Abstract
Background
Aeroallergen testing can improve precision care for persistent asthma. How testing benefits diverse populations of adults with asthma and the importance of the aeroallergen sensitization and test modality used remain poorly understood.
Objective
We evaluated whether aeroallergen testing was associated with a reduction in oral corticosteroid (OCS) bursts.
Methods
We used electronic health record data to conduct a retrospective cohort study of adults with asthma who were prescribed an inhaled corticosteroid and had an allergy/immunology visit in a large health system between January 1, 2017, and June 30, 2022. We used negative binomial regression models to evaluate whether testing was associated with fewer OCS bursts in the 12-month period after an initial visit among all patients and those without chronic obstructive pulmonary disease (COPD) and smoking histories. We then repeated these analyses while considering effects of sensitization to aeroallergen categories and whether the testing was via skin prick or serum-specific IgE.
Results
A total of 684 (48.4%) of 1,412 patients underwent testing. Testing was not associated with fewer bursts overall (incidence rate ratio [IRR] = 0.84 vs no testing, P = .08), but it was among never smokers without COPD (461 of 927 tested, IRR = 0.69, P = .005). Among never smokers without COPD, sensitization to 5-7 aeroallergen categories (IRR = 0.57 vs no test, P = .003) and receipt of skin prick tests (IRR = 0.58 vs no test, P < .0005) were associated with fewer bursts.
Conclusion
Aeroallergen testing was associated with reduced OCS bursts among adults with asthma who were never smokers without COPD. This association varied according to aeroallergen sensitization and test modality used.
Key words: Asthma, aeroallergen testing, asthma exacerbations, electronic health record, epidemiology
Asthma is a common chronic disease in adults and a major public health burden in the United States.1, 2, 3 A key component of asthma care is to reduce symptoms and exacerbations by addressing modifiable risk factors, including aeroallergen exposure in sensitized patients.2,4 Pollens, dust mite, cat, dog, and molds are common allergens and important asthma triggers.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Addressing allergic asthma triggers is beneficial in adults with asthma; most are sensitized to aeroallergens, and aeroallergen exposure is nearly universal in US homes.20, 21, 22, 23 In studies of adults with asthma, aeroallergen testing with allergen avoidance education improved patients’ awareness of perceived asthma triggers and lung function24 and complemented asthma self-management.25 National Heart, Lung, and Blood Institute–led online focus groups of people with asthma that explored patients’ values and priorities related to their asthma found that most patients took steps to mitigate indoor allergen exposure.26 Thus, testing aligns with patients’ strategies to reduce asthma symptoms.
The US clinical guidelines recommend aeroallergen testing for patients with persistent asthma, which is defined as asthma requiring step 2 or higher treatment to maintain control; and the preferred controller treatment at step 2 is an inhaled corticosteroid (ICS)-containing treatment.4,26 However, allergen mitigation may be difficult or costly, and the benefit of testing large numbers of adults with asthma in a real-life setting has not been well studied. In prior work, we found that among 1,789 adults with asthma who established outpatient care in a large health system, who were prescribed an ICS, and who underwent aeroallergen testing, receipt of oral corticosteroid (OCS) bursts was reduced in the 12-month period after testing compared with before.20 Consistent with the clinical focus of providers, testing was more often performed in the context of asthma subspecialty clinics, specifically allergy/immunology and pulmonary, rather than in primary care (odds ratios of 91.3 and 7.1 vs primary care, respectively). However, that study was unable to determine whether improved outcomes were due to allergy testing and testing-based management versus other interventions that may have occurred during specialist visits. Moreover, although many patients with asthma have smoking histories and/or comorbid chronic obstructive pulmonary disease (COPD), 2 studies that evaluated the benefits of aeroallergen testing limited or excluded these patients.24,25 Furthermore, although patients with allergic sensitization to aeroallergens may be expected to benefit the most from testing, this is not well studied. Finally, prior investigations have not evaluated the relative benefits of testing via skin prick tests (SPTs) or serum-specific IgE tests among adults with asthma.
In this study, we sought to determine, among adults with asthma who had an allergy/immunology visit, whether undergoing guideline-concordant aeroallergen testing was associated with fewer OCS bursts after the visit compared with patients who did not undergo testing. We then considered whether the association of testing with OCS bursts differed for patients who did not have COPD and who were never smokers; and whether the association varied according to aeroallergen sensitization or the test modality.
Methods
Study design
We conducted a retrospective cohort study of adults with asthma using electronic health record (EHR) data from Penn Medicine, a large, diverse health system that serves the greater Philadelphia area, from encounters dated January 1, 2015, to June 30, 2023. The University of Pennsylvania institutional review board approved our study.
Study population
We obtained patient-level data and clinical notes for adults (ie, age ≥18 years) who had at least one encounter with an International Classification of Diseases, Tenth Revision (ICD-10), code for asthma (J45∗) in any of their records; received an ICS prescription from January 1, 2016, to June 30, 2023; and had 2 or more outpatient visits at Penn’s primary allergy/immunology clinic from January 1, 2017, to June 30, 2023, for which the index visit occurred on or before June 30, 2022. We chose a 2-visit threshold to ensure that all patients had established allergy/immunology care. Patient-level data included age at the index visit (categorized into 5 levels); sex; race (using EHR categories); ethnicity (ie, Hispanic or Latino status); insurance (categorized as commercial, Medicaid, or Medicare); body mass index (BMI in kg/m2, categorized into the 5 levels used by the Centers for Disease Control and Prevention27); smoking category (never, former, or current); and chronic rhinitis (defined as having any ICD-10 code of J30∗ or J31∗).
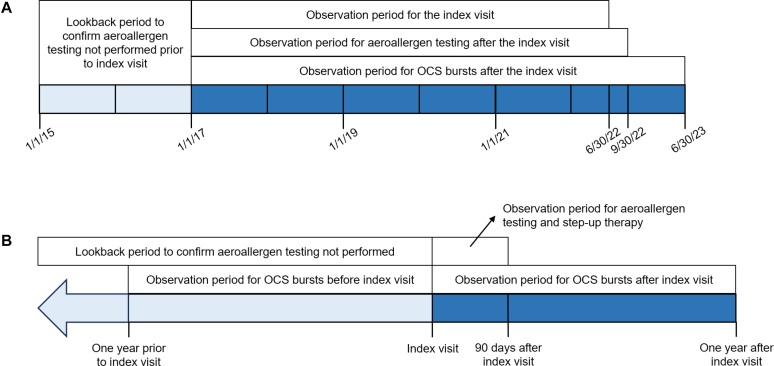
Timeline of observations included
Fig 1 displays timelines of data considered. We chose January 1, 2017, as the date of the earliest possible index visit because in our health system, emergency department (ED) and hospitalization encounter data from some hospitals in 2015 are missing from the data warehouse, whereas encounter data from January 1, 2016, onward is complete.
Fig 1.
A, Overall study period. Shown are observation periods for the index visit, aeroallergen testing after the index visit, counting of OCS bursts after the index visit, and additional lookback period to confirm that aeroallergen testing was not performed. B, Individual-level study period. This timeline is centered on the index visit date for each patient. Shown are the 90-day observation period for aeroallergen testing and step-up therapy after the index visit, the 12-month observation periods for OCS bursts before and after the index visit, and the lookback period to confirm that aeroallergen testing was not performed.
Asthma exacerbation measures
We selected 3 variables to represent asthma exacerbations during the 12-month period before the index visit: OCS bursts, ED visits, and hospitalizations. OCS bursts were categorized into 4 groups: counts of 0, 1, 2-3, and 4 or more; and ED visits and hospitalizations into 2 groups each: counts of 0, and 1 or more. The outcome of OCS bursts in the 12-month period after the index visit was modeled as count data. Definitions for OCS bursts, ED visits, and hospitalizations are described in the Methods section of the Online Repository available at www.jaci-global.org, and lists of ICD-10 codes and chief complaints that were used to deem ED visits and hospitalizations as asthma related are provided in Table E1, also available in the Online Repository.
Aeroallergen testing data
We defined aeroallergen testing as receipt of any SPT or serum-specific IgE test to tree, grass, weed, dust mite, cat, dog, or mold that was recorded in the EHR as structured data, as previously described.20 More details are available in the Methods section of the Online Repository. We chose a 90-day threshold for testing after the index visit so it could occur within the 3-month follow-up interval that is consistent with guideline care.2,4 We considered 2 definitions for testing after the index visit. The first was a binary variable of no testing or any testing. The second was a 3-level test modality variable of (1) no testing, (2) receipt of SPTs (ie, with or without serum-specific IgE tests), or (3) receipt of only serum-specific IgE tests.
Aeroallergen sensitization data
We defined sensitization to aeroallergens as previously described.20 We categorized test results into 7 categories of tree, grass, weed, dust mite, cat, dog, and mold,20 and considered results for patients who had results in all 7 categories. We then created a 3-level aeroallergen sensitization variable of no testing, sensitization to 1-4 categories, or sensitization to 5-7 categories.
Inhaler data
We identified inhaler prescription data during the 12-month period before the index visit and categorized these into 3 groups: (1) no ICS-containing prescriptions, (2) ICS prescriptions only, (3) any ICS/long-acting β-agonist (LABA) prescriptions, and (4) any ICS/LABA plus long-acting muscarinic antagonist (LAMA) prescriptions. We also identified inhaler prescription data in the 90-day period after the index visit to determine whether step-up therapy occurred. More details are provided in the Methods section in the Online Repository.
COPD
We defined COPD as having any ICD-10 code of J41∗, J42∗, J43∗, or J44∗; and prescription of any of LAMA, LABA, or LABA/LAMA inhaler formulations from January 1, 2016, to June 30, 2023. Lists of these inhalers are shown in Table E2 in the Online Repository available at www.jaci-global.org.
Documentation of likely reasons that providers did not perform testing, performed testing, and performed serum-specific IgE testing
An allergy/immunology specialist performed EHR chart review for 50 patients who were categorized as not undergoing testing to determine the likely reason that a test was not performed. A second reviewer who was an allergy/immunology fellow reviewed the same 50 charts, and for any disagreements, the 2 reviewers reached consensus. In addition, the specialist performed chart review for 50 patients with aeroallergen testing to identify the likely reason that it was performed; and for 50 patients with serum-specific IgE testing to identify the likely reason that it was performed (ie, instead of SPTs). Details are provided in the Methods section of the Online Repository.
Statistical analysis
Stata 16.1 was used to perform statistical analysis. We evaluated bivariate associations of patient demographic factors (eg, age, sex, race, and ethnicity), insurance, BMI, smoking, COPD, chronic rhinitis, inhaler category, and exacerbation measures in the 12-month period before the index visit, and receipt of step-up therapy after the index visit with testing versus no testing by Pearson chi-square tests. For the primary analysis, we created a multivariable negative binomial regression model with OCS burst count in the 12-month period after the index visit as the outcome, the binary testing variable as the exposure, and the same variables as in the bivariate associations, except for step-up therapy, included as covariates. We assessed collinearity among the independent variables by computing their variance inflation factors. We repeated the model creation in 3 secondary analyses after (1) restricting the dataset to patients whose index visits and 90-day postvisit periods occurred earlier than Philadelphia’s coronavirus disease 2019 (COVID-19) lockdown date of March 17, 2020; (2) excluding patients with COPD; and (3) excluding both patients with COPD and current or former smokers.
Among patients who either did not undergo testing, or who underwent testing to 7 aeroallergen categories and had sensitization to at least one category, we repeated the primary analysis and each of the 3 secondary analyses using the 3-level aeroallergen sensitization variable.
We then evaluated the same bivariate associations of patient factors with receipt of any SPTs versus only serum-specific IgE tests by Pearson chi-square tests. Finally, we repeated each of the 4 primary and secondary analyses using the 3-level test modality variable.
Results
Patient characteristics
Of 1,412 adults with asthma who appeared to be eligible for testing according to guidelines, 684 (48.4%) underwent testing within 90 days of their index visits, and 728 (51.6%) did not undergo testing. A total of 923 (65.4%) of 1,412 also had an internal medicine or family medicine visit, and 453 (32.1%) of 1,412 also had a pulmonary visit. Patients who underwent testing were more likely to be younger than 45 years (P < .001), Black (P < .001), Hispanic or Latino (P = .02); to not have Medicare insurance (P < .001); and to have chronic rhinitis (P < .001) (Table I). Patients who underwent testing were more likely to have received step-up therapy in the 90-day period after the index visit (P = .03).
Table I.
Patient characteristics related to aeroallergen testing among 1,412 adults with asthma
| Characteristic | Overall (n = 1,412) | No testing (n = 728) | Testing (n = 684) | P value |
|---|---|---|---|---|
| Age | <.001 | |||
| 18-34 years | 444 (31.4) | 183 (25.1) | 261 (38.2) | |
| 35-44 years | 257 (18.2) | 114 (15.7) | 143 (20.9) | |
| 45-54 years | 247 (17.5) | 144 (19.8) | 103 (15.1) | |
| 55-64 years | 226 (16.0) | 135 (18.5) | 91 (13.3) | |
| 65+ years | 238 (16.9) | 152 (20.9) | 86 (12.6) | |
| Sex | .35 | |||
| Male | 308 (21.8) | 166 (22.8) | 142 (20.7) | |
| Female | 1,104 (78.2) | 562 (77.2) | 542 (79.2) | |
| Race | <.001 | |||
| American Indian or Alaskan Native | 8 (0.6) | 0 (0.0) | 8 (1.2) | |
| Asian | 65 (4.6) | 33 (4.5) | 32 (4.7) | |
| Black | 517 (36.6) | 232 (31.9) | 285 (41.7) | |
| Native Hawaiian or other Pacific Islander | 1 (0.1) | 0 (0.0) | 1 (0.1) | |
| White | 821 (58.1) | 463 (63.6) | 358 (52.3) | |
| Ethnicity | .02 | |||
| Not Hispanic or Latino | 1,377 (97.5) | 717 (98.5) | 660 (96.5) | |
| Hispanic or Latino | 35 (2.5) | 11 (1.5) | 24 (3.5) | |
| Insurance | <.001 | |||
| Commercial | 716 (50.7) | 358 (49.2) | 358 (52.3) | |
| Medicaid | 375 (26.6) | 170 (23.4) | 205 (30.0) | |
| Medicare | 321 (22.7) | 200 (27.5) | 121 (17.7) | |
| BMI | .84 | |||
| Normal | 407 (28.8) | 220 (30.2) | 187 (27.3) | |
| Overweight | 413 (29.2) | 207 (28.4) | 206 (30.1) | |
| Class I obesity | 265 (18.8) | 136 (18.7) | 129 (18.8) | |
| Class II obesity | 158 (11.2) | 80 (11.0) | 78 (11.4) | |
| Class III obesity | 169 (12.0) | 85 (11.7) | 84 (12.3) | |
| Smoking category | .23 | |||
| Never | 965 (68.3) | 483 (66.3) | 482 (70.5) | |
| Former | 322 (22.8) | 178 (24.5) | 144 (21.0) | |
| Current | 125 (8.9) | 67 (9.2) | 58 (8.5) | |
| COPD | 111 (7.9) | 57 (7.8) | 54 (7.9) | .96 |
| Chronic rhinitis | 1,328 (94.1) | 652 (89.6) | 676 (98.8) | <.001 |
| Inhaler category in the 12-month period before the index visit | .14 | |||
| No ICS | 724 (51.3) | 352 (48.4) | 372 (54.4) | |
| ICS only | 195 (13.8) | 110 (15.1) | 85 (12.4) | |
| ICS/LABA | 412 (29.2) | 223 (30.6) | 189 (27.6) | |
| ICS/LABA and LAMA | 81 (5.7) | 43 (5.9) | 38 (5.6) | |
| OCS bursts in the 12-month period before the index visit | .70 | |||
| 0 | 930 (65.9) | 484 (66.5) | 446 (65.2) | |
| 1 | 282 (20.0) | 138 (19.0) | 144 (21.1) | |
| 2-3 | 149 (10.6) | 77 (10.6) | 72 (10.5) | |
| 4 or more | 51 (3.6) | 29 (4.0) | 22 (3.2) | |
| Asthma ED visit in the 12-month period before the index visit | 36 (2.5) | 16 (2.2) | 20 (2.9) | .39 |
| Asthma hospitalization in the 12-month period before the index visit | 21 (1.5) | 10 (1.4) | 11 (1.6) | .72 |
| Step-up therapy after the index visit | 491 (34.8) | 234 (32.1) | 257 (37.6) | .03 |
Data are presented as nos. (%). Patients who did not receive any testing (n = 728) were compared with patients who received any SPTs or serum-specific IgE tests on or within 90 days of index allergy/immunology visit (n = 684) by chi-square test.
Documentation of likely reasons that providers did not perform testing, did perform testing, and performed serum-specific IgE testing
The chart reviewers had 84% agreement in identifying the likely reasons that testing was not performed after initial review. After conferring on 8 (16%) of 50 charts that the reviewers disagreed on, consensus was achieved for all of them; the results are shown in Table II. Nineteen patients (38%) had prior test results in their charts, and most (n = 10) underwent testing 2 to 5 years before the index visit. Tests were ordered or planned but not completed for 10 patients (20%). Among 50 patients who underwent testing, the likely reason for testing was determined to be asthma and rhinitis for 26 patients (52%), whereas asthma alone was the likely reason for 5 patients (10%) (Table III). Among 50 patients who received serum-specific IgE tests, no likely reason for why it was performed could be identified for 24 patients (48%); the provider was unable to perform SPTs (eg, due to current antihistamine use) was the reason for 17 patients (34%); and the test-related encounter was a telemedicine visit was the reason for 9 patients (18%) (Table IV).
Table II.
Seven likely reasons why aeroallergen testing was not performed on or before the index visit
| Likely reason | No. (%) |
|---|---|
|
19 (38) |
| Testing between 2 and 5 years of the index visit | 10 (20) |
| Testing >5 years before the index visit | 4 (8) |
| Year of testing not specified | 4 (8) |
| Testing within 2 years of the index visit | 2 (4) |
|
10 (20) |
|
9 (18) |
|
6 (12) |
|
4 (8) |
|
1 (2) |
|
1 (2) |
Reasons are according to chart review of 50 patients who did not have codified aeroallergen testing according to structured EHR data. Reasons are ordered by decreasing percentage.
Table III.
Three likely reasons why aeroallergen testing was performed
| Likely reason | No. (%) |
|---|---|
|
26 (52) |
|
19 (38) |
|
5 (10) |
Reasons are according to chart review of 50 patients who had codified aeroallergen testing according to structured EHR data. Reasons are ordered by decreasing percentage.
Table IV.
Three likely reasons why serum-specific IgE tests were provided instead of SPTs
| Reason | No. (%) |
|---|---|
|
24 (48) |
|
17 (34) |
|
9 (18) |
Reasons are according to chart review of 50 patients with serum-specific IgE test. Two reasons were identified for approximately half of patients, while no clear reason was identified for the remainder.
For example, because of current antihistamine receipt.
Testing was not associated with fewer OCS bursts after the index visit among all patients
According to the primary analysis of 1,412 patients, testing was not associated with fewer OCS bursts (incidence rate ratio [IRR] = 0.84 vs no testing, P = .08) (Table V). The IRRs for all independent variables are shown in Table E4 in the Online Repository available at www.jaci-global.org. The variance inflation factors were less than 1.7, indicating minimal collinearity (see Table E5 in the Online Repository).
Table V.
Effect of aeroallergen testing on OCS burst count in 12-month period after the index allergy/immunology visit
| Characteristic | Variable | Value |
|---|---|---|
| Overall cohort (N = 1,412) | IRR (95% CI) | 0.84 (0.70, 1.02) |
| P value | .08 | |
| Index visit before COVID-19 lockdown (n = 997) | IRR (95% CI) | 0.78 (0.62, 0.98) |
| P value | .04 | |
| Overall cohort, no COPD (n = 1,301) | IRR (95% CI) | 0.85 (0.69, 1.05) |
| P value | .13 | |
| Overall cohort, no COPD and never smokers (n = 927) | IRR (95% CI) | 0.69 (0.54, 0.90) |
| P value | .005 |
IRRs were adjusted for all independent variables (age, sex, race, ethnicity, insurance status, BMI, smoking category, COPD, chronic rhinitis, inhaler category, OCS bursts, asthma ED visits, and asthma hospitalizations in 12-month period before the index visit) in multivariable models using data from full cohort of 1,412 adults with asthma who established outpatient allergy/immunology care. This model was repeated for subgroups of 1,412 patients: 997 whose index visits and postvisit 90-day periods occurred before COVID-19 lockdown (same covariates as overall cohort); 1,176 who did not have COPD (COPD was not included as covariate); and 860 who did not have COPD and were never smokers (COPD and smoking category were not included as covariates).
Influence of COVID-19 pandemic
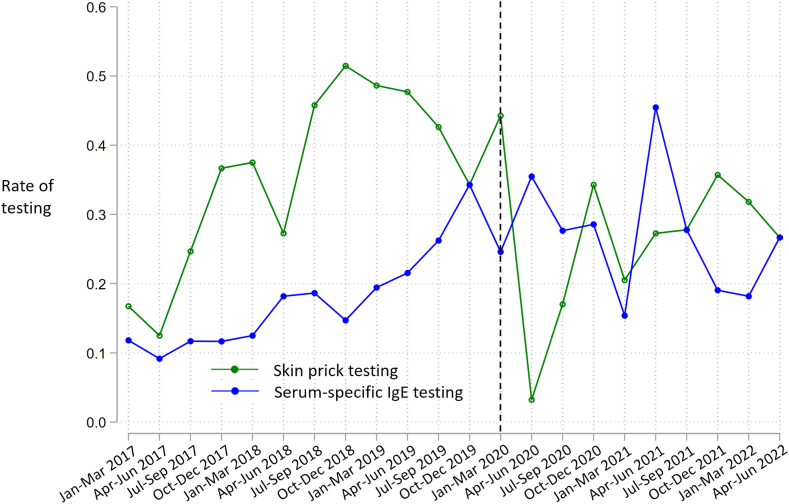
Fig 2 shows the rates of testing via SPTs and serum-specific IgE tests over time. From January 2017 to April 2020, testing via SPTs was performed at a higher rate except for October-December 2019. After the COVID-19 lockdown date, the rate of testing via SPTs declined while the rate via serum-specific IgE tests increased. Thereafter, the rate of testing via SPTs increased. A total of 997 (70.6%) of 1,412 index visits and 90-day postvisit periods occurred before the lockdown date, and for these patients, testing was associated with fewer bursts in the multivariable analysis (IRR = 0.78, P = .04) (Table V).
Fig 2.
Three-month rates of testing via SPTs and serum-specific IgE tests for 668 patients who had an index visit between January 1, 2017, and June 30, 2022, and underwent testing at the visit or up to 90 days after. Rates of testing represent ratios of the number of patients tested to the number of index visits within each 3-month period. The Black dotted line represents 3-month period during which COVID-19 lockdown in Philadelphia occurred.
Testing was associated with fewer OCS bursts among patients who did not have COPD and were never smokers
Among 1,301 (92.1%) of 1,412 patients without COPD, 630 (48.4%) underwent testing, and testing was not associated with fewer OCS bursts (IRR = 0.85, P = .13) (Table V). Among 927 (65.7%) of 1,412 never smokers without COPD, 461 (49.7%) underwent testing, and testing was associated with fewer bursts (IRR = 0.69, P = .005) (Table V).
Sensitization to 5-7 aeroallergen categories was associated with fewer OCS bursts after the index visit among patients who did not have COPD and were never smokers
Among 1,255 patients who either did not undergo testing or who underwent testing to 7 aeroallergen categories and had sensitization to at least one, 292 (23.3%) had sensitization to 1-4 categories and 235 (18.7%) to 5-7 categories. Compared with patients who did not undergo testing, there were no associations of sensitization to 1-4 categories (IRR = 0.85, P = .21) or 5-7 categories (IRR = 0.79, P = .11) with fewer OCS bursts. Results of the primary and 3 secondary analyses are shown in Table VI. Among 830 never smokers without COPD, sensitization to 5-7 categories was associated with fewer bursts (IRR = 0.57, P = .003), whereas sensitization to 1-4 categories was not (IRR = 0.83, P = .25).
Table VI.
Effect of aeroallergen testing on OCS burst count in 12-month period after the index allergy/immunology visit while considering sensitization to 1-4 or 5-7 aeroallergen categories among categories of tree, grass, weed, dust mite, cat, dog, and mold
| Characteristic | Variable | Value |
|---|---|---|
| Overall cohort (N = 1,255) | Sensitization to 1-4 categories | |
| IRR (95% CI) | 0.85 (0.67, 1.09) | |
| P value | .21 | |
| Sensitization to 5-7 categories | ||
| IRR (95% CI) | 0.79 (0.59, 1.05) | |
| P value | .11 | |
| Index visit before COVID-19 lockdown (n = 887) | Sensitization to 1-4 categories | |
| IRR (95% CI) | 0.83 (0.62, 1.13) | |
| P value | .24 | |
| Sensitization to 5-7 categories | ||
| IRR (95% CI) | 0.76 (0.54, 1.06) | |
| P value | .11 | |
| Overall cohort, no COPD (n = 1,162) | Sensitization to 1-4 categories | |
| IRR (95% CI) | 0.89 (0.68, 1.16) | |
| P value | .37 | |
| Sensitization to 5-7 categories | ||
| IRR (95% CI) | 0.79 (0.58, 1.08) | |
| P value | .14 | |
| Overall cohort, no COPD and never smokers (n = 830) | Sensitization to 1-4 categories | |
| IRR (95% CI) | 0.83 (0.61, 1.14) | |
| P value | .25 | |
| Sensitization to 5-7 categories | ||
| IRR (95% CI) | 0.57 (0.39, 0.82) | |
| P value | .003 |
IRRs were adjusted for all independent variables (age, sex, race, ethnicity, insurance status, BMI, smoking category, COPD, chronic rhinitis, inhaler category, OCS bursts, asthma ED visits, and asthma hospitalizations in the 12-month period before the index visit) in multivariable models using data from 1,255 patients among 1,412 in overall cohort who either did not undergo testing or who underwent testing to 7 aeroallergen categories and had sensitization to at least one. This model was repeated for subgroups of 1,255 patients: 887 whose index visits and postvisit 90-day periods occurred before the COVID-19 lockdown (same covariates as overall cohort); 1,162 who did not have COPD (COPD was not included as a covariate); and 830 who did not have COPD and were never smokers (COPD and smoking category were not included as covariates).
Receipt of SPTs was associated with fewer OCS bursts after the index visit
Among 684 patients who underwent testing, 430 (62.9%) received SPTs and 254 (37.1%) received only serum-specific IgE tests. Comparing these 2 subgroups, patients who received SPTs were more likely to be under age 45 (P < .001), to have commercial insurance (P < .001), to have normal or overweight BMI (P < .001), to not have COPD (P = .001), and to not be receiving ICS (P = .006) or to have received OCS bursts (P = .02) in the year before the index visit (see Table E3 in the Online Repository available at www.jaci-global.org). According to the multivariable analyses that used the 3-level test modality variable, the receipt of SPTs was associated with fewer bursts (IRR ≤ 0.78 vs no testing, P ≤ .03 for SPTs vs no testing in each of the primary and secondary analyses), whereas the receipt of only serum-specific IgE tests was not (P > .05 in the primary and secondary analyses) (Table VII).
Table VII.
Effect of the receipt of SPTs and serum-specific IgE tests on OCS burst count in 12-month period after the index allergy/immunology visit
| Characteristic | Variable | Value |
|---|---|---|
| Overall cohort (N = 1,412) | SPTs | |
| IRR (95% CI) | 0.78 (0.62, 0.98) | |
| P value | .03 | |
| Only serum-specific IgE tests | ||
| IRR (95% CI) | 0.93 (0.72, 1.19) | |
| P value | .55 | |
| Index visit before COVID-19 lockdown (n = 997) | SPTs | |
| IRR (95% CI) | 0.74 (0.57, 0.96) | |
| P value | .02 | |
| Only serum-specific IgE tests | ||
| IRR (95% CI) | 0.86 (0.63, 1.18) | |
| P value | .36 | |
| Overall cohort, no COPD (n = 1,301) | SPTs | |
| IRR (95% CI) | 0.76 (0.60, 0.97) | |
| P value | .03 | |
| Only serum-specific IgE tests | ||
| IRR (95% CI) | 0.99 (0.76, 1.30) | |
| P value | .96 | |
| Overall cohort, no COPD and never smokers (n = 927) | SPTs | |
| IRR (95% CI) | 0.58 (0.43, 0.78) | |
| P value | <.0005 | |
| Only serum-specific IgE tests | ||
| IRR (95% CI) | 0.90 (0.65, 1.25) | |
| P value | .54 |
IRRs were adjusted for all independent variables (age, sex, race, ethnicity, insurance status, BMI, smoking category, COPD, chronic rhinitis, inhaler category, OCS bursts, asthma ED visits, and asthma hospitalizations in 12-month period before the index visit) in multivariable models using data from full cohort of 1,412 adults with asthma who established outpatient allergy/immunology care. This model was repeated for subgroups of 1,412 patients: 997 whose index visits and postvisit 90-day periods occurred before COVID-19 lockdown (same covariates as overall cohort); 1,301 who did not have COPD (COPD was not included as a covariate); and 927 who did not have COPD and were never smokers (COPD and smoking category were not included as covariates).
Discussion
In this study of adults with asthma in a large health system who established care in an allergy/immunology clinic and for whom aeroallergen testing appeared to be indicated according to the guidelines, aeroallergen testing was associated with fewer OCS bursts in the 12-month period after the index visit among never smokers without COPD. Within this subgroup, sensitization to 5-7 aeroallergen categories, but not 1-4 categories, was associated with fewer bursts. These findings did not extend to all asthma patients. We also found that testing via SPTs but not serum-specific IgE tests was associated with fewer bursts for the full cohort and in each secondary analysis.
Thirty-eight percent of patients categorized as not having undergone testing had prior test results available in the EHR. While these patients’ testing status may be considered misclassified, an alternative view is that some previously tested patients could benefit from repeat testing. Notably, 20% of patients who underwent chart review had tests ordered or planned but not performed. Insofar as these patients were nonadherent to providers’ recommendations, it is possible that they were less adherent overall (eg, to inhaler therapy) than patients who underwent testing after the index visit. Therefore, differences in adherence between the tested and untested patients, irrespective of the testing itself, may have contributed to differences in OCS bursts after the index visit. Among patients who underwent testing, only 10% had asthma alone—and not rhinitis or both asthma and rhinitis—listed as the likely reason for testing. Most patients with asthma have rhinitis,2,28 which may explain why most patients had both asthma and rhinitis listed as the reason for ordering tests. More research is needed to understand why tests are performed in patients with asthma with and without rhinitis.
Our findings suggest that testing in accordance with the guidelines offers clinical benefit among asthma patients who do not have COPD or a history of smoking. It is possible that patient-driven behavioral changes or greater awareness of asthma triggers were mediators between testing and fewer OCS bursts; this is supported by our finding that among never smokers without COPD, sensitization to 5-7 aeroallergen categories was associated with fewer OCS bursts, whereas sensitization to 1-4 categories was not. Interestingly, we found that patients who underwent testing were more likely to receive step-up therapy after the index visit, offering another possible explanation for fewer bursts. According to expert consensus in the Global Initiative for Asthma guidelines, allergen exposure, if sensitized, is a rationale for step-up treatment.2 However, we were unable to measure whether the test results prompted step-up therapy. It is also possible that the need for step-up therapy motivated providers to order tests or that both step-up therapy and testing comprised a comprehensive care strategy. More research is needed to evaluate whether aeroallergen testing is independently associated with clinical benefit and whether step-up inhaler therapy mediates this association.
We observed that before the COVID-19 lockdown in Philadelphia, SPTs were performed at a higher rate than serum-specific IgE tests, but these rates became more similar after March 2020, presumably due to the rise in telemedicine encounters. This is supported by the finding in the chart review that 18% of patients likely received serum-specific IgE tests because the test-related visit was a telemedicine encounter. Prior research has found numerous changes in asthma-related health care utilization during the pandemic, including an increase in telemedicine and reductions in asthma exacerbations.29, 30, 31, 32, 33, 34, 35, 36, 37, 38 We performed a secondary analysis restricting the index visit dates to those that occurred before the lockdown and observed greater benefit from testing than in the overall cohort. This may reflect a differential benefit according to test type: patients whose index visits occurred before the lockdown received SPTs at a higher rate.
Asthma associated with comorbid COPD or smoking represents difficult-to-treat subtypes with distinct patterns of airway inflammation and airway remodeling.39,40 Our results may reflect less effectiveness of testing among patients with asthma and smoking-related comorbidity. Alternatively, because patients with COPD or smoking histories were older, and because older adults with asthma are less likely to be sensitized to aeroallergens,20,21,41 older age may underlie their relative lack of benefit from testing. Prospective studies are needed to clarify these relationships.
Testing via SPTs may be more beneficial than via serum-specific IgE tests because the former offers immediate results that can be incorporated into management plans at the same visit. However, a caveat of comparing the 2 test modalities is that compared with patients who received SPTs, patients who received only serum-specific IgE tests were more likely to have COPD, be prescribed more controller inhalers, and have had more OCS bursts before the index visit. The reasons for these patient differences in test selection are unknown, although for patients with more severe or uncontrolled asthma, providers may deprioritize a 20-minute skin test procedure in favor of other tests at the visit (eg, spirometry), or they may choose to bundle serum-specific IgE tests with other serologic tests. It is possible that residual confounding related to patient selection accounted for the observed differences in OCS bursts after the index visit according to test type. Specifically, we were unable to account for asthma control, environmental exposures, and genetic risk factors for asthma exacerbations.
This study has several additional limitations. Persistent asthma is a clinical diagnosis, but we used ICS prescriptions to classify asthma as persistent, which may misclassify asthma that has been over- or undertreated. However, because misclassification usually biases toward the null, our results suggest a benefit of aeroallergen testing among patients in the cohort whose disease was correctly classified as persistent asthma and thus should have, according to the guidelines, undergone testing. Although airflow obstruction is a risk factor for asthma exacerbations2 and aeroallergen testing with allergen avoidance education has been found to improve lung function,24 we were unable to incorporate pulmonary function testing results due to inadequate data capture (eg, testing not documented as being performed or lung volumes not accompanied by flow volume curves). Our results may not be generalizable to other health systems. However, our findings within a diverse, predominantly urban cohort of adults with asthma may generalize to similar health systems. It is possible that patients who underwent aeroallergen testing were more likely to have received asthma-related education, and this education may have improved outcomes regardless of the testing. Finally, we were unable to count OCS prescriptions issued by providers outside Penn.
In summary, we found that in our diverse health system where asthma morbidity is high and asthma disparities exist, patients with asthma who were never smokers without COPD who underwent aeroallergen testing in the context of an outpatient allergy/immunology visit had fewer OCS bursts in the 12-month period after testing. In addition, among all patients with asthma, the receipt of SPTs was associated with fewer OCS bursts in the 12-month period after testing.
Clinical implication.
Among adults with asthma, aeroallergen testing may improve asthma outcomes for never smokers without COPD and for patients who receive SPTs.
Disclosure statement
Research reported in this publication was supported by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute under awards R01HL162354 and K24HL115354; and the National Institute of Environmental Health Sciences under awards T32ES019851 and P30ES013508. Additional funding was provided by the Pennsylvania Allergy Education Research Fund and the Patient-Centered Outcomes Research Institute 25 under award AS-1307-05218. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the report. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding agencies.
Disclosure of potential conflict of interest: K. H. Morales owns stock in Altria Group, British American Tobacco, and Phillip Morris International. The rest of the authors declare that they have no relevant conflicts of interest.
Acknowledgments
We thank Sam Chiu, Victoria Rautman, and Mario Gallone from the University of Pennsylvania Data Analytics Center and Penn Data Store for extracting the EHR data used for this project.
Supplementary data
References
- 1.Centers for Disease Control and Prevention Uncontrolled asthma among adults. https://www.cdc.gov/asthma/asthma_stats/uncontrolled-asthma-adults-2019.htm Available at:
- 2.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention (2023 update) https://ginasthma.org/archived-reports/ Available at:
- 3.Nurmagambetov T., Kuwahara R., Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15:348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 4.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report, 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Olivieri M., Heinrich J., Schlünssen V., Antó J.M., Forsberg B., Janson C., et al. The risk of respiratory symptoms on allergen exposure increases with increasing specific IgE levels. Allergy. 2016;71:859–868. doi: 10.1111/all.12841. [DOI] [PubMed] [Google Scholar]
- 6.Douglass J.A., Lodge C., Chan S., Doherty A., Tan J.A., Jin C., et al. Thunderstorm asthma in seasonal allergic rhinitis: the TAISAR study. J Allergy Clin Immunol. 2022;149:1607–1616. doi: 10.1016/j.jaci.2021.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Grant T., Aloe C., Perzanowski M., Phipatanakul W., Bollinger M.E., Miller R., et al. Mouse sensitization and exposure are associated with asthma severity in urban children. J Allergy Clin Immunol Pract. 2017;5:1008–1014.e1. doi: 10.1016/j.jaip.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenstreich D.L., Eggleston P., Kattan M., Baker D., Slavin R.G., Gergen P., et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 9.Rabito F.A., Carlson J.C., He H., Werthmann D., Schal C. A single intervention for cockroach control reduces cockroach exposure and asthma morbidity in children. J Allergy Clin Immunol. 2017;140:565–570. doi: 10.1016/j.jaci.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Mistry H., Ajsivinac Soberanis H.M., Kyyaly M.A., Azim A., Barber C., Knight D., et al. The clinical implications of Aspergillus fumigatus sensitization in difficult-to-treat asthma patients. J Allergy Clin Immunol Pract. 2021;9:4254–4267.e10. doi: 10.1016/j.jaip.2021.08.038. [DOI] [PubMed] [Google Scholar]
- 11.Pulimood T.B., Corden J.M., Bryden C., Sharples L., Nasser S.M. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 2007;120:610–617. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 12.Arroyave W.D., Rabito F.A., Carlson J.C. The relationship between a specific IgE level and asthma outcomes: results from the 2005-2006 National Health and Nutrition Examination Survey. J Allergy Clin Immunol Pract. 2013;1:501–508. doi: 10.1016/j.jaip.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Osborne M.L., Pedula K.L., O’Hollaren M., Ettinger K.M., Stibolt T., Buist A.S., et al. Assessing future need for acute care in adult asthmatics: the Profile of Asthma Risk Study: a prospective health maintenance organization-based study. Chest. 2007;132:1151–1161. doi: 10.1378/chest.05-3084. [DOI] [PubMed] [Google Scholar]
- 14.Gergen P.J., Mitchell H.E., Calatroni A., Sever M.L., Cohn R.D., Salo P.M., et al. Sensitization and exposure to pets: the effect on asthma morbidity in the US population. J Allergy Clin Immunol Pract. 2018;6:101–107.e2. doi: 10.1016/j.jaip.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh K.J., Yii A.C.A., Lapperre T.S., Chan A.K., Chew F.T., Chotirmall S.H., et al. Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy. 2017;10:131–140. doi: 10.2147/JAA.S130459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Driscoll B.R., Hopkinson L.C., Denning D.W. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med. 2005;5:4. doi: 10.1186/1471-2466-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medrek S.K., Kao C.C., Yang D.H., Hanania N.A., Parulekar A.D. Fungal sensitization is associated with increased risk of life-threatening asthma. J Allergy Clin Immunol Pract. 2017;5:1025–1031.e2. doi: 10.1016/j.jaip.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Fairs A., Agbetile J., Hargadon B., Bourne M., Monteiro W.R., Brightling C.E., et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. 2010;182:1362–1368. doi: 10.1164/rccm.201001-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salo P.M., Arbes S.J., Crockett P.W., Thorne P.S., Cohn R.D., Zeldin D.C. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol. 2008;121:678–684.e2. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleeson P., Morales K., Buckey T., Fadugba O., Apter A.J., Himes B. Factors associated with aeroallergen testing among adults with asthma in a large health system. J Allergy Clin Immunol Glob. 2023;2 doi: 10.1016/j.jacig.2023.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teague W.G., Phillips B.R., Fahy J.V., Wenzel S.E., Fitzpatrick A.M., Moore W.C., et al. Baseline Features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6:545–554.e4. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw D.E., Sousa A.R., Fowler S.J., Fleming L.J., Roberts G., Corfield J., et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46:1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 23.Salo P.M., Wilkerson J., Rose K.M., Cohn R.D., Calatroni A., Mitchell H.E., et al. Bedroom allergen exposures in US households. J Allergy Clin Immunol. 2018;141:1870–1879.e14. doi: 10.1016/j.jaci.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobb C., Ritz T., Rowlands G., Griffiths C. Effects of allergen and trigger factor avoidance advice in primary care on asthma control: a randomized-controlled trial. Clin Exp Allergy. 2010;40:143–152. doi: 10.1111/j.1365-2222.2009.03350.x. [DOI] [PubMed] [Google Scholar]
- 25.Janson S.L., McGrath K.W., Covington J.K., Cheng S.C., Boushey H.A. Individualized asthma self-management improves medication adherence and markers of asthma control. J Allergy Clin Immunol. 2009;123:840–846. doi: 10.1016/j.jaci.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC) Cloutier M.M., Baptist A.P., Blake K.V., Brooks E.G., Bryant-Stephens T., et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146:1217–1270. doi: 10.1016/j.jaci.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Defining adult overweight and obesity. https://www.cdc.gov/bmi/adult-calculator/bmi-categories.html?CDC_AAref_Val=https://www.cdc.gov/obesity/basics/adult-defining.html Available at:
- 28.Cruz A.A., Popov T., Pawankar R., Annesi-Maesano I., Fokkens W., Kemp J., et al. Common characteristics of upper and lower airways in rhinitis and asthma: ARIA update, in collaboration with GA2LEN. Allergy. 2007;62(suppl 84):1–41. doi: 10.1111/j.1398-9995.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- 29.Kouis P., Lemonaris M., Xenophontos E., Panayiotou A., Yiallouros P.K. The impact of COVID-19 lockdown measures on symptoms control in children with asthma: a systematic review and meta-analysis of observational cohort studies. Pediatr Pulmonol. 2023;58:3213–3226. doi: 10.1002/ppul.26646. [DOI] [PubMed] [Google Scholar]
- 30.Chan A.H.Y., Tomlin A., Chan E., Harrison J., Beyene K.A. Effect of the COVID-19 pandemic on asthma exacerbations in New Zealand: an interrupted time series analysis. J Allergy Clin Immunol Glob. 2023;2 doi: 10.1016/j.jacig.2023.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah S.A., Quint J.K., Sheikh A. Impact of COVID-19 pandemic on asthma exacerbations: retrospective cohort study of over 500,000 patients in a national English primary care database. Lancet Reg Health Eur. 2022;19 doi: 10.1016/j.lanepe.2022.100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye D., Gates A., Radhakrishnan L., Mirabelli M.C., Flanders W.D., Sircar K. Changes in asthma emergency department visits in the United States during the COVID-19 pandemic. J Asthma. 2023;60:1601–1607. doi: 10.1080/02770903.2023.2165445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salciccioli J.D., She L., Tulchinsky A., Rockhold F., Cardet J.C., Israel E. Effect of COVID-19 on asthma exacerbation. J Allergy Clin Immunol Pract. 2021;9:2896–2899.e1. doi: 10.1016/j.jaip.2021.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenyon C.C., Hill D.A., Henrickson S.E., Bryant-Stephens T.C., Zorc J.J. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020;8:2774–2776.e1. doi: 10.1016/j.jaip.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadopoulos N.G., Mathioudakis A.G., Custovic A., Deschildre A., Phipatanakul W., Wong G., et al. Childhood asthma outcomes during the COVID-19 pandemic: findings from the PeARL multi-national cohort. Allergy. 2021;76:1765–1775. doi: 10.1111/all.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sayed S., Diwadkar A.R., Dudley J.W., O’Brien J., Dvorin D., Kenyon C.C., et al. COVID-19 pandemic–related reductions in pediatric asthma exacerbations corresponded with an overall decrease in respiratory viral infections. J Allergy Clin Immunol Pract. 2022;10:91–99.e12. doi: 10.1016/j.jaip.2021.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simoneau T., Greco K.F., Hammond A., Nelson K., Gaffin J.M. Impact of the COVID-19 pandemic on pediatric emergency department use for asthma. Ann Am Thorac Soc. 2021;18:717–719. doi: 10.1513/AnnalsATS.202007-765RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taquechel K., Diwadkar A.R., Sayed S., Dudley J.W., Grundmeier R.W., Kenyon C.C., et al. Pediatric asthma health care utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8:3378–3387.e11. doi: 10.1016/j.jaip.2020.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson N.C. The role of smoking in asthma and chronic obstructive pulmonary disease overlap. Immunol Allergy Clin North Am. 2022;42:615–630. doi: 10.1016/j.iac.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Kuruvilla M.E., Lee F.E.H., Lee G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56:219–233. doi: 10.1007/s12016-018-8712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lefaudeux D., De Meulder B., Loza M.J., Peffer N., Rowe A., Baribaud F., et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol. 2017;139:1797–1807. doi: 10.1016/j.jaci.2016.08.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.