Abstract
Background
The optimal glycosylated hemoglobin (HbA1c) target in type 2 diabetes mellitus (T2DM) patients remains controversial, especially in patients with concomitant coronary heart disease (CHD). This study aimed to investigate the correlation between baseline HbA1c and long-term prognosis in CHD patients with T2DM.
Methods
The study enrolled 6,839 CHD patients with T2DM and measured HbA1c at admission in a multicenter prospective observational cohort. Patients were divided into two groups according to baseline HbA1c levels: optimal glycemic control group (HbA1c < 7.0 %, n = 3023) and poor glycemic control group (HbA1c ≥ 7.0 %, n = 3816). The study endpoints were all-cause death and major adverse cardiac and cerebrovascular events (MACCEs).
Results
The median follow-up period was 2.1 years. During this period, 229 (3.3 %) all-cause deaths, 165 (2.4 %) cardiac deaths, and 759 (11.1 %) MACCEs occurred. Unadjusted Kaplan–Meier analysis showed that the incidences of all-cause death, cardiac death, non-fatal MI, unplanned revascularization, and MACCEs were significantly lower in the HbA1c < 7.0 % group than in the HbA1c ≥ 7.0 % group (P < 0.05). Multivariate Cox hazard analysis indicated that the incidences of all-cause death, cardiac death and MACCEs were significantly lower in the HbA1c < 7.0 % group compared to the HbA1c ≥ 7.0 % group [all-cause death: hazard ratio (HR) 1.969, 95 % confidence interval (CI) 1.421–2.729; cardiac death: HR 2.515, 95 % CI 1.647–3.839; MACCEs: HR 1.345, 95 % CI 1.150–1.573; P < 0.001].
Conclusions
Baseline HbA1c level was associated with all-cause death, cardiac death, and MACCEs in CHD patients with T2DM.
Keywords: Coronary heart disease, Type 2 diabetes mellitus, Glycemic control, Glycosylated hemoglobin, Adverse outcomes
1. Introduction
Type 2 diabetes mellitus (T2DM) is a major global health threat and imposes a substantial global economic burden [1,2]. It is well-established that T2DM is a major risk factor for macrovascular and microvascular disease, with the progression of these complications largely dependent on the long-term blood glucose levels [3]. Glycosylated hemoglobin (HbA1c), a biomarker reflecting blood glucose levels over approximately 2–3 months [4], is widely used for diagnosing DM and assessing glycemic control [5]. Accumulated evidences have recommended routine monitoring of HbA1c in the management of diabetes patients [6,7].
Many studies have indicated that poor glycemic control, as measured by HbA1c levels, increased the risk of microvascular complications [8,9]. However, several landmark trials have demonstrated that intensive glucose control did not significantly reduce adverse cardiovascular events in long-standing T2DM patients [[10], [11], [12]]. Although most current guidelines recommend maintaining HbA1c levels below 6.5 % or 7.0 % to prevent adverse cardiovascular outcomes in patients with T2DM [6,7], the optimal HbA1c target remains a subject of intense debate, especially in patients with concomitant macrovascular complications [13,14].
Cardiovascular disease (CVD) is the leading cause of mortality in China [15], with coronary heart disease (CHD) being the predominant type of CVD. In recent years, abnormal glucose metabolism has attracted widespread attention in patients with CHD. However, the relationship between HbA1c levels and long-term prognosis in CHD patients remains unclear, with some studies reporting conflicting results [[16], [17], [18]]. Therefore, we aim to evaluate the predictive value of baseline HbA1c in mortality and major adverse cardiac and cerebrovascular events (MACCEs) in CHD patients with T2DM within the PROMISE cohort study.
2. Methods
2.1. Study design and population
The present study utilized data from the PRospective Observational Multi-center cohort for ISchemic and hEmorrhage risk in coronary artery disease patients (PROMISE), which was designed to develop ischemic and bleeding risk scores specifically for Chinese populations. This study was conducted at Fuwai Hospital, National Center for Cardiovascular Diseases in Beijing, and eight regional tertiary medical centers in mainland China, including General Hospital of Northern Theater Command of Chinese People's Liberation Army (Shenyang), Peking Union Medical College Hospital (Beijing), Peking University Third Hospital (Beijing), the First Hospital of Lanzhou University (Lanzhou), the First Affiliated Hospital of Zhejiang University (Hangzhou), Guangdong Cardiovascular Institute (Guangzhou), Xinxiang Central Hospital (Xinxiang), and the First Hospital of Qinhuangdao (Qinhuangdao). A total of 18,701 hospitalized patients with CHD were recruited in the PROMISE cohort study between January 2015 and May 2019. Inclusion criteria required patients to be at least 18 years old, diagnosed with CHD, indicated for at least one antiplatelet drug, and willing to sign informed consent. Exclusion criteria included a life expectancy of less than 6 months or concurrent participation in another interventional clinical trial. The PROMISE study complied with the Declaration of Helsinki and received approval from the Ethics Committee of Fuwai Hospital (protocol code: No. 2017-860). All participants provided written informed consent.
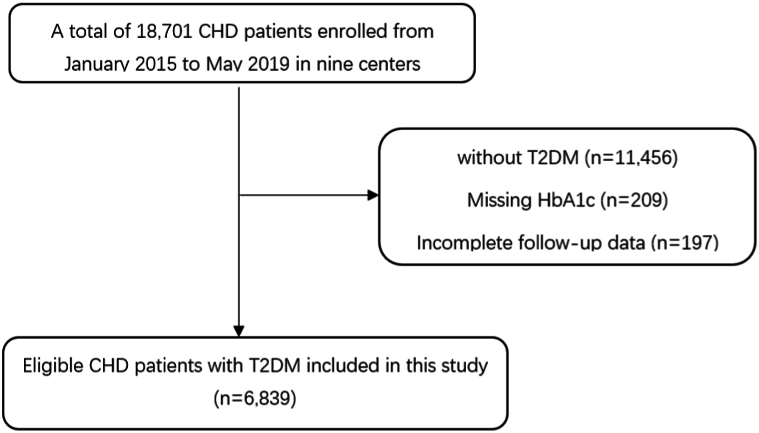
The participant selection process for the present study is depicted in Fig. 1. Patients were excluded from the present analysis if they (1) were not diagnosed with T2DM; (2) lacked baseline data on HbA1c; or (3) had incomplete follow-up data. Ultimately, 6,839 CHD patients with T2DM were included. These patients were further divided into two groups according to baseline HbA1c levels: baseline HbA1c < 7.0 % (n = 3,023) and baseline HbA1c ≥ 7.0 % (n = 3,816). T2DM was defined as fasting blood glucose ≥7.0 mmol/L (126.00 mg/dL), HbA1c ≥ 6.5 % (48 mmol/mol), blood glucose ≥11.1 mmol/L in the 2-h oral glucose tolerance test, oral anti-diabetes drugs or insulin use, or self-reported diabetes.
Fig. 1.
Flowchart of the study. CHD, coronary heart disease; T2DM, type 2 diabetes mellitus; HbA1c, glycosylated hemoglobin.
2.2. Blood sampling and data collection
Fasting blood samples were drawn in the morning as part of routine clinical practice. Blood glucose was assayed using an enzymatic hexokinase method. HbA1c was measured with automated glycohemoglobin analyzers (Tosoh HLC-723G8, Tokyo, Japan). Baseline data on demographic and clinical characteristics, laboratory results, and medications were obtained from the electronic medical records of each clinical center and verified by well-trained independent clinical research coordinators.
2.3. Endpoints and follow-up
The primary endpoint was all-cause death. Secondary endpoints were MACCEs, a composite of cardiac death, non-fatal myocardial infarction (MI), unplanned revascularization, and stroke, as well as each component of the composite endpoint. Unplanned revascularization was defined as PCI or CABG for any lesion driven by ischemic symptoms or events. Cardiac death was defined as death due to a proximate cardiovascular cause or any death without an unequivocal non-cardiovascular cause. MI was diagnosed in accordance with the contemporaneous Universal Definition of Myocardial Infarction [19,20]. Strokes included ischemic and hemorrhagic strokes, classified according to the World Health Organization classification of diseases [21].
Medication lists and outcome data were collected through outpatient visits, telephone interviews, text messages, and letters by an independent group of clinical research coordinators 1 and 2 years after discharge. Investigator training, telephone recording, and an online follow-up system were applied to ensure comparable follow-up across sites. Two independent cardiologists adjudicated endpoint events, and any disagreement was resolved by consensus.
2.4. Statistical analysis
Continuous variables were presented as mean ± SD or median and interquartile range (if variables were skew distribution). Categorical variables were presented as frequency and percentage. Cumulative incidences between groups were estimated using Kaplan–Meier curves and compared by log-rank test. Univariable and multivariable Cox regression models were applied to identify the independent associations between HbA1c and MACCEs or all-cause death. Covariates for adjustment in the multivariable model include age, sex, acute coronary syndrome (ACS), prior MI, current smoking, body mass index (BMI), hypertension, dyslipidemia, left ventricular ejection fraction (LVEF), serum creatinine, the synergy between percutaneous coronary intervention with Taxus and cardiac surgery (SYNTAX) score, and previous percutaneous coronary intervention (PCI). Subgroup analyses were performed to assess the robustness of the results using interaction tests in groups stratified by age, sex, BMI, clinical presentation, dyslipidemia, current smoking, hypertension, prior MI, and SYNTAX score. For all analyses, statistical significance was defined as two-sided p values < 0.05. Statistical analyses were performed using SPSS 22.0 for Windows (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Baseline characteristics of the population
Baseline clinical characteristics are listed in Table 1. Among the 6839 participants, the mean age was 61.8 years, and the mean duration since T2DM diagnosis was 9.5 years earlier. The mean BMI was 26.1 kg/m°2. At baseline, nearly 24 % of the patients were receiving insulin. Patients with high baseline HbA1c (HbA1c ≥ 7 %) were more likely to be female, obese, present with ACS, have a longer duration of T2DM, a higher rate of current smoking, a higher rate of prior MI, a greater proportion receiving PCI treatment, and exhibit higher SYNTAX preoperative scores, and high sensitivity C- reactive protein (hs-CRP) levels compared to patients with low baseline HbA1c (HbA1c < 7 %) (all P < 0.05). However, there were no significant differences between the two groups in age, history of early-onset CHD, the use of lipid-lowering drugs, antihypertensive drugs, and antiplatelet therapy, or the proportion of prior stroke and peripheral arterial disease (all P > 0.05, Table 1).
Table 1.
Baseline characteristics in CHD patients with T2DM.
| Characteristics | Total |
Baseline HbA1c < 7 % |
Baseline HbA1c ≥ 7 % |
P value |
|---|---|---|---|---|
| N = 6839 | N = 3023 | N = 3816 | ||
| Demographics | ||||
| Age, years | 61.8 ± 10.0 | 61.7 ± 10.2 | 61.8 ± 9.9 | 0.876 |
| Male sex, n (%) | 4932 (72.1) | 2273 (75.2) | 2659 (69.7) | <0.001 |
| BMI, kg/m2 | 26.1 ± 3.3 | 25.9 ± 3.2 | 26.4 ± 3.3 | <0.001 |
| Duration of T2DM | 9.5 ± 7.1 | 8.7 ± 6.9 | 10.2 ± 7.2 | <0.001 |
| Diagnosis on admission, n (%) | ||||
| ACS | 2901 (42.4) | 1116 (36.9) | 1785 (46.8) | <0.001 |
| STEMI | 1556 (22.8) | 596 (19.7) | 960 (25.2) | <0.001 |
| NSTEMI | 710 (10.4) | 297 (9.8) | 413 (10.8) | 0.179 |
| UAP | 635 (9.3) | 223 (7.4) | 412 (10.8) | <0.001 |
| SAP | 3938 (57.6) | 1907 (63.1) | 2031 (53.2) | <0.001 |
| Medical history, n (%) | ||||
| Current Smoking | 1272 (18.6) | 432 (14.3) | 840 (22.0) | <0.001 |
| Hypertension | 4813 (70.4) | 2192 (72.5) | 2621 (68.7) | 0.001 |
| Dyslipidemia | 5361 (78.4) | 2460 (81.4) | 2901 (76.0) | <0.001 |
| CKD | 202 (3.0) | 92 (3.0) | 110 (2.9) | 0.697 |
| COPD | 94 (1.4) | 50 (1.7) | 44 (1.2) | 0.077 |
| Prior stroke | 1227 (17.9) | 543 (18.0) | 684 (17.9) | 0.968 |
| Prior MI | 1352 (19.8) | 538 (17.8) | 814 (21.3) | <0.001 |
| Prior PCI | 1952 (28.5) | 889 (29.4) | 1063 (27.9) | 0.158 |
| Prior CABG | 213 (3.1) | 101 (3.3) | 112 (2.9) | 0.337 |
| PAD | 456 (6.7) | 212 (7.0) | 244 (6.4) | 0.308 |
| Laboratory variables | ||||
| HbA1c, % | 7.4 ± 1.5 | 6.2 ± 0.5 | 8.4 ± 1.3 | <0.001 |
| FBG, mmol/L | 8.0 ± 3.3 | 6.4 ± 2.2 | 9.4 ± 3.5 | <0.001 |
| SCR, mmol/L | 84.0 ± 32.6 | 84.9 ± 40.9 | 83.2 ± 24.1 | 0.034 |
| hs-CRP, mg/L | 3.9 ± 5.6 | 3.4 ± 5.7 | 4.3 ± 5.6 | <0.001 |
| LVEF, % | 59.3 ± 8.2 | 59.9 ± 7.8 | 58.9 ± 8.5 | <0.001 |
| Medications, n (%) | ||||
| Insulin | 1635 (23.9) | 278 (9.2) | 1393 (36.5) | <0.001 |
| Lipid-lowering drugs | 6532 (95.9) | 2882 (95.6) | 3650 (96.1) | 0.374 |
| ACEI/ARB | 3886 (57.0) | 1727 (57.3) | 2159 (56.8) | 0.689 |
| CCB | 3046 (44.7) | 1467 (48.7) | 1579 (41.6) | <0.001 |
| β-blocker | 5517 (81.0) | 2461 (81.7) | 3056 (80.4) | 0.199 |
| aspirin | 6544 (96.0) | 2878 (95.5) | 3666 (96.5) | 0.038 |
| P2Y12 antagonist | 6515 (95.6) | 2951 (97.9) | 3564 (93.8) | <0.001 |
CHD, coronary heart disease; T2DM, type2 diabetes mellitus; HbA1c, glycosylated hemoglobin; BMI, Body mass index; ACS, acute coronary syndrome; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; UAP, unstable angina pectoris; SAP, stable angina pectoris; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; PAD, peripheral artery disease; FBG, fasting blood glucose; SCR, serum creatinine; hs-CRP, high sensitive-C reaction protein; LVEF, left ventricular ejection fraction; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor antagonists; CCB, calcium channel blocker; P2Y12 antagonist, P2Y12 receptor antagonist.
3.2. Severity of coronary artery lesions in the population
A total of 98.5 % of enrolled patients completed coronary angiography, of which 70.6 % underwent PCI. Patients in the high baseline HbA1c group had a higher proportion of triple-vessel lesions, a higher SYNTAX score, and a higher proportion of coronary stenting placement compared to those in the low baseline HbA1c group (all P < 0.05, Table 2).
Table 2.
Severity of coronary artery lesions in CHD patients with T2DM.
| Characteristics | total |
Baseline HbA1c < 7 % |
Baseline HbA1c ≥ 7 % |
P value |
|---|---|---|---|---|
| N = 6839 | N = 3023 | N = 3816 | ||
| CAG, n (%) | 6738 (98.5) | 2986 (98.8) | 3752 (98.3) | 0.123 |
| lesions involving vessels, n (%) | ||||
| LM | 750 (11.0) | 333 (11.1) | 417 (11.0) | 0.967 |
| Single lesion | 1561 (23.2) | 803 (26.9) | 758 (20.2) | <0.001 |
| Bilateral lesions | 1879 (27.9) | 894 (29.9) | 985 (26.3) | 0.001 |
| Triple vessel | 3294 (48.9) | 1288 (43.1) | 2006 (53.5) | <0.001 |
| PCI, n (%) | 4831 (70.6) | 2091 (69.2) | 2740 (71.8) | 0.018 |
| Stenting | 4375 (64.0) | 1880 (62.2) | 2495 (65.4) | 0.006 |
| PTCA | 316 (4.6) | 147 (4.9) | 169 (4.4) | 0.396 |
| DCB | 107 (1.6) | 48 (1.6) | 59 (1.5) | 0.890 |
| SYNTAX score, n (%) | 14.0 ± 9.4 | 13.0 ± 9.0 | 14.8 ± 9.6 | <0.001 |
| 0-22 | 5672 (82.9) | 2590 (85.7) | 3082 (80.8) | <0.001 |
| 23-32 | 898 (13.1) | 343 (11.3) | 555 (14.5) | <0.001 |
| ≥33 | 1167 (17.1) | 433 (14.3) | 734 (19.2) | <0.001 |
CHD, coronary heart disease; T2DM, type2 diabetes mellitus; HbA1c, glycosylated hemoglobin; CAG, Coronary angiography; LM, left main disease; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; DCB, dug coated balloon; SYNTAX, the synergy between percutaneous coronary intervention with Taxus and cardiac surgery.
3.3. Association of baseline HbA1c level with adverse clinical outcomes and subgroup analyses
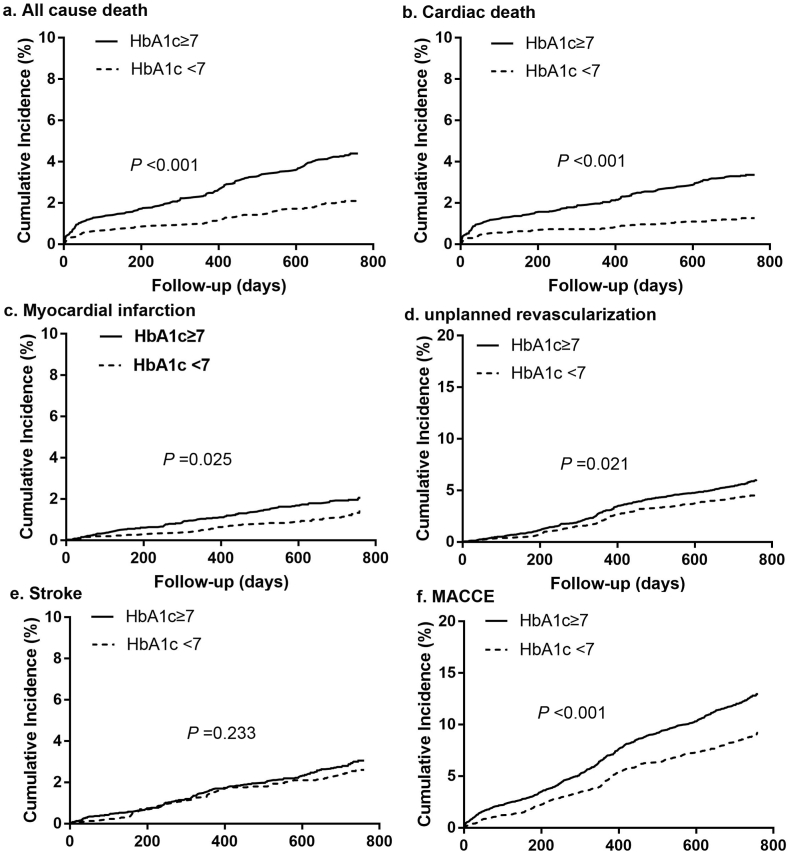
During the follow-up period (median: 2.1 years, interquartile range: 2.0–2.1 years), 759 MACCEs (11.1 %) and 229 all-cause deaths (3.3 %) occurred in CHD patients with T2DM. Patients in the high baseline HbA1c group had significantly higher rates of all-cause death, cardiac death, non-fatal MI, unplanned revascularization, and MACCEs compared to patients in the low baseline HbA1c group (all P for trend < 0.05, Table 3). There was no significant difference in stroke between the high baseline HbA1c group and the low baseline HbA1c group (P > 0.05, Table 3). Kaplan-Meier cumulative survival curves for all-cause death and MACCEs over the 2-year follow-up period were shown in Fig. 2.
Table 3.
Clinical outcomes in CHD patients with T2DM during follow-up.
| Variables |
total |
Baseline HbA1c < 7 % |
Baseline HbA1c ≥ 7 % |
P value |
|---|---|---|---|---|
| n (%) | N = 6839 | N = 3023 | N = 3816 | |
| All-cause death | 229 (3.3) | 63 (2.1) | 166 (4.4) | <0.001 |
| Cardiac death | 165 (2.4) | 38 (1.3) | 127 (3.3) | <0.001 |
| Non-fatal MI | 117 (1.7) | 40 (1.3) | 77 (2.0) | 0.028 |
| Stroke | 193 (2.8) | 77 (2.5) | 116 (3.0) | 0.222 |
| Unplanned revascularization | 357 (5.2) | 138 (4.6) | 219 (5.7) | 0.030 |
| MACCE | 759 (11.1) | 271 (9.0) | 488 (12.8) | <0.001 |
CHD, coronary heart disease; T2DM, type2 diabetes mellitus; HbA1c, glycosylated hemoglobin; MI, myocardial infarction; MACCE, major adverse cardiac and cerebrovascular events.
Fig. 2.
Kaplan-Meier survival curves for 2-year primary and secondary outcomes in CHD patients with T2DM. (a) All-cause death, (b) cardiac death, (c) myocardial infarction, (d) unplanned revascularization, (e) stroke, and (f) MACCE. HbA1c, glycosylated hemoglobin; MACCE, major adverse cardiac and cerebrovascular events. CHD, coronary heart disease; T2DM, type 2 diabetes mellitus.
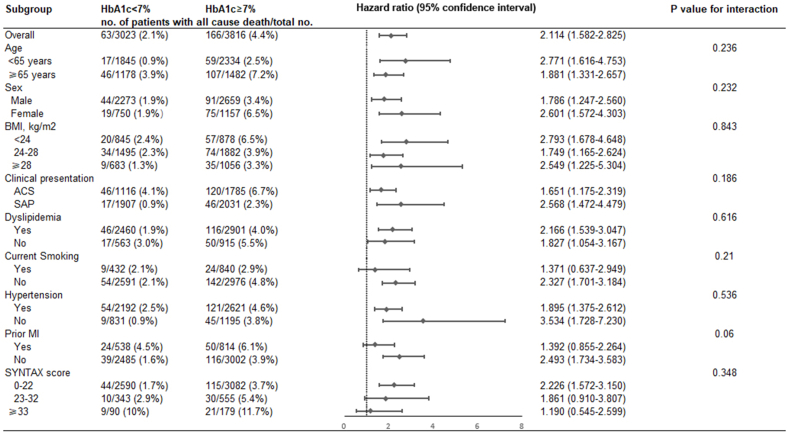
Both before and after adjusting for multiple covariates, the risk of all-cause death, cardiac death, and MACCEs in the high baseline HbA1c group were significantly higher than those in the low baseline HbA1c group [Before adjustment: all-cause death: hazard ratio (HR) 2.114, 95 % confidence interval (CI) 1.582–2.825, P < 0.001; cardiac death: HR 2.678, 95 % CI: 1.864–3.848, P < 0.001; MACCEs: HR 1.459, 95 % CI: 1.258–1.692, P < 0.001. After adjustment: all-cause death: HR 1.969, 95 % CI: 1.421–2.729, P < 0.001; cardiac death: HR 2.515, 95 % CI 1.647–3.839, P < 0.001; MACCEs: HR 1.345, 95 % CI 1.150–1.573, P < 0.001; Table 4]. Before adjusting for covariates, the risk of non-fatal MI and unplanned revascularization in the high baseline HbA1c group were significantly higher than the low baseline HbA1c group (non-fatal MI: HR 1.548, 95 % CI 1.057–2.268, P = 0.025; unplanned revascularization: HR 1.284, 95 % CI 1.038–1.589, P = 0.021; Table 4). However, there was no significant difference between the two groups in the risk of MI and unplanned revascularization after adjusting for multiple covariates (MI: HR 1.384, 95 % CI 0.921–2.081, P = 0.118; unplanned revascularization: HR 1.192, 95 % CI 0.957–1.484, P = 0.117; Table 4). Similarly, there was no difference between the two groups in the risk of stroke before and after adjusting for covariates (all P for trend > 0.05, Table 4). The effect of baseline HbA1c level on all-cause death was consistent across subgroups (All p for interaction > 0.05) (Fig. 3).
Table 4.
Risks of 2-year primary and secondary outcomes in CHD patients with T2DM.
| Variables | HR (95%CI) | P value | Adjusteda HR (95 % CI) | P value |
|---|---|---|---|---|
| All-cause death | 2.114 (1.582–2.825) | <0.001 | 1.969 (1.421–2.729) | <0.001 |
| Cardiac death | 2.678 (1.864–3.848) | <0.001 | 2.515 (1.647–3.839) | <0.001 |
| Non-fatal MI | 1.548 (1.057–2.268) | 0.025 | 1.384 (0.921–2.081) | 0.118 |
| Stroke | 1.192 (0.893–1.592) | 0.233 | 1.199 (0.885–1.626) | 0.242 |
| unplanned Revascularization | 1.284 (1.038–1.589) | 0.021 | 1.192 (0.957–1.484) | 0.117 |
| MACCE | 1.459 (1.258–1.692) | <0.001 | 1.345 (1.150–1.573) | <0.001 |
CHD, coronary heart disease; T2DM, type2 diabetes mellitus; HbA1c, glycosylated hemoglobin; MI, myocardial infarction; MACCE, major adverse cardiac and cerebrovascular events; HR, hazard ratio; CI, confidence interval.
Adjusted for age, sex, ACS, prior myocardial infarction, current smoking, body mass index, hypertension, dyslipidemia, left ventricular ejection fraction, Serum creatinine, SYNTAX score, percutaneous coronary intervention.
Fig. 3.
Subgroup analyses for the primary outcome. HbA1c, glycosylated hemoglobin; BMI, Body mass index; ACS, acute coronary syndrome; SAP, stable angina pectoris; MI, myocardial infarction; SYNTAX, the synergy between percutaneous coronary intervention with Taxus and cardiac surgery.
4. Discussion
The results of the present study showed that baseline glycemic control, as indicated by HbA1c, was significantly associated with all-cause death, cardiac death, and MACCEs in CHD patients with T2DM, both before and after adjusting for multiple covariates. However, there was no significant difference between high and low baseline HbA1c groups in the rates of non-fatal MI and unplanned revascularization after adjusting for multiple covariates. In our study, CHD patients with T2DM who had poor baseline glycemic control, as indicated by higher HbA1c levels, exhibited more severe coronary artery lesions and a higher proportion of triple-vessel lesions compared to those with optimal baseline glycemic control.
In recent years, the impact of abnormal glucose metabolism on CHD patients has gained widespread attention, and maintaining optimal blood glucose control is essential. HbA1c measurement has been widely used in the diagnosis, evaluation of treatment efficacy, and risk stratification of patients with diabetes. However, the relationship between HbA1c levels and clinical outcomes in CHD patients with T2DM remains controversial.
Ike et al. [22] found that in patients with diabetes who underwent PCI, the clinical outcomes in the HbA1c < 6.9 % group were better than in the HbA1c ≥ 6.9 % group. However, after multivariate adjustment, there was no difference in clinical outcomes between the two groups, suggesting that glycemic control initiated at the time of PCI did not lead to improved outcomes at follow-up. Similarly, Lemesle et al. [16] reported that the one-year incidence of MACEs was similar between the high HbA1c (> 7 %) and low HbA1c (≤ 7 %) groups (23.7 % vs. 20.8 %; P = 0.45), indicating that HbA1c may not be a reliable predictor of cardiac events in diabetic patients undergoing PCI. Singla et al. [23] also found that after adjusting for baseline differences, glycemic control by HbA1c had no independent influence on one-year MACEs in diabetic patients with AMI after successful stent placement. Tian et al. [24] also reported that the benefit concluded from the ADVANCE study was unrelated to differences in baseline HbA1c levels among patients. However, Hwang et al. [25] found that HbA1c < 7.0 %, measured 2 years after PCI, was associated with a reduced rate of MACCEs, suggesting that high HbA1c levels may identify the diabetic patients with PCI at increased risk of adverse events, especially repeat revascularization. Corpus et al. [26] found that optimal glycemic control (preprocedural HbA1c ≤ 7 %) was associated with a lower rate of revascularization, cardiac rehospitalization, and recurrent angina in diabetic patients undergoing elective PCI. Kassaian et al. [27] reported that good glycemic control (HbA1c ≤ 7 %) was associated with lower MACEs in diabetic patients after PCI. Similarly, Sharma et al. [28] found that patients with diabetes who were not on insulin with well-controlled glycemic levels (HbA1c ≤ 7 %) had significantly lower long-term mortality after PCI, whereas insulin users had similar mortality rates across different HbA1c categories.
In the present study, baseline HbA1c ≥ 7 % in CHD patients with T2DM was also associated with a higher rate of MACCEs during the 2-years follow-up period, which aligns with previous findings [25,27]. The benefit of baseline glycemic control in our study was primarily due to a reduction in long-term mortality (including all-cause death and cardiac death), which represents a reduction in hard clinical endpoints. Meanwhile, unplanned revascularization and non-fatal MI showed only a downward trend in patients with optimal glycemic control. Previous studies had shown that poor glycemic control increases the risk of repeat revascularization, cardiac rehospitalization, and recurrent angina, which are typically softer but still clinically relevant endpoints [25,26,29].
Previous studies indicated that elevated baseline HbA1c levels are an independent predictor of the severity of CAD and are associated with triple-vessel disease [17]. In our study, we similarly found that high baseline HbA1c was associated with the severity of CAD and a higher incidence of triple-vessel disease in CHD patients with T2DM. Some studies have also demonstrated that controlling HbA1c level < 7.0 % yields significant benefits by attenuating the progression of coronary artery calcification in asymptomatic patients with diabetes, thereby reducing the incidence of cardiovascular diseases [14]. Poor glycemic control is closely related to severe endothelial dysfunction, which exacerbates the progression and outcomes of atherosclerosis [30].
4.1. Limitation
This study has several limitations. Firstly, it explored the association between baseline HbA1c levels and cardiovascular adverse events during follow-up; however, HbA1c levels were not measured repeatedly during follow-up, and they may have fluctuated over time. Secondly, as this is an observational study, certain unmeasured risk factors (such as lifestyle) may have introduced residual confounding effects on long-term mortality. Although our analysis had adjusted for common cardiovascular risk factors, statistical adjustments to take account of risk factors cannot enable the observational study to attribute causality. Additionally, this study excluded only patients with a life expectancy of less than 6 months and did not fully exclude those with malignancies or cachexia, which may introduce potential confounding. Finally, long-term data on lipid-lowering and antihypertensive regimens, such as low-density lipoprotein cholesterol and blood pressure levels during follow-up, as well as the intensity and dosage of statins and antihypertensive drugs, are limited.
5. Conclusions
Baseline HbA1c level was significantly associated with the risk of all-cause death, cardiac death, and MACCEs in CHD patients with T2DM: For these patients, optimal glycemic control (baseline HbA1c < 7.0 %) may lead to a better prognosis. Our data suggest that high baseline HbA1c levels may identify CHD patients with T2DM at increased risk of adverse events, especially all-cause death and cardiac death. Further study is warranted to determine whether effective HbA1c control improves prognosis in CHD patients with T2DM.
CRediT authorship contribution statement
Xiao-Fang Tang: Writing – original draft, Data curation, Conceptualization. Qin-Xue Li: Data curation, Conceptualization. Ya-Ling Han: Supervision, Funding acquisition. Xiao-Zeng Wang: Supervision, Project administration, Funding acquisition, Conceptualization. Ying Song: Project administration, Methodology. Zheng Zhang: Project administration, Conceptualization. Jing-Jing Xu: Project administration, Methodology. Zhen-Yu Liu: Project administration, Methodology, Data curation. Yan Chen: Investigation, Formal analysis. Yong-Zhen Zhang: Investigation, Data curation. Pei Zhu: Methodology, Investigation, Data curation. Xiao-Gang Guo: Project administration, Conceptualization. Lin Jiang: Project administration, Data curation. Zhi-Fang Wang: Methodology, Conceptualization. Ru Liu: Project administration, Methodology. Qing-Sheng Wang: Resources, Data curation. Yi Yao: Project administration, Data curation. Ying-Qing Feng: Investigation, Formal analysis. Xue-Yan Zhao: Validation, Supervision, Conceptualization. Jin-Qing Yuan: Validation, Supervision, Funding acquisition.
Data availability statement
Data will be made available on request.
Ethics statement
The PROMISE study complied with the principles of the Declaration of Helsinki, and received approval from the Ethics Committee of Fuwai Hospital (protocol code: No. 2017-860). All participants provided written informed consent before enrollment.
Consent for publication
Not applicable.
Funding
The study was supported by the National High Level Hospital Clinical Research Funding (grant number 2023-GSP-QN-16), the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences [grant numbers NCRC2022003], the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) [grant numbers 2023-I2M-1-002], and the National Key Research and Development Program of China (grant Numbers: 2016YFC1301300, 2016YFC1301301).
Declaration of competing interest
The authors declare that the study has no competing interests.
Acknowledgments
We were grateful to the Department of Cardiology, Fuwai Hospital, the Chinese Academy of Medical Sciences, and the National Center for Cardiovascular Disease for help in recruiting patients. We thank all staff members who contributed to the study.
Contributor Information
Xue-Yan Zhao, Email: zhao_xueyan@sina.com.
Jin-Qing Yuan, Email: dr_jinqingyuan@126.com.
References
- 1.Bommer C., Sagalova V., Heesemann E., Manne-Goehler J., Atun R., Barnighausen T., Davies J., Vollmer S. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41(5):963–970. doi: 10.2337/dc17-1962. [DOI] [PubMed] [Google Scholar]
- 2.Bommer C., Heesemann E., Sagalova V., Manne-Goehler J., Atun R., Barnighausen T., Vollmer S. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017;5(6):423–430. doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- 3.Stratton I.M., Adler A.I., Neil H.A., Matthews D.R., Manley S.E., Cull C.A., Hadden D., Turner R.C., Holman R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan D.M., Singer D.E., Hurxthal K., Goodson J.D. The clinical information value of the glycosylated hemoglobin assay. N. Engl. J. Med. 1984;310(6):341–346. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 5.Prasad K. Does HbA1cc play a role in the development of cardiovascular diseases? Curr. Pharmaceut. Des. 2018;24(24):2876–2882. doi: 10.2174/1381612824666180903121957. [DOI] [PubMed] [Google Scholar]
- 6.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes A. 6. Glycemic targets: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S73–S84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 8.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 9.Ohkubo Y., Kishikawa H., Araki E., Miyata T., Isami S., Motoyoshi S., Kojima Y., Furuyoshi N., Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res. Clin. Pract. 1995;28(2):103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 10.Action to Control Cardiovascular Risk in Diabetes Study G., Gerstein H.C., Miller M.E., Byington R.P., Goff D.C., Jr., Bigger J.T., Buse J.B., Cushman W.C., Genuth S., Ismail-Beigi F., et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group A.C., Patel A., MacMahon S., Chalmers J., Neal B., Billot L., Woodward M., Marre M., Cooper M., Glasziou P., et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 12.Duckworth W., Abraira C., Moritz T., Reda D., Emanuele N., Reaven P.D., Zieve F.J., Marks J., Davis S.N., Hayward R., et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 13.Qaseem A., Wilt T.J., Kansagara D., Horwitch C., Barry M.J., Forciea M.A., Clinical Guidelines Committee of the American College of P, Fitterman N., Balzer K., Boyd C., et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann. Intern. Med. 2018;168(8):569–576. doi: 10.7326/M17-0939. [DOI] [PubMed] [Google Scholar]
- 14.Laiteerapong N., Ham S.A., Gao Y., Moffet H.H., Liu J.Y., Huang E.S., Karter A.J. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the diabetes & aging study) Diabetes Care. 2019;42(3):416–426. doi: 10.2337/dc17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao D., Liu J., Wang M., Zhang X., Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 2019;16(4):203–212. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 16.Lemesle G., Bonello L., de Labriolle A., Maluenda G., Syed A.I., Collins S.D., Ben-Dor I., Torguson R., Kaneshige K., Xue Z., et al. Prognostic value of hemoglobin A1C levels in patients with diabetes mellitus undergoing percutaneous coronary intervention with stent implantation. Am. J. Cardiol. 2009;104(1):41–45. doi: 10.1016/j.amjcard.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 17.Hong L.F., Li X.L., Guo Y.L., Luo S.H., Zhu C.G., Qing P., Xu R.X., Wu N.Q., Li J.J. Glycosylated hemoglobin A1c as a marker predicting the severity of coronary artery disease and early outcome in patients with stable angina. Lipids Health Dis. 2014;13:89. doi: 10.1186/1476-511X-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.She J., Deng Y., Wu Y., Xia Y., Li H., Liang X., Shi R., Yuan Z. Hemoglobin A(1c) is associated with severity of coronary artery stenosis but not with long term clinical outcomes in diabetic and nondiabetic patients with acute myocardial infarction undergoing primary angioplasty. Cardiovasc. Diabetol. 2017;16(1):97. doi: 10.1186/s12933-017-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thygesen K., Alpert J.S., Jaffe A.S., Simoons M.L., Chaitman B.R., White H.D., Joint ESCAAHAWHFTFftUDoMI. Katus H.A., Lindahl B., Morrow D.A., et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 20.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D. Executive group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the universal definition of myocardial I: fourth universal definition of myocardial infarction (2018) J. Am. Coll. Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 21.Rajakulendran S., Dua T., Harper M., Shakir R. The classification of neurological disorders in the 11th revision of the International Classification of Diseases (ICD-11) J. Neurol. Neurosurg. Psychiatry. 2014;85(9):952–953. doi: 10.1136/jnnp-2013-307093. [DOI] [PubMed] [Google Scholar]
- 22.Ike A., Nishikawa H., Shirai K., Mori K., Kuwano T., Fukuda Y., Takamiya Y., Yanagi D., Kubota K., Tsuchiya Y., et al. Impact of glycemic control on the clinical outcome in diabetic patients with percutaneous coronary intervention--from the FU-registry. Circ. J. 2011;75(4):791–799. doi: 10.1253/circj.cj-10-0474. [DOI] [PubMed] [Google Scholar]
- 23.Singla A., Orshaw P., Boura J., Harjai K.J. Glycosylated hemoglobin and outcomes in diabetic patients with acute myocardial infarction after successful revascularization with stent placement: findings from the guthrie health off-label stent (GHOST) investigators. J. Intervent. Cardiol. 2012;25(3):262–269. doi: 10.1111/j.1540-8183.2011.00715.x. [DOI] [PubMed] [Google Scholar]
- 24.Tian J., Ohkuma T., Cooper M., Harrap S., Mancia G., Poulter N., Wang J.G., Zoungas S., Woodward M., Chalmers J. Effects of intensive glycemic control on clinical outcomes among patients with type 2 diabetes with different levels of cardiovascular risk and hemoglobin A(1c) in the ADVANCE trial. Diabetes Care. 2020;43(6):1293–1299. doi: 10.2337/dc19-1817. [DOI] [PubMed] [Google Scholar]
- 25.Hwang J.K., Lee S.H., Song Y.B., Ahn J., Carriere K., Jang M.J., Park T.K., Choi S.H., Yang J.H., Choi J.H., et al. Glycemic control status after percutaneous coronary intervention and long-term clinical outcomes in patients with type 2 diabetes mellitus. Circ. Cardiovasc. Interv. 2017;10(4) doi: 10.1161/CIRCINTERVENTIONS.116.004157. [DOI] [PubMed] [Google Scholar]
- 26.Corpus R.A., George P.B., House J.A., Dixon S.R., Ajluni S.C., Devlin W.H., Timmis G.C., Balasubramaniam M., O'Neill W.W. Optimal glycemic control is associated with a lower rate of target vessel revascularization in treated type II diabetic patients undergoing elective percutaneous coronary intervention. J. Am. Coll. Cardiol. 2004;43(1):8–14. doi: 10.1016/j.jacc.2003.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Kassaian S.E., Goodarzynejad H., Boroumand M.A., Salarifar M., Masoudkabir F., Mohajeri-Tehrani M.R., Pourhoseini H., Sadeghian S., Ramezanpour N., Alidoosti M., et al. Glycosylated hemoglobin (HbA1c) levels and clinical outcomes in diabetic patients following coronary artery stenting. Cardiovasc. Diabetol. 2012;11:82. doi: 10.1186/1475-2840-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma P.K., Agarwal S., Ellis S.G., Goel S.S., Cho L., Tuzcu E.M., Lincoff A.M., Kapadia S.R. Association of glycemic control with mortality in patients with diabetes mellitus undergoing percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2014;7(4):503–509. doi: 10.1161/CIRCINTERVENTIONS.113.001107. [DOI] [PubMed] [Google Scholar]
- 29.Menon V., Kumar A., Patel D.R., John J.S., Wolski K.E., McErlean E., Riesmeyer J.S., Weerakkody G., Ruotolo G., Cremer P.C., et al. Impact of baseline glycemic control on residual cardiovascular risk in patients with diabetes mellitus and high-risk vascular disease treated with statin therapy. J. Am. Heart Assoc. 2020;9(1) doi: 10.1161/JAHA.119.014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simova I.I., Denchev S.V., Dimitrov S.I., Ivanova R. Endothelial function in patients with and without diabetes mellitus with different degrees of coronary artery stenosis. J. Clin. Ultrasound. 2009;37(1):35–39. doi: 10.1002/jcu.20532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.