Abstract
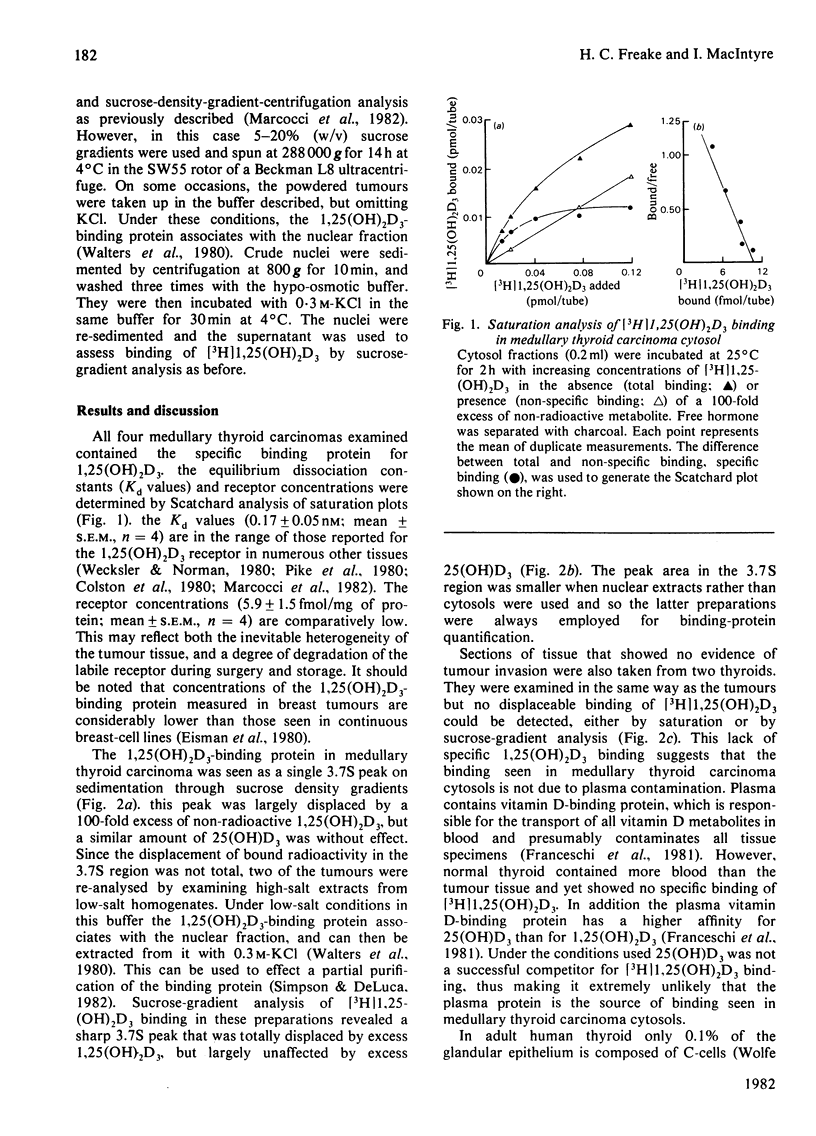
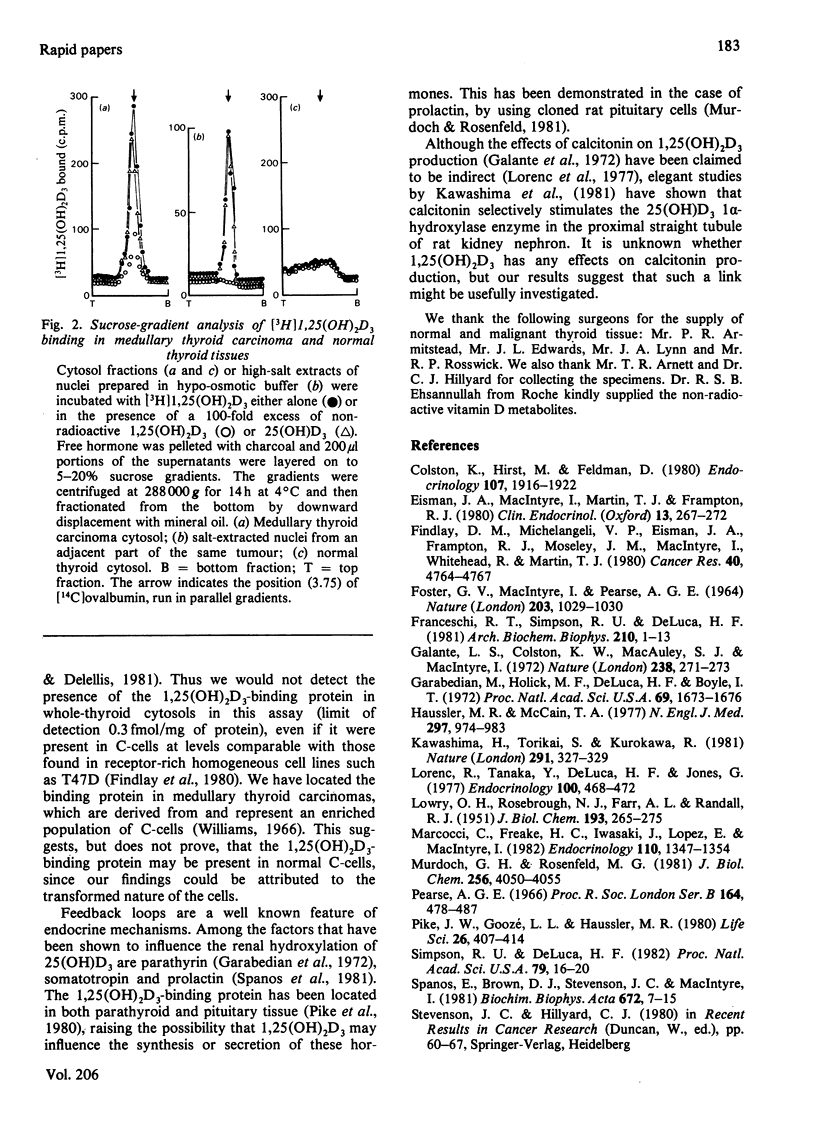
A specific 1,25-dihydroxycholecalciferol-binding protein has been detected in high-salt cytosols prepared from human medullary thyroid carcinomas. The binding protein had the same equilibrium dissociation constant (Kd = 0.17 +/- 0.05 nM; n = 4) and sedimentation coefficient on sucrose gradients (3.7S) as than seen in established vitamin D target tissues. This protein was not detected in normal thyroid cytosols, which may reflect the low proportion of C-cells within the gland.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colston K., Hirt M., Feldman D. Organ distribution of the cytoplasmic 1,25-dihydroxycholecalciferol receptor in various mouse tissues. Endocrinology. 1980 Dec;107(6):1916–1922. doi: 10.1210/endo-107-6-1916. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Macintyre I., Martin T. J., Frampton R. J., King R. J. Normal and malignant breast tissue is a target organ for 1,25-(0H)2 vitamin D3. Clin Endocrinol (Oxf) 1980 Sep;13(3):267–272. doi: 10.1111/j.1365-2265.1980.tb01053.x. [DOI] [PubMed] [Google Scholar]
- FOSTER G. V., MACINTYRE I., PEARSE A. G. CALCITONIN PRODUCTION AND THE MITOCHONDRION-RICH CELLS OF THE DOG THYROID. Nature. 1964 Sep 5;203:1029–1030. doi: 10.1038/2031029a0. [DOI] [PubMed] [Google Scholar]
- Findlay D. M., Michelangeli V. P., Eisman J. A., Frampton R. J., Moseley J. M., MacIntyre I., Whitehead R., Martin T. J. Calcitonin and 1,25-dihydroxyvitamin D3 receptors in human breast cancer cell lines. Cancer Res. 1980 Dec;40(12):4764–4767. [PubMed] [Google Scholar]
- Galante L., Colston K. W., MacAuley S. J., MacIntyre I. Effect of calcitonin on vitamin D metabolism. Nature. 1972 Aug 4;238(5362):271–273. doi: 10.1038/238271a0. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R., McCain T. A. Basic and clinical concepts related to vitamin D metabolism and action (first of two parts). N Engl J Med. 1977 Nov 3;297(18):974–983. doi: 10.1056/NEJM197711032971804. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in proximal straight tubule of rat kidney. Nature. 1981 May 28;291(5813):327–329. doi: 10.1038/291327a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorenc R., Tanaka Y., DeLuca H. F., Jones G. Lack of effect of calcitonin on the regulation of vitamin D metabolism in the rat. Endocrinology. 1977 Feb;100(2):468–472. doi: 10.1210/endo-100-2-468. [DOI] [PubMed] [Google Scholar]
- Marcocci C., Freake H. C., Iwasaki J., Lopez E., MacIntyre I. Demonstration and organ distribution of the 1,25-dihydroxyvitamin D3-binding protein in fish (A. anguilla). Endocrinology. 1982 Apr;110(4):1347–1354. doi: 10.1210/endo-110-4-1347. [DOI] [PubMed] [Google Scholar]
- Murdoch G. H., Rosenfeld M. G. Regulation of pituitary function and prolactin production in the GH4 cell line by vitamin D. J Biol Chem. 1981 Apr 25;256(8):4050–4053. [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry of the thyroid C cells and their relationship to calcitonin. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):478–487. doi: 10.1098/rspb.1966.0044. [DOI] [PubMed] [Google Scholar]
- Pike J. W., Goozé L. L., Haussler M. R. Biochemical evidence for 1,25-dihydroxyvitamin D receptor macromolecules in parathyroid, pancreatic, pituitary, and placental tissues. Life Sci. 1980 Feb 4;26(5):407–414. doi: 10.1016/0024-3205(80)90158-7. [DOI] [PubMed] [Google Scholar]
- Simpson R. U., DeLuca H. F. Purification of chicken intestinal receptor for 1 alpha, 25-dihydroxyvitamin D3 to apparent homogeneity. Proc Natl Acad Sci U S A. 1982 Jan;79(1):16–20. doi: 10.1073/pnas.79.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos E., Brown D. J., Stevenson J. C., MacIntyre I. Stimulation of 1,25-dihydroxycholecalciferol production by prolactin and related peptides in intact renal cell preparations in vitro. Biochim Biophys Acta. 1981 Jan 7;672(1):7–15. doi: 10.1016/0304-4165(81)90273-7. [DOI] [PubMed] [Google Scholar]
- Stevenson J. C., Hillyard C. J. Thyroid cancer: tumour markers. Recent Results Cancer Res. 1980;73:60–67. [PubMed] [Google Scholar]
- Walters M. R., Hunziker W., Norman A. W. Unoccupied 1,25-dihydroxyvitamin D3 receptors. Nuclear/cytosol ratio depends on ionic strength. J Biol Chem. 1980 Jul 25;255(14):6799–6805. [PubMed] [Google Scholar]
- Wecksler W. R., Norman A. W. Biochemical properties of 1 alpha, 25-dihydroxyvitamin D receptors. J Steroid Biochem. 1980 Aug;13(8):977–989. doi: 10.1016/0022-4731(80)90173-9. [DOI] [PubMed] [Google Scholar]
- Williams E. D. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol. 1966 Mar;19(2):114–118. doi: 10.1136/jcp.19.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]


