α) expressing POCs with Zol treatment (p = 0.0058). On the other hand, CXCR4 mRNA expression was significantly inhibited in Zol-treated HUVECs (p = 0.0063). CXCR4 protein expression and activity showed a corresponding dose-dependent downregulation HUVEC surface treated with conditioned media from POC treated with Zol (p = 0.008 and 0.03, respectively). Similar inhibition was observed of HUVEC migration (p = 0.0012), and tube formation (p < 0.0001), effects that were reversed with SDF-1. Finally, there was a significant reduction of CD31+ HUVECs in Alveolar bone of Zol-treated rats (p = 0.0071), confirmed by significantly lower percentage of blood vessel volume (p = 0.026), and marginally lower vessel number (p = 0.062) in the alveolar bone.
Conclusion
Pre-osteoclasts play a crucial role in the initial angiogenic response in alveolar bone following dental extraction. Disruption of this process may be a predisposing factor to osteonecrosis.
Keywords: Angiogenesis, Osteoclasts, SDF1, CXCR4, Zoledronate, Osteonecrosis
Highlights
-
•
Pre-osteoclasts play a central role in initiating angiogenesis in bone
-
•
Pre-osteoclast-driven SDF1/CXCR4 axis plays a key role in bone angiogenesis
-
•
Chronic inhibition of osteoclasts results in an impairment in post-traumatic angiogenesis in alveolar bone following dental extraction, contributing to the induction of MRONJ.
1. Introduction
Medication-related osteonecrosis of Jaw (MRONJ) is an uncommon, yet severe, complication in patients on long-term treatment with anti-resorptive and anti-angiogenic medications. Along with atypical femoral fractures (AFF), MRONJ has caused significant concern for both patients and physicians, contributing to a decline in the prescription rates of anti-resorptive medications, especially bisphosphonates (BP), in what has been called a “crisis in osteoporosis treatment.” (Khosla and Shane, 2016)
Most MRONJ occurrences typically involved a triggering factor, typically an invasive dental trauma or chronic periodontitis (Ruggiero et al., 2014). One common feature between the two conditions is the presence of a substantial challenge to bone regeneration. Previous studies from our group and others' have established that failure to launch an effective regenerative response is one of multiple factors that converge to trigger the osteonecrosis cascade in these cases (Elsayed et al., 2018, Elsayed et al., 2020). Successful regeneration requires the efficient cellular interplay between bone, immune system, and blood vessels at the very early stage (Dimitriou et al., 2011). The detailed mechanisms of such integration remain not clearly understood.
The anti-resorptive action of BP has been attributed largely to inhibiting osteoclast-dependent bone resorption (Rodan and Fleisch, 1996; Colucci et al., 1998). BP-treated osteoclasts show altered morphology, cytoskeletal arrangement, and impaired proliferation, differentiation, and gene expression (Russell, 2011).
Stromal cell-derived factor 1 / C-X-C motif chemokine receptor 4 (SDF-1; CXCL12/CXCR4) pathway has emerged as an important player in bone regeneration and remodeling (Xu et al., 2017). SDF-1 is constitutively expressed by bone-marrow stromal cells, such as perivascular and bone-lining stromal cells and surrounding niche (Petit et al., 2007). Recently, SDF-1 has been shown to be expressed by osteoclast (TRAP+) cell lineage (Kollet et al., 2006). The cognate receptors for SDF-1, CXCR4, and CXCR7 are widely expressed by many cells. However, studies suggested that SDF-1 primarily induce neoangiogenesis by stimulating CXCR4+ pro-angiogenic cells (Ratajczak et al., 2006). More evidence suggested that SDF1/CXCR4 signaling could be a major regulator of angiogenesis during regeneration and repair in various tissues (Askari et al., 2003; Walter et al., 2005; Yamaguchi et al., 2003; Deshane et al., 2007). It has recently been shown that SDF1 mediated the migration and tube formation by endothelial progenitor cells via Akt signaling (Cun et al., 2021). SDF-1α was also demonstrated to play an important role in revascularization of ischemic hind limbs, through recruitment of CXCR4+ hemangiocytes (Jin et al., 2006).
Interestingly, subcutaneous injection of SDF-1α was shown to induce both recruitment of monocytes and formation of neovascularization foci in mice, strongly suggesting an angiogenic role by monocyte lineage cells involving SDF-1/CXCR4 signaling (Salcedo et al., 1999). SDF1 protein and mRNA are expressed by monocyte-lineage cells, while CXCR4 is widely expressed in endothelial cells in the same tissue (Pablos et al., 1999).
The bone microenvironment is a complex and dynamic niche, where osteogenesis and angiogenesis interact to maintain skeletal integrity (Redlich and Smolen, 2012; Diomede et al., 2020). The continuous and condition-sensitive integration of cellular function between bone and blood vessels is key for bone growth, remodeling, and regeneration. Dysregulation of osteogenesis-angiogenesis coupling is implicated in various bone disorders, including osteoporosis, osteonecrosis, rheumatoid arthritis, and bone cancers (Kanczler and Oreffo, 2008). More specifically, hyperactive osteoclasts combined with dysregulated angiogenesis are thought to play a role in rheumatoid arthritis (Cackowski et al., 2010). On the other hand, both inhibition of osteoclasts and impaired angiogenesis have been detected in osteonecrosis of the jaw (Howie et al., 2015a).
Osteoclastogenesis involves the differentiation of mononuclear hematopoietic progenitors to multinucleated cells via the tightly regulated RANK/RANKL/OPG signaling pathway (Redlich and Smolen, 2012). RANKL, expressed by stromal cells, binds to the RANK extracellular receptor, triggering a cascade effect leading to differentiation (Boyle et al., 2003). During the early phase of bone regeneration, osteoclasts are recruited from the bloodstream, attach to bone surfaces at the site, and start breaking down and digesting necrotic bone, paving the way for subsequent osteogenesis by bone-forming osteoblasts (Boyle et al., 2003). Adequate vascular perfusion is essential at this early stage, since it ensures adequate supply and activation conditions of both osteoclast and osteoblast precursors. However, the next critical step that determines the success or failure of regeneration is angiogenesis: maintaining adequate oxygen supply, mineral homeostasis, and removal of necrotic products, and guarding against contamination by microorganisms, where necessary (Diomede et al., 2020).
We have previously shown that alveolar bone in Zol-treated rats show reduced post-extraction vascularity in alveolar bone (Guevarra et al., 2015). In this study, we postulated that matrix-bound bisphosphonates (BPs) disrupt the crosstalk between osteoclastogenesis and angiogenesis by inhibiting the SDF-1/CXCR4 axis. We suggest that the SDF-1/CXCR4 pathway is crucial for the coupling of osteoclastogenesis and angiogenesis, and its interference could contribute significantly to complications related to bisphosphonates.
2. Material and methods
2.1. RAW 264.7 cell culture
RAW 264.7 monocyte cell line (American Type Culture Collection ATCC, Manassas, VA, USA) is an established cell line that can be reliably differentiated into osteoclasts. Cells were cultured in Dulbecco's Modified Eagle Medium DMEM (Thermo Fisher Scientific, NY, USA), containing 10 % fetal bovine serum (FBS; Atlanta biological by R&D system, GA, USA), 100UmL−1 penicillin, and 100μgmL−1 streptomycin at 37 °C in a humidified atmosphere of 5 % CO2 in air. For pre-osteoclast differentiation, RAW 264.7 cells were cultured in DMEM containing 10 % FBS and 50ngmL−1 RANKL and the culture medium was changed every 2 days until the 5th day of differentiation.
2.2. Endothelial cell proliferation assay
We determined the effect of different Zol doses on the proliferation of endothelial cells. Human umbilical vein endothelial cells (HUVEC; LONZA, Basel, Switzerland) cell were seeded in 96-well microplates (5 × 103 per well) and treated with 0 μM, 1 μM, 10 μM, and 50 μM Zoledronate (Zol; Selleckchem, Houston, TX, USA) for 1 h, 3 h, and 6 h, three replicates-each. Next, MTT solution (5 mg/mL in H2O) was added to the well, followed by the addition of 0.3 mL of dimethyl sulfoxide to dissolve the MTT-formazan. Then, microplates were incubated for 1 h at 37 °C under a humidified atmosphere that was comprised of 5 % CO2, lysed, and then absorbance values were obtained using Synergy H1 microplate reader (BioTek, Winooski, VT, USA) by subtracting the background absorbance at 690 nm from absorbance at 570 nm. Absorbance values were converted to percent viability using untreated controls as baseline.
2.3. Conditioned media preparation
To test the effect of osteoclast signaling on endothelial cell differentiation, conditioned media from Zol-treated and untreated pre-osteoclasts were prepared and collected. Media were stored at −80 °C after a centrifugation at 2500 rpm for 10 min at 4 °C. Conditioned media were collected at the 5th day of osteoclast differentiation process. Conditioned medium from pre-osteoclasts (POC-CM) with serum were collected for co-culturing with HUVECs. Serum-free (POC-CM) were collected for migration assay.
2.4. Effect of Zol on viability of RAW 264.7 cells
The PrestoBlue (Life Technologies, Carlsbad, CA, USA) reagent was used at 10 % volume:volume ratio in cell media (10 μL in 100 μL sample), followed by a 2-hour incubation at 37 °C. Cultured RAW 264.7 cells were incubated over different concentrations Zol-treated bone matrix 24 well plate. The DNA concentration ng/ml) was measures as an indicator for cell viability. Total well absorbance was measured at 570 nm; with reference wavelength of 600 nm using the FLUOstar Omega (BMG Labtech, Cary, NC, USA). Media only control was treated with the reagent similarly and the average absorbance value was deducted from all the wells.
2.5. Flow cytometry
Extracellular staining for expression of RANK and CD14 (Biolegend, SanDiego, USA) was done on ice, in Flow Cytometry Staining Buffer (Affymetrix, eBioscience, Thermo fisher scientific, Waltham MA, USA). Blocking of FC receptor (FCR) using mouse FcR blocking reagent (MACS/Miltenyi Biotec Inc., San Diego, CA, USA) was done for 15 min on ice and protected from light, this was followed by adding the fluorophore conjugated antibody (with the recommended concentration) on ice for 30 min, after which cells were washed. After the last wash the cells were fixed for 1 h using Fixation Buffer (affymetrix, eBioscience, Thermofisher scientific, Waltham MA, USA), after which cells were washed twice with permeabilization buffer using fix/perm concentrate (eBioScience, San Diego, CA, USA), before incubation with antibodies for intracellular staining of CXCL-12 (BD Biosciences, Franklin Lakes, NJ, USA) for 1 h at room temperature while protected from light. Finally, cells were washed twice with permeabilization buffer and re-suspended in flow cytometry buffer and run through a four-color flow cytometer (FACS Calibur, BD Biosciences, Franklin Lakes, NJ, USA) and data were collected using CellQuest software. Gating excluded dead cells and debris using forward and side scatter plots.
2.6. Immunofluorescent assessment (IF) of HUVEC proliferation
HUVEC with and without treatment of SDF-1α (100 ng/mL), POCs-CM CTRL, POCs-CM (10 μM), AMD3100 (400 ng/mL) or the combination of POCs-CM (10 μM) + SDF-1α (100 ng/mL) were subjected to immunofluorescent (IF) staining to detect cell proliferation marker Ki76 as an indicator of angiogenesis and CD31 was used as a marker for endothelial cells. IF staining was done using our published protocol (Samra et al., 2023; Tawfik et al., 2021; Mohamed et al., 2017).Briefly, HUVEC cells were fixed for 10 min in 4 % paraformaldehyde then was washed with PBS, followed by blocking nonspecific reaction with Power Block (BioGenex, Fremont, CA, USA, Cat. # BS-1310-25) for 1 h. Then cells were incubated with anti-Ki67 (Abcam, Cambridge, MA, USA, Cat#ab15580) and anti CD31 (Novus Biologicals, NB100–2284) for 3 h at 37 °C. Then, cells were washed 3 times with PBS containing 0.3 % Triton-X. This was followed by incubation with appropriate secondary antibodies (Alexa Fluor and Texas Red Avidin, Invitrogen, Eugene, Oregon) and were cover-slipped with nuclear staining DAPI (Sigma-Aldrich Chemical Corp., St. Louis, MO, USA). Fluorescent microscope with high-resolution camera using Zeiss Axiovision digital image processing software (version 4.8) was used for images capturing.
2.7. Wound healing (migration) assay using live-cell microscopy
The wound healing assay serves as a conventional in vitro method for investigating the coordinated movement of cells in two dimensions (Ilina and Friedl, 2009). A gap devoid of cells was generated within a dense cell monolayer through physical exclusion or by eliminating cells via mechanical, means. The presence of this cell-free space prompts the migration of cells into the void. Herein we compared the cell migration of HUVEC in different groups; SDF-1α (100 ng/mL), POCs-CM CTRL, POCs-CM (10 μM), AMD3100 (400 ng/mL) or the combination of POCs-CM (10 μM) + SDF-1α (100 ng/mL). This was followed by a series of illustrative images captured during a wound healing assay conducted on a fully formed endothelial monolayer.
2.8. Endothelial cell tube formation assay
The assessment of POCs conditioned media on HUVECs' ability to form vascular-like structures on growth factor-reduced Matrigel as an indicator for angiogenesis, was performed as previously described (Mohamed et al., 2017; Francescone et al., 2011), 24-well culture plates were coated with Matrigel according to the manufacturer's instructions. HUVEC (5 × 104) were seeded on the coated surface of solidified matrix with POC conditioned media Serum-starved HUVEC cells (5 × 104) per each well were seeded into on the coated surface of solidified matrix and treated with our different groups; SDF-1α (100 ng/mL), POCs-CM CTRL, POCs-CM (10 μM), AMD3100 (400 ng/mL) or the combination of POCs-CM (10 μM) + SDF-1α (100 ng/mL) Images were taken from 6 different well/group 6–7 h later. The analysis was made by using plugin “Angiogenesis Analyzer” for software ImageJ (Schneider et al., 2012).
2.9. Western blotting analysis
HUVEC were cultured with different concentration of Zol [0 μM, 0.1 μM, 1 μΜ, 10 μM] for 3 h. Then the cells were lysed in buffer and total protein contents were determined using the BCA protein Assay Kit. Equal amounts of protein were subjected to Mini-PROTEAN TGX precast electrophoresis gels (Bio-Rad Life Science research, Hercules, California) and transferred to PVDF membranes (Merk Millipore, Burlington, Massachusetts, USA). The membranes were blocked with 5 % non-fat milk and probed overnight with 1:500 dilution of Human CXCR4 Antibody (MAB173-100, R&D systems, Minneapolis, MN), with phospho-Akt and pan-Akt (Cell Signaling Technology, Inc., Danvers, MA, USA). Then, the membrane was incubated with 1:10000 dilution of anti-mouse IgG, HRP-linked Antibody (Cell Signaling Technology, Inc., Danvers, MA, USA). The membrane was developed with SuperSignal™ West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, NY, USA). CXCR-4 protein expression levels and its phosphorylation (p-Akt) were detected by Gel Doc™ XR+ Gel Documentation System. (Bio-Rad, Hercules, California, USA). Beta Actin Loading Control Monoclonal Antibody (BA3R), DyLight 680 (Invitrogen, Carlsbad, CA, USA) was used for normalization.
2.10. Real-time polymerase chain reaction (real-time PCR)
CXCR4 and CXCL-12 (SDF-1α) mRNA expression levels in both Zol-treated HUVECs and POCs were determined by real-time PCR. Total RNA was isolated by using RNeasy Mini Kit (Qiagen Sciences Inc., Germantown, MD). RNA was reverse-transcribed using iScript cDNA synthesis kits (Bio-Rad, Hercules, CA) and the resultant cDNA analyzed by qRT-PCR in a 20 mL reaction volume containing Supermix and primer-probe sets obtained from Applied Biosystems StepOnePlus machine (Thermo Fisher Scientific, NY, USA). 18S was used as an internal control for normalization.
2.11. In-vivo vascular changes
The study was carried out in a strict accordance with Institutional Animal Care and Use Committee (IACUC) of Augusta University and was approved by Animal Ethical and Welfare Committee of Augusta University. Sixteen Sprague-Dawley rats were divided equally into 2 main groups (Zol-treated and saline-treated, for 13 weeks). Then, unilateral extraction of 1st and 2nd molar was performed. A week after, 150 cc of Brite-Vu contrast dye was perfused through a catheter into the ascending aorta connected to a pump. The animals were euthanized, and the mandibles were harvested and fixed in 4 % paraformaldehyde in phosphate-buffered saline (PBS) for 48 h. The mandibles were kept in EDTA solution to be decalcified for 5–7 weeks.
2.12. Microcomputed tomography (microCT) analysis
In order to visualize the in-vivo vascular changes, the mandible was scanned after being fully decalcified. The visualization and 3-dimensional analysis of the perfused demineralized right and left hemimandible was performed. The samples were scanned with an in vitro micro-CT device -Skyscan 1172, (SkyScan, Aartlesaar, Belgium) with scanning parameters of: 40 kV, 102 uA, exposure 560 ms/frame, average of 3 frames per projection, and 0.25 mm filter. The specimens were scanned at high resolution (500 × 500 pixels). Reconstructions for X-ray projections were made with Skyscan Nrecon-software (v. 1.7.3.1, Brüker micro-CT, Kontich, Belgium). Ring artefact and beam hardening corrections were applied in reconstruction.
2.13. Immunohistochemistry for the micro-vasculature in alveolar bone
Tissue specimens were fixed with 4 % paraformaldehyde in phosphate-buffered saline (PBS) and decalcified in ethylene diamine tetra-acetic acid (EDTA). The specimens were dehydrated with ethanol, and treated with xylene, then embedded in paraffin. The specimens were cut into 5 μm thick frontal sections using a microtome. Sections were stained with hematoxylin and eosin (HE) for histological observation and prepared for immunohistochemistry. The slides were stained for endothelial cell marker Anti-CD31 antibody (Abcam, Cambridge, MA, USA). The number of CD31+ ECs in alveolar bone was analyzed by for software Image J.
2.14. Statistical analysis
Statistical analysis was done using GraphPad Prism software version6 (GraphPad Software, La Jolla, CA, USA). Data values were reported as means ± SD. Normality assumption was evaluated using the Shapiro-Wilk test. When normality assumptions were not met, an alternative non-parametric test was done. A one-way ANOVA test with significance defined as p value <0.05, a confidence level of 95 % confidence interval.
3. Results
3.1. Differentiating pre-osteoclasts express SDF-1, which is inhibited by zoledronate treatment
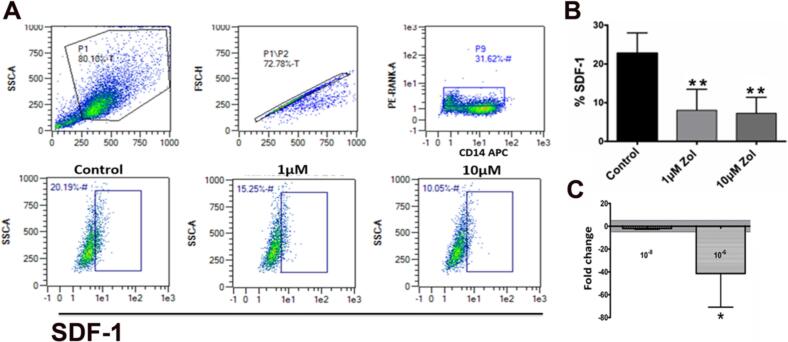
CD14 is a surface marker characteristic of differentiating myeloid lineage cells (Zamani et al., 2013). RANK is an essential surface marker for pre-osteoclast before differentiating into multinucleated osteoclasts (Asagiri and Takayanagi, 2007). In this study, CD14+/RANK+ Cells were stained with CXCL12 antibody to identify SDF-1- secreting pre-osteoclasts and the effect of interrupting their differentiation by zoledronate (Zol) on SDF-1α expression. The results revealed a dose-dependent reduction in SDF-1α (CXCL-12) expression in POCs. The percentage of SDF-1a in untreated CD14+/RANK+ cells was 39.15 % and was reduced to 11.02 % and 7.34 %, with 1 μM and 10 μM Zol, respectively (p = 0.006; Fig. 1a and b). SDF-1 mRNA expression level was measured in Zol-treated (10−8 M and10−6M) POCs. The results showed reduction in gene expression, as compared to untreated HUVECs (p = 0.02; Fig. 1c).
Fig. 1.
Pre-osteoclast produce SDF-1 during differentiation. A, B: Flowcytometry analysis demonstrates that the percentage of pre-osteoclasts (CD14+/RANKL+) expressing SDF1 decreased with increase in Zol treatment dose; C: RT-PCR- mRNA expression of SDF-1a expression in Zol-treated POCs (*p = 0.02; **p = 0.006).
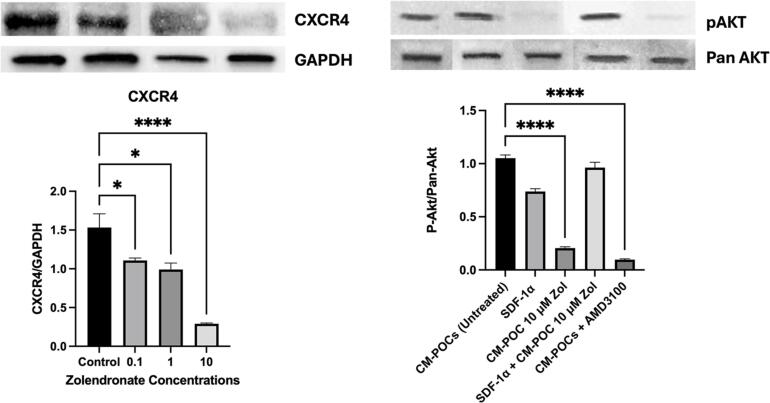
The CXCR4 mRNA expression was significantly inhibited in Zol-treated HUVECs (10−10 M, 10−8 M, and 10−6 M), compared to untreated HUVECs (p = 0.0063). Western blotting analysis data showed a dose-dependent downregulation of CXCR4 protein expression by HUVEC- treated with Zol (0.1 μM, 1 μM, 10 μM; p = 0.008; Fig. 2, left panel). Importantly, conditioned media from pre-osteoclasts (POC-CM) treated with Zol caused a significant inhibition in the CXCR4 activity (p-Akt/Pan-Akt) in HUVECs compared to untreated POC-CM. This inhibitory effect was corrected by adding SDF-1α (100 ng/mL) to the media. Pan-Akt expression was used as loading control (p = 0.03; Fig. 2, right panel).
Fig. 2.
Differentiating endothelial cells express CXCR4 receptors, which are also activated by conditioned medium of differentiating pre-osteoclasts. CXCR4 expression (left panel) and activation (right panel) takes place when ECs are treated with media from differentiating osteoclasts, an effect that was inhibited when osteoclasts were treated with Zol or the SDF1 inhibitor AMD3100, Receptor activation was rescued with adding SDF1 to the conditioned media.
3.2. MTT assay [effect of Zol on proliferation of HUVECs]
The cytotoxicity of Zol doses on HUVECs was first determined by MTT assay after 1, 3, and 6 h. There was no significant reduction in proliferation at 1 μm, 10 μm at the 1 (p = 0.18), 3 (p = 0.22), and 6 h (p = 0.221) points compared to control (Supplemental Fig. S1).
3.2.1. In vitro assessment of Zol treatment on HUVECs angiogenesis
Angiogenesis is a biological phenomenon in which new capillaries emerge from pre-existing vessels. Essentially, the growth of angiogenic sprouts is contingent upon proliferation, migration, and differentiation of endothelial cells (HUVECs).
-
1-
Cell proliferation assessment.
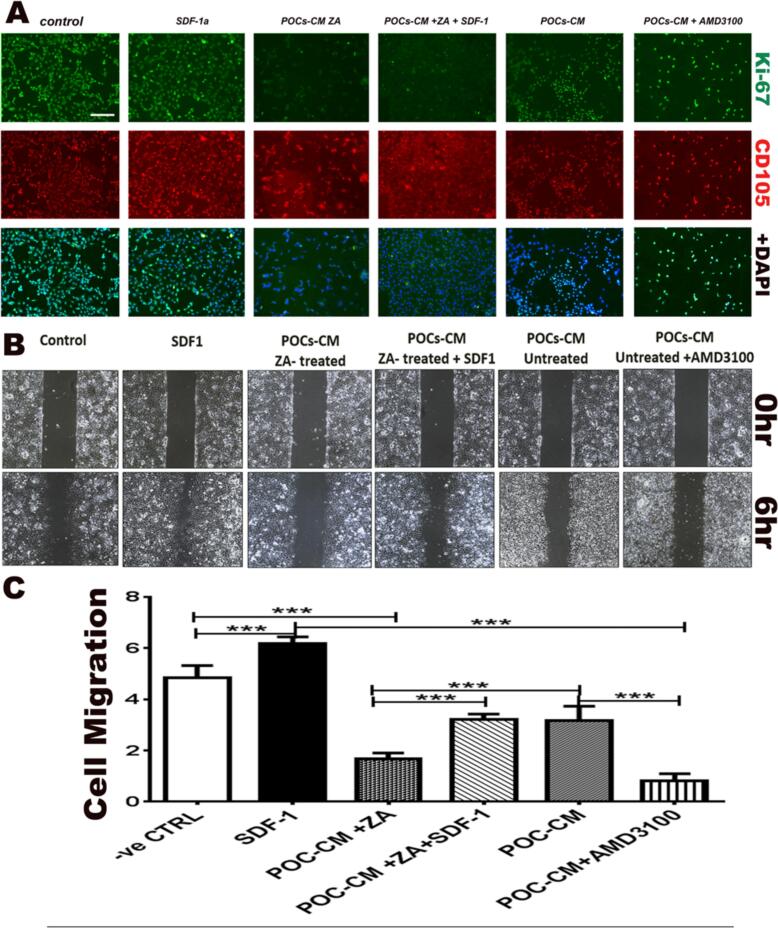
We evaluated the immunofluorescence expression of Ki67 as a marker of cell proliferation in HUVECs cells to check the effect of POC-stimulated HUVECs on cell proliferation (Fig. 3A). Expression CD31(endothelial cell marker) and Ki67(proliferation marker) was compared in HUVECs under 6 conditions: 1) normal medium; 2) SDF-1 (100 ng/mL); 3) conditioned medium from untreated POCs (POCs-CM); 4) conditioned medium from POCs treated with 10 μM Zol (POCs-CM-Zol); 5) POC-CM-Zol plus SDF-1; and 6) POC-CM-Zol plus AMD3100 (CXCR4 inhibitor; 400 ng/mL). An endothelial cell marker (CD31, red) was used in combination with Ki67 (proliferation marker; green). SDF-1 increased cell proliferation. Direct SDF1 treatment stimulated proliferation, while CM from POC treated with Zol inhibited proliferation, so did the CXCR4 blocker AMD3100. The inhibitory effect of Zol on POC was partially reversed by adding SDF1 to the conditioned media.
-
2-
Migration Assay
Fig. 3.
Endothelial cell proliferation and differentiation is stimulated by SDF1 or media from differentiating osteoclasts and inhibited when CXCR4 is blocked or the osteoclast differentiation is inhibited with Zol. A: Immunofluorescence expression of Ki67 (marker of cell proliferation, green) and endothelial cell marker (CD31, red) in HUVECs treated with normal media, SDF-1 (100 ng/mL), conditioned media from untreated POCs (POCs-CM), plus Zol (10 μM), POC-CM-Zol plus SDF-1, and POC-CM-Zol plus AMD3100 (400 ng/mL) showed that direct treatment with SDF-1 increased EC proliferation. Proliferation was inhibited with the addition of Zol or CXCR4 inhibitor AMD3100 and rescued with the addition of SDF1 to the media. B: Wound healing assay to assess the migration of endothelial cells mirrored the results of the proliferation assay. Direct treatment with SDF-1 (100 ng/mL) had a significant stimulatory effect on migration. Conditioned media from Zol-treated POC (10 μM) resulted in severe inhibition of HUVEC migration (p = 0.0012), an effect that was reversed when SDF-1 was added media (p = 0.0067). Moreover, HUVEC treatment with CXCR4 blocker AMD3100 (400 ng/mL) resulted in inhibited migration. C: Statistics from the migration assay.
The migration of endothelial cells plays a crucial role in the process of angiogenesis to check the effect of POCs stimulated HUVECs on cell migration, the wound healing assay was used for assessing the migration of cells in a two-dimensional space by creating an artificial gap in a densely packed cell monolayer, and then monitoring cell movement. Similar to proliferation results, direct treatment with SDF1 caused significant increase in HUVEC migration (p = 0.0012; Fig. 3B and C), while CM from POC treated with Zol (10 μM) resulted in inhibition of HUVEC migration, an effect that was partially reversed when SDF-1 was added to the POC-CM-Zol media (p = 0.0067). Similarly, HUVEC treatment with CXCR4 blocker AMD3100 (400 ng/mL) inhibited migration (Fig. 3C).
-
3-
Tube formation assay
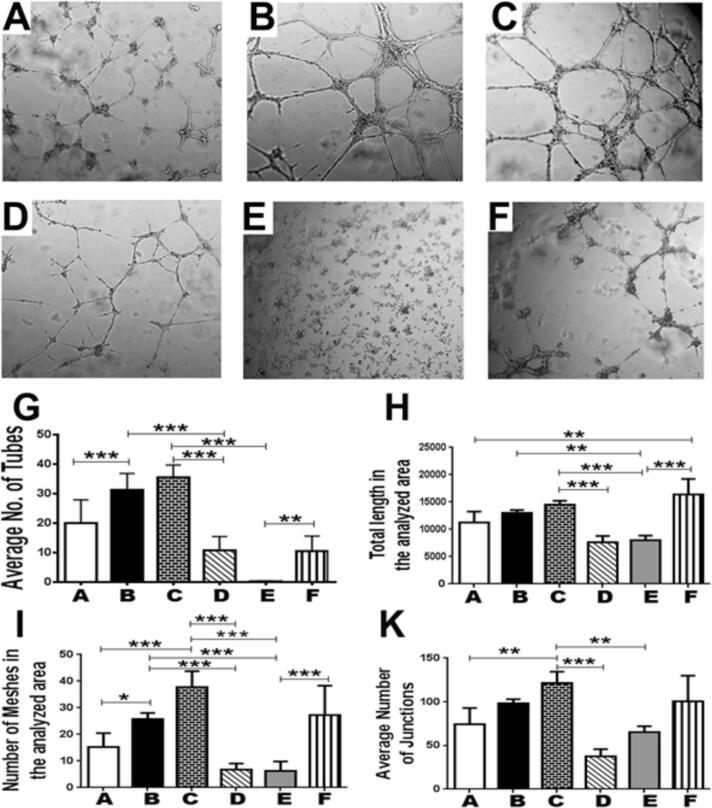
HUVECs in stock medium containing 10 ng/mL VEGF were planted on Matrigel and the parameters of tube formation were compared between groups. Tube formation was stimulated by direct treatment of HUVEC with SDF-1α or the POC-CM (Fig. 4, groups A, B, and C). Pre-treatment of POC with 10 μM Zol (Fig. 4, group D) inhibited tube formation (p < 0.0001), an effect that was partially reversed by adding SDF-1 to CM of Zol-treated POC (p < 0.0001; Fig. 4, group F). On the other hand, blocking SDF-1α/CXCR4 using 400 ng/mL AMD3100 significantly inhibited tube formation (31.6 ± 7.3 vs 9.8 ± 5.06; p < 0.0001; (Fig. 4, group E).
Fig. 4.
In vitro endothelial cell vessel formation is stimulated by SDF1 or media from differentiating osteoclasts and inhibited when CXCR4 is blocked or osteoclast differentiation is inhibited. (A) Control; (B) SDF-1; (C) POC-CM; (D) POC_Zol_CM; (E) POC_CM_AMD3100; (F) POC_Zol_CM_SDF-1. In-vitro tube formation by HUVECs was stimulated by: Direct treatment of HUVEC with SDF-1α or POC-CM. Pre-treatment of POC with 10 μM Zol (D) inhibited tube formation (p < 0.0001), an effect that was partially reversed by adding SDF-1 to CM of Zol-treated POC (p < 0.0001). On the other hand, blocking SDF-1α/CXCR4 using 400 ng/mL AMD3100 severely inhibited tube formation (E; p < 0.0001).
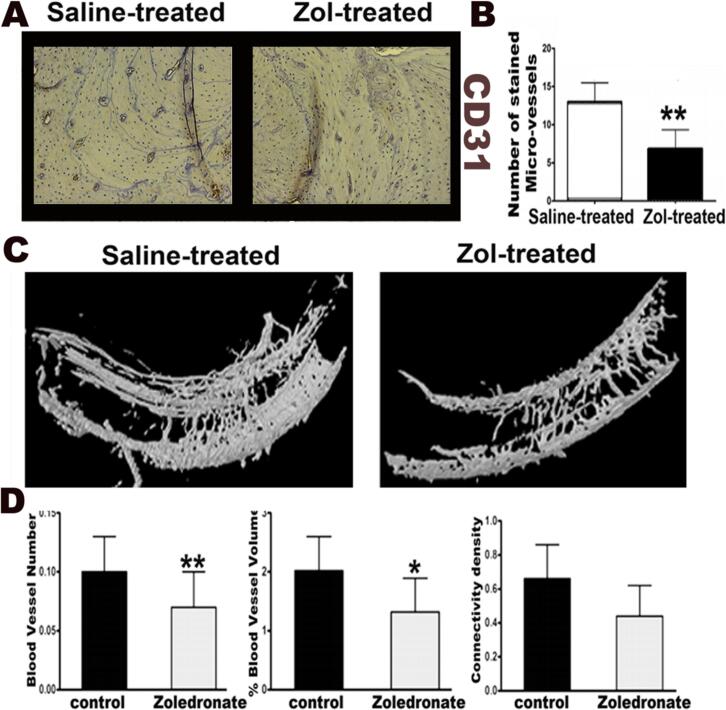
3.3. Inhibition of post-extraction angiogenesis in alveolar bone of Zol-treated rats
There was a significant reduction of CD31+ ECs in alveolar bone of Zol treated rats, as compared to saline treated group (p = 0.0071; Fig. 5). Zol-treated animals showed significantly lower percentage of blood vessel volume (p = 0.026), as well as a marginally lower vessel number (p = 0.062) in the alveolar bone, as compared to saline treated animals. (Fig. 5)
Fig. 5.
Rats treated with osteoclast inhibitor Zoledronate showed impaired angiogenesis after dental extraction. A and B: Histologic section from alveolar bone 8 weeks following surgical molar extraction showing a significant reduction of CD31+ by ECs in Alveolar bone of Zol-treated rats, as compared to saline treated group (p = 0.0071). C and D: MicroCT analysis of contrast-infused jaws from Zol-treated animals showed significantly lower percentage of blood vessel volume (p = 0.026), as well as a marginally lower vessel number (p = 0.062) in the alveolar bone following dental extraction, as compared to saline treated animals.
4. Discussion
Data from the present study suggest that the secretion of SDF-1 by pre-osteoclasts could be instrumental in regulating early angiogenesis that is crucial for subsequent bone rejuvenation. During osteoclast differentiation, SDF-1α was expressed and secreted prior to maturation into multi-nucleated, bone-resorbing osteoclasts. While pre-osteoclast-derived SDF-1 caused inhibition of endothelial cell proliferation and migration, it stimulated their differentiation, evident in tube formation. Blocking CXCR4 receptors in endothelial cells arrested their proliferation, migration, and differentiation, while SDF-1 alone stimulated all these processes. Furthermore, inhibiting osteoclast differentiation with zoledronate (Zol) also inhibited their SDF-1 mediated angiogenic action.
Stromal-cell-derived factor 1 alpha (SDF1α; CXCL12) has been shown to regulate angiogenesis (Petit et al., 2007). SDF1 recruits endothelial progenitor cells to sites of vascular damage through the CXCR4 pathway (Petit et al., 2007; Teicher and Fricker, 2010; Yang et al., 2018). It is also expressed in areas of inflammation and bone damage, where it is thought to play a role in the induction of osteoclastogenesis (Cackowski et al., 2010; Shahnazari et al., 2013).
SDF-1 is an essential chemokine for the bone microenvironment, inducing several effects via its alpha-chemokine receptor (CXCR4). SDF-1/CXCR4 pathway has been known for being involved in promoting chemotaxis and recruitment of osteoclast precursors (Yu et al., 2003) as well as endothelial progenitor cells (Zheng et al., 2007) from the bone marrow. Recently, it has been shown that SDF-1 promotes angiogenesis through stimulating CXCR4+ pro-angiogenic cells during embryogenesis and oncogenesis (Ratajczak et al., 2006).
Results from the present study suggest that Zol-induced osteoclast inhibition further disrupts their pro-angiogenic effect by diminishing SDF-1 signaling in the alveolar bone microenvironment during early socket regeneration. Our earlier studies demonstrated that alveolar bone was uniquely vulnerable to the negative effects of anti-resorptive medications (Elsayed et al., 2018, Elsayed et al., 2020, Elsayed et al., 2021; Howie et al., 2015a, Howie et al., 2015b). Compromising angiogenesis would add another challenge that could eventually push the bone down an osteonecrosis path.
In our in-vitro experiments, direct Zol treatment of TRAP+ cell lineage resulted in a dose-dependent decrease in both mRNA expression of SDF-1 and intracellular staining of SDF-1. Moreover, Zol treatment of endothelial cells led to the downregulation of CXCR4 expression and activity. Notably, the function of endothelial cells was compromised when treated with conditioned media from Zol-treated TRAP+ cell lineage. This compromised effect was partially alleviated with SDF-1a, a partiality attributed to the compromised CXCR4 activity and signaling pathway.
Zol-treated animals exhibited lower alveolar blood vessel volume and numbers, compared to saline-treated animals. It is worth mentioning that bisphosphonate, especially high potency intravenous forms such as zoledronate, have a strong bone-targeting property, which relies on their mechanically-stable, P-C-P bond, which replaces the less stable inorganic pyrophosphate (P-O-P). Through this configuration, bisphosphonate molecules bind to divalent ions, such as Ca2+, while being resistant to enzymatic or chemical breakdown (Morris and Einhorn, 2005) These molecules accumulate in the bone matrix for years, even after treatment cessation (Roelofs et al., 2012; Weiss et al., 2008; Papapoulos and Cremers, 2007). In a previous study, we demonstrated that matrix-bound bisphosphonates were both biologically accessible and active; and that they played a significant role in the pathogenesis of medication-related osteonecrosis of the jaw (MRONJ) (Elsayed et al., 2018). It is yet to be determined whether the inhibition of osteoclast-related angiogenic response is unique to zoledronate or common to other anti-resorptive medications, especially ones that do not accumulate in the matrix.
This study provides evidence that impaired angiogenesis contributes to the induction of MRONJ. However, previous studies have established that a myriad of conditions must be present for MRONJ to occur. These factors, all triggered by long-term anti-resorptive treatment, can only occur at the same time in certain areas, such as alveolar bone. That is why MRONJ is rare outside the oral cavity. These factors include inhibition of osteoclast resorption of necrotic bone after a traumatic event or periodontitis (Howie et al., 2015a), stimulation of bacterial colonization, paired with an impairment of innate immune response (Elsayed et al., 2020, Elsayed et al., 2021), and the inhibition of angiogenesis. Two added localization factors would be that alveolar bone is a preferred location for long-term accumulation of Zol in the bone matrix (Elsayed et al., 2018), and the uniquely challenging healing environment in the oral cavity after trauma or inflammation (Elsayed et al., 2018; Howie et al., 2015b).
In summary, SDF-1/CXCR4 signaling pathway plays an important in the cross talk between osteoclasts and vascular precursor cells. Following trauma, the lack of osteoclast-derived SDF-1 could be a mechanism of impaired bone healing. Specifically, inhibition of osteoclast differentiation by zoledronate disrupts this critical intercellular mechanism, further advancing the progression towards osteonecrosis in alveolar bone following dental trauma. Further studies are needed to characterize the role of osteoclast-mediated angiogenesis in bone regeneration, remodeling, inflammation, and oncogenesis.
The following is the supplementary data related to this article.
Cultured HUVEC were incubated with different concentrations of Zol (1 μm, 10 μm and 50 μm) for 1, 3, and 6 h. The absorbance at a wavelength 450 nm was measured to represent relative cell proliferation, [n = 6]. There was no significant reduction in proliferation at 1 μm, 10 μm at the 1 (p = 0.18), 3 (p = 0.22), and 6 h (p = 0.221) points compared to control. There was a decrease at 50 μM after 3 and 6 h (p < 0.01).
CRediT authorship contribution statement
Mohamed Awad: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Elizabeth Taylor-Diaz: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Amany Tawfik: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Khaled Hussein: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ahmed Elmansi: Writing – review & editing, Methodology, Investigation. Mahmoud Elashiry: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. Ranya Elsayed: Writing – review & editing, Methodology, Formal analysis, Data curation, Conceptualization. Linah Shahoumi: Writing – review & editing, Methodology, Data curation. James Borke: Writing – review & editing, Visualization, Supervision, Methodology, Investigation, Conceptualization. William Hill: Writing – review & editing, Supervision, Resources, Methodology, Investigation, Conceptualization. Fanglong Dong: Formal analysis.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mohammed Elsalanty reports financial support was provided by National Institutes of Health. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Asagiri M., Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Askari A.T., Unzek S., Popovic Z.B., Goldman C.K., Forudi F., Kiedrowski M., Rovner A., Ellis S.G., Thomas J.D., DiCorleto P.E., Topol E.J., Penn M.S. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Cackowski F.C., Anderson J.L., Patrene K.D., Choksi R.J., Shapiro S.D., Windle J.J., Blair H.C., Roodman G.D. Osteoclasts are important for bone angiogenesis. Blood. 2010;115(1):140–149. doi: 10.1182/blood-2009-08-237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci S., Minielli V., Zambonin G., Cirulli N., Mori G., Serra M., Patella V., Zambonin Zallone A., Grano M. Alendronate reduces adhesion of human osteoclast-like cells to bone and bone protein-coated surfaces. Calcif. Tissue Int. 1998;63(3):230–235. doi: 10.1007/s002239900519. [DOI] [PubMed] [Google Scholar]
- Cun Y., Diao B., Zhang Z., Wang G., Yu J., Ma L., Rao Z. Role of the stromal cell derived factor-1 in the biological functions of endothelial progenitor cells and its underlying mechanisms. Exp. Ther. Med. 2021;21(1):39. doi: 10.3892/etm.2020.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshane J., Chen S., Caballero S., Grochot-Przeczek A., Was H., Li Calzi S., Lach R., Hock T.D., Chen B., Hill-Kapturczak N., Siegal G.P., Dulak J., Jozkowicz A., Grant M.B., Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J. Exp. Med. 2007;204(3):605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou R., Jones E., McGonagle D., Giannoudis P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011;9(1):66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede F., Marconi G.D., Fonticoli L., Pizzicanella J., Merciaro I., Bramanti P., Mazzon E., Trubiani O. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int. J. Mol. Sci. 2020;21(9) doi: 10.3390/ijms21093242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed R., Abraham P., Awad M.E., Kurago Z., Baladhandayutham B., Whitford G.M., Pashley D.H., McKenna C.E., Elsalanty M.E. Removal of matrix-bound zoledronate prevents post-extraction osteonecrosis of the jaw by rescuing osteoclast function. Bone. 2018;110:141–149. doi: 10.1016/j.bone.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed R., Kurago Z., Cutler C.W., Arce R.M., Gerber J., Celis E., Sultan H., Elashiry M., Meghil M., Sun C., Auersvald C.M., Awad M.E., Zeitoun R., Elsayed R., Eldin M.E.M., Isales C., Elsalanty M.E. Role of dendritic cell-mediated immune response in oral homeostasis: a new mechanism of osteonecrosis of the jaw. FASEB J. 2020;34(2):2595–2608. doi: 10.1096/fj.201901819RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed R., El-Awady A., Cutler C., Kurago Z., Elashiry M., Sun C., Bloomquist R., Meghil M.M., Elsalanty M.E. Matrix-Bound Zolzoledronate Enhances the Biofilm Colonization of Hydroxyapatite. 2021;10(11) doi: 10.3390/antibiotics10111380. Effects on Osteonecrosis, Antibiotics (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francescone I.R., Faibish M., Shao R. A matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J. Vis. Exp. 2011;55 doi: 10.3791/3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevarra C.S., Borke J.L., Stevens M.R., Bisch F.C., Zakhary I., Messer R., Gerlach R.C., Elsalanty M.E. Vascular alterations in the Sprague-Dawley rat mandible during intravenous bisphosphonate therapy. J. Oral Implantol. 2015;41(2):e24–e29. doi: 10.1563/AAID-JOI-D-13-00074. [DOI] [PubMed] [Google Scholar]
- Howie R.N., Borke J.L., Kurago Z., Daoudi A., Cray J., Zakhary I.E., Brown T.L., Raley J.N., Tran L.T., Messer R., Medani F., Elsalanty M.E. A model for osteonecrosis of the jaw with Zoledronate treatment following repeated major trauma. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0132520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie R.N., Bhattacharyya M., Salama M.E., Refaey M.E., Isales C., Borke J., Daoudi A., Medani F., Elsalanty M.E. Removal of pamidronate from bone in rats using systemic and local chelation. Arch. Oral Biol. 2015;60(12):1699–1707. doi: 10.1016/j.archoralbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilina O., Friedl P. Mechanisms of collective cell migration at a glance. J. Cell Sci. 2009;122(Pt 18):3203–3208. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- Jin D.K., Shido K., Kopp H.G., Petit I., Shmelkov S.V., Young L.M., Hooper A.T., Amano H., Avecilla S.T., Heissig B., Hattori K., Zhang F., Hicklin D.J., Wu Y., Zhu Z., Dunn A., Salari H., Werb Z., Hackett N.R., Crystal R.G., Lyden D., Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat. Med. 2006;12(5):557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanczler J.M., Oreffo R.O. Osteogenesis and angiogenesis: the potential for engineering bone. Eur. Cell. Mater. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- Khosla S., Shane E. A crisis in the treatment of osteoporosis. J. Bone Miner. Res. 2016;31(8):1485–1487. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- Kollet O., Dar A., Shivtiel S., Kalinkovich A., Lapid K., Sztainberg Y., Tesio M., Samstein R.M., Goichberg P., Spiegel A., Elson A., Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 2006;12(6):657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Mohamed R., Sharma I., Ibrahim A.S., Saleh H., Elsherbiny N.M., Fulzele S., Elmasry K., Smith S.B., Al-Shabrawey M., Tawfik A. Hyperhomocysteinemia alters retinal endothelial cells barrier function and angiogenic potential via activation of oxidative stress. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-09731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.D., Einhorn T.A. Bisphosphonates in orthopaedic surgery, The Journal of bone and joint surgery. American. 2005;87(7):1609–1618. doi: 10.2106/JBJS.D.03032. [DOI] [PubMed] [Google Scholar]
- Pablos J.L., Amara A., Bouloc A., Santiago B., Caruz A., Galindo M., Delaunay T., Virelizier J.L., Arenzana-Seisdedos F. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am. J. Pathol. 1999;155(5):1577–1586. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapoulos S.E., Cremers S.C.L.M. Prolonged bisphosphonate release after treatment in children. N. Engl. J. Med. 2007;356(10):1075–1076. doi: 10.1056/NEJMc062792. [DOI] [PubMed] [Google Scholar]
- Petit I., Jin D., Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28(7):299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak M.Z., Zuba-Surma E., Kucia M., Reca R., Wojakowski W., Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20(11):1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- Redlich K., Smolen J.S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012;11(3):234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- Rodan G.A., Fleisch H.A. Bisphosphonates: mechanisms of action. J. Clin. Investig. 1996;97(12):2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs A.J., Stewart C.A., Sun S., Blazewska K.M., Kashemirov B.A., McKenna C.E., Russell R.G., Rogers M.J., Lundy M.W., Ebetino F.H., Coxon F.P. Influence of bone affinity on the skeletal distribution of fluorescently labeled bisphosphonates in vivo. J. Bone Miner. Res. 2012;27(4):835–847. doi: 10.1002/jbmr.1543. [DOI] [PubMed] [Google Scholar]
- S. Ruggiero, T.B. Dodson, J. Fantasia, R. Goodday, T. Aghaloo, B. Mehrotra, F. O'Ryan, Medication-Related Osteonecrosis of the Jaw—2014 Update, Special Committee on Medication-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons (AAOMS), http://www.aaoms.org/docs/position_papers/mronj_position_paper.pdf?pdf=MRONJ-Position-Paper, 2014. [DOI] [PubMed]
- Russell R.G. Bisphosphonates: the first 40 years. Bone. 2011;49(1):2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Salcedo R., Wasserman K., Young H.A., Grimm M.C., Howard O.M., Anver M.R., Kleinman H.K., Murphy W.J., Oppenheim J.J. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal-derived factor-1alpha. Am. J. Pathol. 1999;154(4):1125–1135. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samra Y.A., Zaidi Y., Rajpurohit P., Raghavan R., Cai L., Kaddour-Djebbar I., Tawfik A. Warburg effect as a novel mechanism for homocysteine-induced features of age-related macular degeneration. Int. J. Mol. Sci. 2023;24(2) doi: 10.3390/ijms24021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnazari M., Chu V., Wronski T.J., Nissenson R.A., Halloran B.P. CXCL12/CXCR4 signaling in the osteoblast regulates the mesenchymal stem cell and osteoclast lineage populations. FASEB J. 2013;27(9):3505–3513. doi: 10.1096/fj.12-225763. [DOI] [PubMed] [Google Scholar]
- Tawfik A., Mohamed R., Kira D., Alhusban S., Al-Shabrawey M. N-methyl-D-aspartate receptor activation, novel mechanism of homocysteine-induced blood-retinal barrier dysfunction. J. Mol. Med. (Berl) 2021;99(1):119–130. doi: 10.1007/s00109-020-02000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B.A., Fricker S.P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 2010;16(11):2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- Walter D.H., Haendeler J., Reinhold J., Rochwalsky U., Seeger F., Honold J., Hoffmann J., Urbich C., Lehmann R., Arenzana-Seisdesdos F., Aicher A., Heeschen C., Fichtlscherer S., Zeiher A.M., Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ. Res. 2005;97(11):1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- Weiss H.M., Pfaar U., Schweitzer A., Wiegand H., Skerjanec A., Schran H. Biodistribution and plasma protein binding of zoledronic acid. Drug Metab. Dispos. 2008;36(10):2043–2049. doi: 10.1124/dmd.108.021071. [DOI] [PubMed] [Google Scholar]
- Xu J., Chen Y., Liu Y., Zhang J., Kang Q., Ho K., Chai Y., Li G. Effect of SDF-1/Cxcr4 signaling antagonist AMD3100 on bone mineralization in distraction osteogenesis. Calcif. Tissue Int. 2017;100(6):641–652. doi: 10.1007/s00223-017-0249-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J., Kusano K.F., Masuo O., Kawamoto A., Silver M., Murasawa S., Bosch-Marce M., Masuda H., Losordo D.W., Isner J.M., Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107(9):1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Yang F., Xue F., Guan J., Zhang Z., Yin J., Kang Q. Stromal-cell-derived factor (SDF) 1-alpha overexpression promotes bone regeneration by osteogenesis and angiogenesis in osteonecrosis of the femoral head. Cell. Physiol. Biochem. 2018;46(6):2561–2575. doi: 10.1159/000489684. [DOI] [PubMed] [Google Scholar]
- Yu X., Huang Y., Collin-Osdoby P., Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J. Bone Miner. Res. 2003;18(8):1404–1418. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- Zamani F., Zare Shahneh F., Aghebati-Maleki L., Baradaran B. Induction of CD14 expression and differentiation to monocytes or mature macrophages in Promyelocytic cell lines: new approach, Adv. Pharm. Bull. 2013;3(2):329–332. doi: 10.5681/apb.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Fu G., Dai T., Huang H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2007;50(3):274–280. doi: 10.1097/FJC.0b013e318093ec8f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cultured HUVEC were incubated with different concentrations of Zol (1 μm, 10 μm and 50 μm) for 1, 3, and 6 h. The absorbance at a wavelength 450 nm was measured to represent relative cell proliferation, [n = 6]. There was no significant reduction in proliferation at 1 μm, 10 μm at the 1 (p = 0.18), 3 (p = 0.22), and 6 h (p = 0.221) points compared to control. There was a decrease at 50 μM after 3 and 6 h (p < 0.01).
Data Availability Statement
Data will be made available on request.