Abstract
Background
The intestinal tract, as the main place for nutrient digestion and absorption, is closely related to the health of livestock and poultry. Melatonin secreted by the pineal gland acts as an endocrine transport signaling molecule to regulate intestinal function. However, the effect on intestinal function after pineal removal is unclear.
Methods
We raised 24 chicks under 400-700 nm white light with or without pinealectomy for 21 days. We used electron microscopy, HE staining, PAS staining, immunohistochemistry, immunofluorescence staining and western blot to detect intestinal physical and to explore the effect of melatonin secreted by the pineal gland on the intestinal mucosa barrier function.
Results
The results showed that after pineal gland removal, the structure of the intestinal villi is severely damaged. Moreover, there was an obviously down-regulation in the villi length, the number of goblet cells and and its secretion of MUC2 protein, the expression level of tight junction proteins (Occludin and ZO-1) and lysozyme secreted by paneth cells, number of PCNA positive cells and macrophage, and an up-regualtion in crypt depth and apoptosis level after pinealectomy, suggesting pinealectomy-mediated melatonin level decrease damaged the intestinal physical, chemical and immune barriers.
Conclusion
Our findings provide new theoretical support for the future use of melatonin in intestinal development and new ideas about the relationship between endocrine hormone and intestinal physiology.
Keywords: Pineal, Melatonin, Intestinal barrier
Introduction
In recent years, the demand for chicken meat and eggs has increased exponentially; the production of chicken meat will increase from 117 million tons to 132 million tons in 2026 (Van Boeckel et al., 2015). Not only that, people's demand for chicken quality has gradually increased. Recently, a series of studies found that the gut homeostasis is closely related to metabolite process (Cui et al., 2017), immunocompetence maintenance (Yeoman et al., 2012), body growth and development (Pan and Yu, 2014) and overall health (Diaz Carrasco et al., 2019), suggesting that gut health is a key indicator of host health. However, poultry intestinal injuries can directly reduce the quality and quantity of meat, egg and milk products, which not only causes economic losses to the livestock and poultry farming industry, but also jeopardizes human food safety. Therefore, studying and improving intestinal health is critical to livestock and human health.
The intestinal tract is the host's digestive system, but also the largest bacterial reservoir, known as the body's "second brain". Intestinal homeostasis is due to a healthy gut barrier structure prevents bacteria and toxins in the lumen of the gut from entering the bloodstream and other tissues and organs (Kong et al., 2017). Although gut microbiota is of great benefit to the host, it is still perceived as foreign by immune cells. Therefore, an elaborate barrier system can isolate gut microbiota from host immune cells thereby avoiding inflammatory responses triggered by gut microbiota. Broadly speaking, the intestinal mucosal barrier consists of intestinal mechanical barrier, intestinal chemical barrier, intestinal microbial barrier and intestinal immune barrier (Gao et al., 2023). These four intestinal barriers are functionally and structurally closely related, but at the same time relatively independent, and together they constitute a complex and orderly intestinal ecology, and damage to the structure or function of any one or more of these barriers will affect the intestinal tract and the health of the organism to varying degrees.
Melatonin, as a neurohormone secreted by the pineal gland that can enter the blood circulation, not only regulates sleep, but also reaches the intestinal tract directly and exerts a protective effect on intestinal health (Wang et al., 2023). In addition, it has been found that melatonin also acts as an endocrine transmitter signaling molecule that transmit signals from the intestinal microbiota to the host, thereby regulating the intestinal microbiota clock, metabolite rhythms, and immune functions (Gao et al., 2019). However, whether melatonin regulates intestinal homeostasis needs to be further explored. Therefore, in the current study, we removed the pineal gland of chicken to investigate the effect of melatonin on the intestinal mucosa barrier function in chicken.
Materials and methods
Animals and treatments
All experiments were approved by the Animal Welfare Committee of the Agricultural
Research Organization, China Agricultural University (Approval No. 20171114-2). A total of 24 newly hatched male chicks (Arbor Acres Broilers), which were purchased from Beijing Huadu Breeding Co., Ltd. (Beijing, China), were raised in 400-700 nm white light group (WL) under an LED system (Zhongshan Junsheng Lighting Technology Co., Ltd., Zhongshan, China) for 21 days. The light intensity was around 15 Lux, and the light regime was 12 light: 12 dark (light on at 08:30 and off at 20:30). The chickens had ad libitum access to feed and water, and the diets were formulated to meet the nutrient recommendations for poultry (NRC, 1994). The temperature in the chicken house was set at 32 °C for the first week and then reduced to 30 °C in the second week, and the relative humidity was maintained at 60 % for the entire period.
Half of the chickens raised under WL will have their pinealectomy surgery (Pinx). The Pinx was conducted at 3 days after hatching (P3) as follows (Li et al., 2015): chicks were anaesthetized by intraperitoneal injection of pentobarbital sodium (30-40 µg/g body weight) and then were placed on a stereotaxic apparatus (ALCBIO, Shanghai, China). One midsagittal incision was made in the skin above the cranium, and a small portion of the skull was removed using a dental drill to expose the pineal gland. The meninges were cut away using microsurgical scissors, the pineal gland was removed with forceps, and the opening was packed with gelfoam to reduce bleeding. The skullcap was placed back on the chick, and the wound was then closed with surgical sutures and treated with a topical antibiotic ointment.
After 21 days, the chicks were quickly sacrificed by decapitation after feeding. Their small intestinal tissue (duodenum, jejunum and ileum) were harvested.
Transmission electron microscope (TEM)
After rinsing with PBS, specimens were embedded in 30 % sucrose in PBS, then embedded in OCT complex (Sakura Finetechnical; Sakura Finetek, Tokyo, Japan), snap-frozen in liquid nitrogen, and set in −80°C. Frozen samples were cut into 20-μm-thick sections using a CM 1850 cryosectioner (Leica Microsystems, Wetzlar, Germany). The small intestinal tissue was oriented using a light microscope and the frozen sections were placed on MAS-coated glass slides (S9441, Matsumani glass, Osaka, Japan). Frozen sections were washed with 0.1 M phosphate buffer, postfixed with 1 % osmium tetroxide in 0.1 M phosphate buffer for 2 h, and then embedded with Epon-812. Ultrathin sections were prepared and mounted on copper grids, stained with uranyl acetate and lead citrate, and then examined by TEM (H-7100, Hitachi, Tokyo, Japan). Images were used to visualize small intestinal villi.
Histological staining
The small intestinal tissue (duodenum, jejunum and ileum) were immediately fixed in 4 % paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4, 4°C) for 48 h and embedded in paraffin for sectioning (5 μm, cross-section). The tissue sections were stained with hematoxylin-eosin (HE) or periodic acid-schiff (PAS). At least 60 random fields in six sections of each sample were photographed at 400 × magnification with a microscope (BX51; Olympus, Tokyo, Japan). The five longest villi per field and a total of 300 longest villi (in each sample) were analyzed per treatment. The villus length (V), crypt depth (C) and villus length/crypt depth (V/C) ratio from HE-staining and the number of goblet cells per 100 absorb cells from PAS-staining were measured.
Western blot analysis
Portions of the small intestinal segments (n = 6) were rapidly homogenized in liquid nitrogen and stored at −80°C for western blotting analysis. Total protein was extracted using lysis buffer (62.5 mmol/L Tris‐HCl, 2 % SDS, 10 % glycerol; pH 6.8). After centrifugation at 12 000 g for 10 minutes at 4°C, the supernatants were collected. The protein concentration was determined using a bicinchoninic acid (BCA) kit (Beyotime, P0012). A sample of 20 µg of protein was electrophoresed using 10 % sodium dodecyl sulphate‐polyacrylamide gelelectrophoresis. After electrotransferring the samples onto a polyvinylidene difluoride membrane (Millipore), they were blocked with 5 % skim milk in 1 × Tris‐buffered saline (TBS) with Tween (TBST) for 2 hours at room temperature, and the membranes were incubated with the monoclonal rabbit anti‐mouse primary antibodies (GAPDH, 1:2000; MUC2, 1:1000; Bax, 1:1000; Bcl2, 1:1000; Caspase3, 1:1000. Abcam) overnight at 4°C. After washing with TBST, they were incubated with horseradish peroxidase‐conjugated goat anti‐rabbit IgG (1:5000, CW0103; CoWin Biotech Co., Inc) for 2 hours at 37°C. The immunoblots were performed using an eECL western blotting kit (CW0049; CoWin Biotech Co., Inc). The bands obtained in the blots were scanned and measured using ImageJ (version 4.0.2; Scion Corp.). Data are expressed as the IOD of the bands, and the results were obtained from three repeated experiments.
Immunohistochemistry and immunofluorescence staining
Small intestinal segments was cut into 5 μm cryosections and incubated with the immunohistochemistry and immunofluorescence staining and incubated with the following primary antibodies: Lysozyme (Abcam, Cambridge, UK, ab108508; 1:1000), PCNA (Abcam, Cambridge, UK, ab29; 1:10000), Lgr5 (Abcam, Cambridge, UK, ab219107; 1:400), Occludin (Abcam, Cambridge, UK, ab216327; 1:200), ZO-1 (Abcam, Cambridge, UK, ab307799; 1:500) and F4/80 (Abcam, Cambridge, UK, ab300421; 1:500) for small intestinal segment.
For immunofluorescence staining, tissues were embedded using these secondary antibodies: goat anti-rabbit FITC 1:100 (BOSTER, BA1090, Wuhan, China). 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) (Thermo Fisher Scientific, D3571, New York, USA) staining was used to highlight nuclei. For immunohistochemical staining, sections were washed with 0.01 mol/L phosphate buffer saline PBS (pH 7.4) and incubated with biotinylated goat anti-rabbit IgG (1:200; Sigma-Aldrich, USA) for 2 h at room temperature. After washing, tissues were incubated with streptavidin-horseradish peroxidase (1:250; Sigma-Aldrich, USA) for 2 h at room temperature. Immunoreactivity was visualized by incubating the tissue sections in 0.01 mol/L PBS containing 0.05 % 3′, 3‐diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, USA) and 0.003 % hydrogen peroxide for 10 min in the dark. The sections were then stained with haematoxylin and mounted. Control slides without the primary antibody were examined in all cases. Immunoreactive cells were presented with yellow‐brown staining in the cell. The positive cells were counted in 25 random fields from five cross‐sections in each sample. The mean integral optical density (IOD) of positive cells was then determined.
Statistical analysis of data
Data analysis was performed using SPSS statistical software (version 10.0; SPSS, Inc., Chicago, IL, USA). Data are expressed as mean ± SD. Differences among groups were statistically analyzed using multiway ANOVA followed by one-way ANOVA, which was used to determine the variance of differences among groups and expressed as follows: different lowercase letters or different uppercase letters: p < 0.05; different letters and one containing uppercase letters and the other lowercase letters: p < 0.01; same letter: p > 0.05.
Results
Pinealectomy damages the intestinal structures
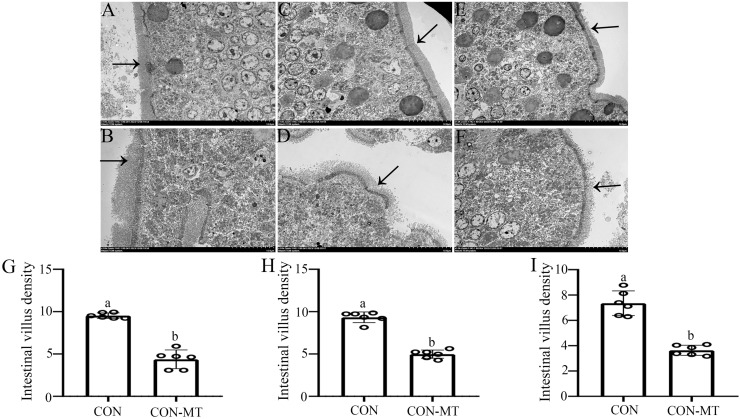
To investigate whether pinealectomy resulted in an intestinal structures damage, we observed the structure of the intestinal villi of broiler chickens under electron microscope of control and pinealectomy groups. Electron microscopy results showed that the villi in the duodenum, jejunum, and ileum of broilers in the control group were structurally intact, continuous, and densely distributed (Fig. 1A, C, E); after pinealectomy, the villi in these three segments of the intestines were reduced in number, sparsely arranged, and severely fractured (Fig. 1B, D, F). Statistically, the density of intestinal villi was significantly reduced by 32.6 % (p = 0.017, Fig. 1G) in duodenum, 30.1 % (p = 0.027, Fig. 1H) in jejunum and 40.2 % (p = 0.011, Fig. 1I) in ileum, respectively, in the pinealectomy group compared to the control group.
Fig. 1.
Pinealectomy damages the intestinal structures. (A-F) Electron microscopy of the intestinal tract in (A, B) duodenum, (C, D) jejunum and (E, F) ileum of CON and CON-MT groups, respectively (scale: 10 µm). Intestinal villus density was measured in duodenum (G), jejunum (H) and ileum (I) of CON and CON-MT groups, respectively. Values are presented as the means ± SE. Differences were assessed by ANOVA and denoted as follows: different lowercase letters: p < 0.05; different uppercase letters: p < 0.01; same letter: p > 0.05.
Pinealectomy damages the intestinal morphology
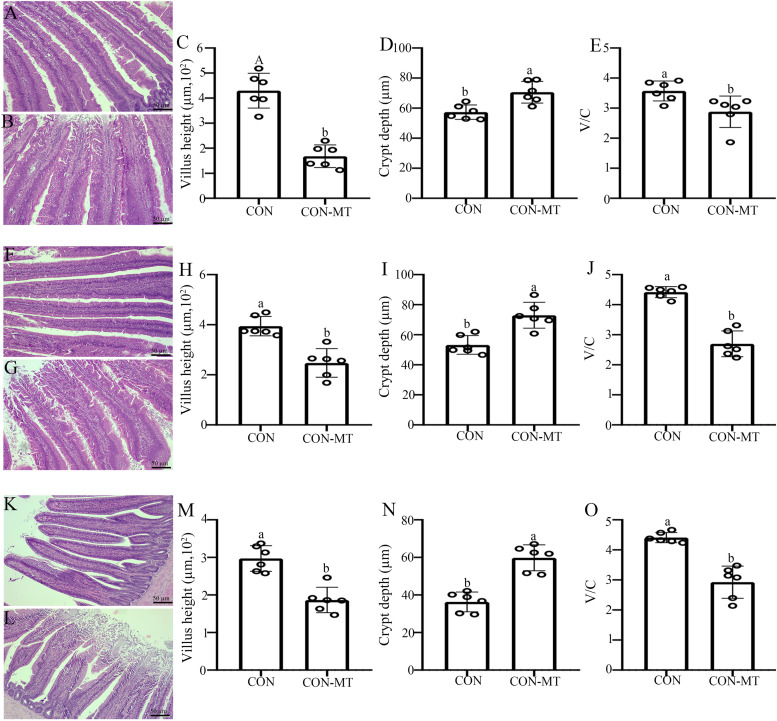
To further investigate whether pinealectomy resulted in an intestinal structures damage, we examined the changes in intestinal mucosal barrier function in duodenum, jejunum and ileum at control and pinealectomy group. The HE staining results showed that the intestinal segment of the pinealectomy group had obvious congestion, particularly in the top and middle villi in ileum, compared with control group (Fig. 2A, B, F, G, K, L).
Fig. 2.
Pinealectomy injurys the intestinal morphology. HE staining in (A, B) duodenum, (F, G) jejunum and (K, L) ileum of CON and CON-MT groups, respectively (scale: 50 µm). Villus height (C, H, M), crypt depth (D, I, N) and V/C ratio (E, J, O) were measured in duodenum, jejunum and ileum of CON and CON-MT groups, respectively. Values are presented as the means ± SE. Differences were assessed by ANOVA and denoted as follows: different lowercase letters: p < 0.05; different uppercase letters: p < 0.01; same letter: p > 0.05.
The length of the villi was significantly decreased by 62.5 % (p = 0.000, Fig. 2C) in duodenum, 20.5 % (p = 0.026, Fig. 2H) in jejunum and 25.2 % (p = 0.032, Fig. 2M) in ileum, respectively, in the pinealectomy group. Meanwhile, the crypt depth was markedly increased by 31.6 % (p = 0.015, Fig. 2D) in duodenum, 38.7 % (p = 0.026, Fig. 2I) in jejunum and 41.8 % (p = 0.010, Fig. 2N) in ileum, which resulted in a notable decrease in the V/C ratio (19.8–25.4 %, p = 0.013–0.042, Fig. 2E, J, O) compared with that of the CON group. These results showed that pinealectomy had different degrees of damage to the three small intestines. From the V/C ratio, the damage of the ileum was the most serious, followed by the jejunum and the duodenum.
Pinealectomy damages the chemical intestinal barrier
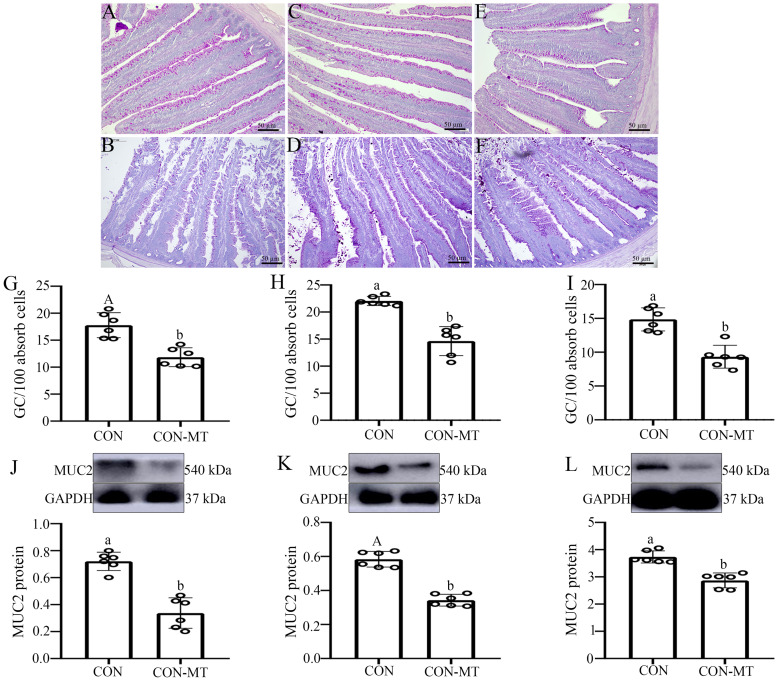
We further explored whether pinealectomy causes chemical intestinal barrier damage. PAS staining showed that the goblet cells were red and spread between intestinal villi and intestinal epithelial cells, mostly in the lower half of the villi (Fig. 3A-F). The number of goblet cells per 100 absorb cells was substantially decreased by 33.9 % (p = 0.001, Fig. 3G) in duodenum, 35.7 % (p = 0.025, Fig. 3H) in jejunum and 32.9 % (p = 0.010, Fig. 3I) in ileum of the pinealectomy group. MUC2 is an important mucin secreted by goblet cells. The presence of intestinal MUC2 is important for maintaining the stability of the intestinal luminal microenvironment and the normal function of intestinal epithelial cells. A similar effect was observed in the expression of MUC2, which was significantly decreased by 52.5 % (p = 0.012, Fig. 3J) in duodenum, 42.5 % (p = 0.000, Fig. 3K) in jejunum and 35.7 % (p = 0.034, Fig. 3L) in ileum in the pinealectomy group compared with that of the CON group.
Fig. 3.
Pinealectomy damages the chemical intestinal Barrier. (A-F) PAS staining in (A, B) duodenum, (C, D) jejunum and (E, F) ileum of CON and CON-MT groups, respectively (scale: 50 µm). Number of goblet cells per 100 absorb cells in (G) duodenum, (H) jejunum and (I) ileum of CON and CON-MT groups. The relative GAPDH protein level of MUC2 protein production were measured in (J) duodenum, (K) jejunum and (L) ileum of CON and CON-MT groups, respectively. Values are presented as the means ± SE. Differences were assessed by ANOVA and denoted as follows: different lowercase letters: p < 0.05; different uppercase letters: p < 0.01; same letter: p > 0.05.
Pinealectomy damages the physical intestinal barrier
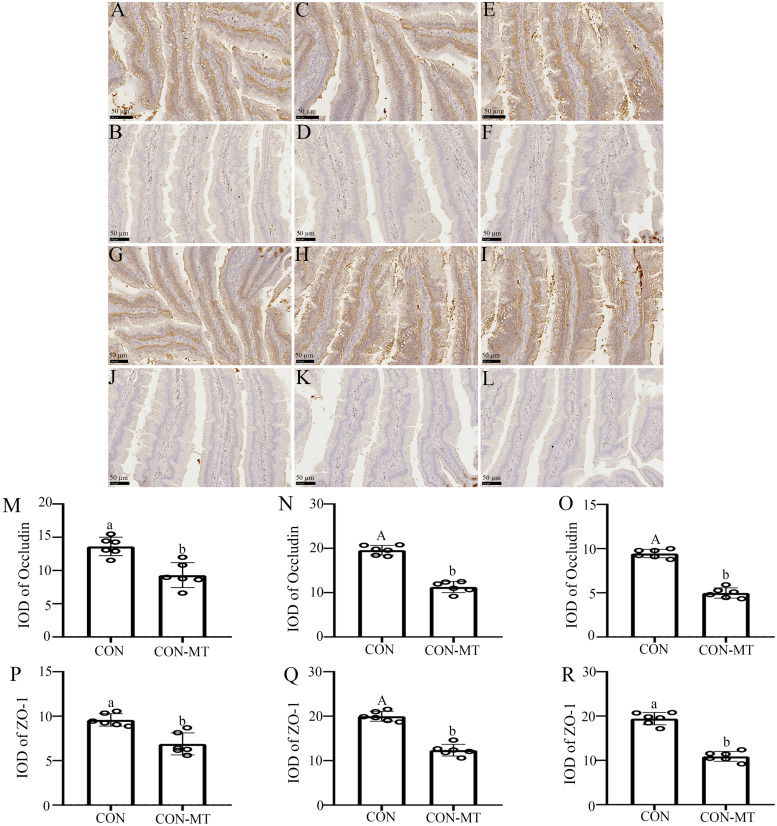
We further explored whether pinealectomy would have an effect on the physical intestinal barrier. Immunohistochemical staining showed that pinealectomy changed the distribution patterns of two tight junction proteins (Occludin and ZO-1, Fig. 4A-L). Statistically, data showed the expression level of Occludin in the pinealectomy group decreased by 45.3 % (p = 0.016, Fig. 4M), 58.5 % (p = 0.000, Fig. 4N) and 58.3 % (p = 0.000, Fig. 4O) in duodenum, jejunum and ileum compared with the CON group. Moreover, the expression level of ZO-1 in the pinealectomy group decreased by 37.3 % (p = 0.026, Fig. 4P), 48.1 % (p = 0.004, Fig. 4Q) and 54.2 % (p = 0.010, Fig. 4R) in duodenum, jejunum and ileum compared with the CON group.
Fig. 4.
Pinealectomy damages the Physical Intestinal Barrier. Immunohistochemical staining of Occludin (A-F) and ZO-1 (G-L) in duodenum (A, B, G, J), jejunum (C, D, H, K) and ileum (E, F, I, L) of CON and CON-MT groups, respectively (scale: 50 µm). IOD of Occludin and ZO-1 in duodenum (M, P), jejunum (N, Q) and ileum (O, R) of CON and CON-MT groups, respectively. Values are presented as the means ± SE. Differences were assessed by ANOVA and denoted as follows: different lowercase letters: p < 0.05; different uppercase letters: p < 0.01; same letter: p > 0.05.
Pinealectomy damages the immunological intestinal barrier
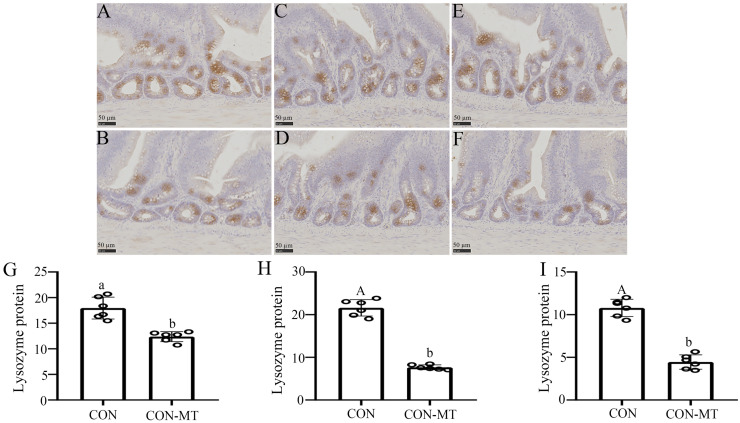
Paneth cells are a rare type of cell in the small intestines that provide host defense against microbial invasion. Upon invasion of the body by bacteria or bacterial antigens, paneth cells secrete antimicrobial molecules such as defensins into the villi of the lumen of the small intestine to help maintain the gastrointestinal barrier. Therefore, we explored the effect of pinealectomy on paneth cells by assaying the expression level of lysozyme in small intestines (Fig. 5A-F). Statistically, the IOD of lysozyme was decreased by 49.9 % (p = 0.035, Fig. 5G) in duodenum, 72.6 % (p = 0.003, Fig. 5H) in jejunum and 77.3 % (p = 0.002, Fig. 5I) in ileum in the pinealectomy group compared with the CON group. The results showed a significant decrease in the amount of lysozyme secreted by intestinal paneth cells after pinealectomy.
Fig. 5.
Pinealectomy damages the immunological Intestinal Barrier. (A-F) Immunohistochemical staining of Lysozyme (scale: 50 µm) in (A, B) duodenum, (C, D) jejunum and (E, F) ileum of CON and CON-MT groups, respectively. IOD of Lysozyme in (G) duodenum, (H) jejunum and (I) ileum of CON and CON-MT groups, respectively. Values are presented as the means ± SE. Differences were assessed by ANOVA and denoted as follows: different lowercase letters: p < 0.05; different uppercase letters: p < 0.01; same letter: p > 0.05.
Pinealectomy inhibits the proliferative viability of intestinal stem cells
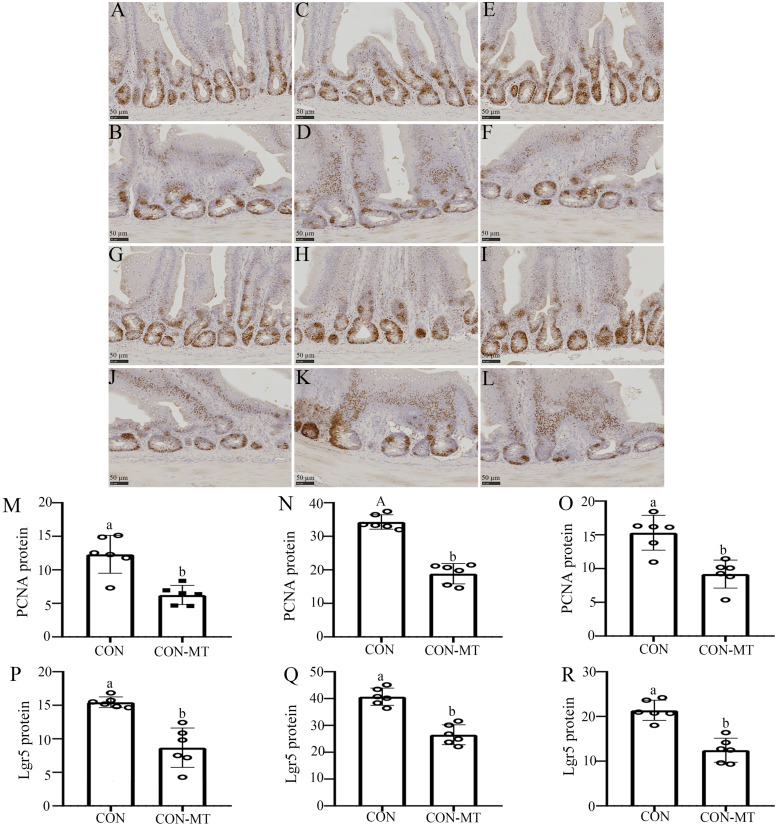
Enterocyte proliferation was detected by PCNA immunohistochemistry (Fig. 6A-F). Statistically, the IOD of PCNA positive cells was decreased by 32.1 % (p = 0.045, Fig. 6M) in duodenum, 45.3 % (p = 0.000, Fig. 6N) in jejunum and 33.2 % (p = 0.031, Fig. 6O) in ileum in the pinealectomy group compared with the CON group. Further, the intestinal stem cell marker was Lgr5, and we further directly examined the effect of pinealectomy on intestinal stem cell numbers, were detected by immunohistochemistry (Fig. 6G-L). The data showed there was a down-regulation in the IOD of Lgr5 by 40.1 % (p = 0.026, Fig. 6P) in duodenum, 36.7 % (p = 0.032, Fig. 6Q) in jejunum and 45.8 % (p = 0.022, Fig. 6R) in ileum in the pinealectomy group compared with the CON group.
Fig. 6.
Pinealectomy inhibits the proliferative viability of intestinal stem cells. Immunohistochemical staining (scale: 50 µm) of PCNA (A-F) and Lgr5 (G-L) in duodenum, jejunum and ileum of CON and CON-MT groups, respectively. IOD of PCNA and Lgr5 in (M, P) duodenum, (N, Q) jejunum and (O, R) ileum of CON and CON-MT groups, respectively. Values are presented as the means ± SE. Differences were assessed by ANOVA and denoted as follows: different lowercase letters: p < 0.05; different uppercase letters: p < 0.01; same letter: p > 0.05.
Pinealectomy suppresses the immune function of the small intestines
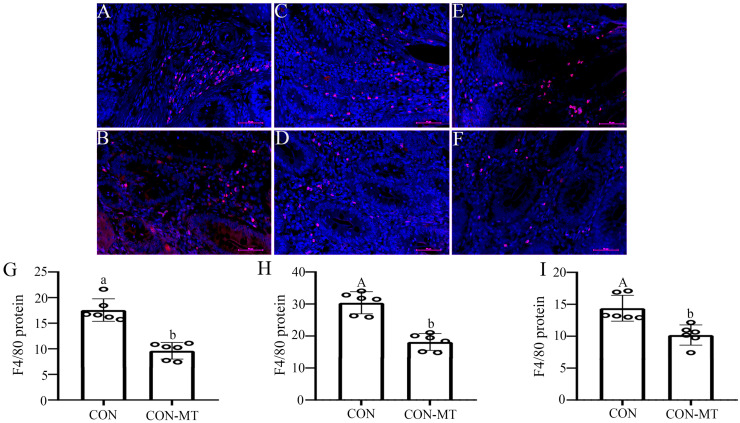
Macrophages are one of the core members of innate immune cells, which not only play a key role in intestinal immunomodulation, but also in the processes of angiogenesis, epithelial cell proliferation, stem cell differentiation, and myofibroblast activation in the intestinal tract. We therefore also tested the effect of pinealectomy on the number of intestinal macrophages via using immunofluorescence in small intestines of pinealectomy group and control group (Fig. 7A-F). The results showed that the number of intestinal macrophages decreased in the duodenum, jejunum and ileum, respectively, after pinealectomy by 42.8 % (p = 0.025, Fig. 7G), 37.6 % (p = 0.000, Fig. 7H) and 30.2 % (p = 0.000, Fig. 7I).
Fig. 7.
Pinealectomy suppresses the immune function of the small intestines. Immunofluorescence staining of F4/80 (scale: 50 µm) in (A, B) duodenum, (C, D) jejunum and (E, F) ileum of CON and CON-MT groups, respectively. IOD of Lysozyme in (G) duodenum, (H) jejunum and (I) ileum of CON and CON-MT groups, respectively. Values are presented as the means ± SE. Differences were assessed by ANOVA and denoted as follows: different lowercase letters: p < 0.05; different uppercase letters: p < 0.01; same letter: p > 0.05.
Pinealectomy increases the apoptosis level in intestinal epithelial cells
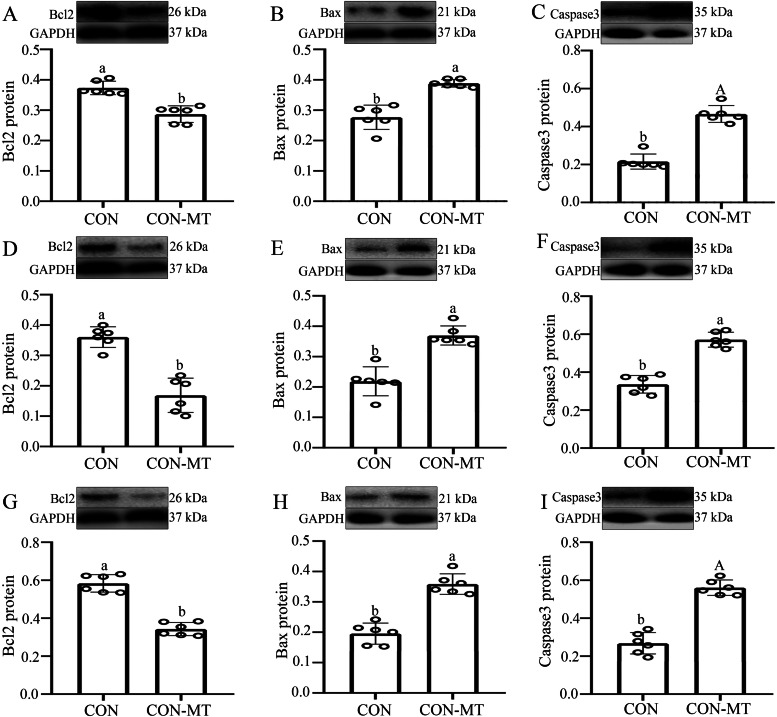
The homeostasis of intestinal epithelial cells lies in the dynamic balance between cell proliferation and apoptosis, so we further examined the apoptosis level (Bcl2, Bax and Caspase3) in intestinal epithelial cells after pinealectomy. Data suggested that the expression level of Bcl2 protein significantly decreased by 21.2 % (p = 0.033, Fig. 8A), 22.7 % (p = 0.025, Fig. 8D) and 32.8 % (p = 0.032, Fig. 8G) in duodenum, jejunum and ileum in the pinealectomy group compared with the CON group. Moreover, compared with CON group, the expression level of Bax and Casapse3 proteins obviously increased by 22.5-43.5 % (p = 0.000-0.015, Fig. 8B, C), 27.8-30.1 % (p = 0.013-0.028, Fig. 8E, F) and 33.7-40.6 % (p = 0.003-0.018, Fig. 8H, I) in duodenum, jejunum and ileum in the pinealectomy group.
Fig. 8.
Pinealectomy increases the apoptosis level in intestinal epithelial cells. The relative GAPDH protein level of Bcl2 (A, D, G), Bax (B, E, H) and Caspase3 (C, F, I) proteins production were measured in duodenum (A-C), jejunum (D-F) and ileum (G-I) of CON and CON-MT groups, respectively. Values are presented as the means ± SE. Differences were assessed by ANOVA and denoted as follows: different lowercase letters: p < 0.05; different uppercase letters: p < 0.01; same letter: p > 0.05.
Discussion
The intestinal mucosal barrier is responsible for the absorption of nutrients and excretion of wastes, and also provides an effective defense against pathogen invasion (Ramanan and Cadwell, 2016). Therefore, the promotion of intestinal development is essential to maintain the health of the organism and reduce the increase in disease incidence (Cho and Yoon, 2014). Intestinal mucosal barriers mainly include mechanical, biological, chemical and immunological barriers (Chairakaki and Greece, 2009), of which mechanical barriers are an important component of intestinal mucosal immunity. The villus height and crypt depth represent the degree of maturation and differentiation of the small intestinal mucosa (Fukumori et al., 2022), and therefore, the V/C ratio is considered an important index for evaluating the mechanical barrier of the small intestines (Dang et al., 2023). The present study showed that the small intestinal villi of control group had a neat and regular morphology, and the epithelial cells were neatly arranged. After pinealectomy, the villi height in the duodenum, jejunum and ileum decreased to different degrees, and the number of villi in the small intestine decreased, shortened and thickened, fused with each other, and the morphology of the villi was very irregular, and there were villi breakage phenomenon, and villi fragments of varying sizes appeared in the lumen of the intestine, and the glands of the small intestine shortened and thinned, and became atrophic. Similarly, reduced melatonin levels caused by 4 weeks of acute sleep deprivation also caused a significant reduction in villus height in the ileum of rats (Ren et al., 2018).
In addition, the results of PAS staining in this experiment showed that the number of goblet cells in the small intestines decreased to different degrees after pinealectomy, suggesting that pinealectomy-induced melatonin level decreased also caused damage to the intestinal immune barrier. The decrease in the number of goblet cells may be due to the large consumption of goblet cells during the reconstruction of the intestinal mucosa, as well as the necrosis and autophagy of the cells under the stimulation of stress. Naama et al. (2023) also found that the number of goblet cells in the intestinal mucosa decreased significantly after endoplasmic reticulum stress. In pinealectomy group, the decrease in the number of goblet cells was accompanied by a decrease in the expression of MUC2, an O-glycosylated protein secreted by the goblet cells in the intestine, which serves as an important immune barrier through the formation of mucus (Parlato and Yeretssian, 2014). Our data also showed that the expression of Occludin and ZO-1 was significantly reduced in all intestinal segments after pinealectomy. Similarly, the increase in intestinal permeability induced by restraint stress has been associated with the temporary redistribution of Occludin and ZO-1 (Mazzon et al., 2002). Moreover, intestinal mucosal immune-associated cells are the first line of defense against various pathogenic microorganisms entering the intestinal mucosa. Macrophages are the gatekeepers of intestinal immunity, and paneth cells can resist the invasion of pathogenic bacteria by secreting lysozyme. Our results showed that after pinealectomy, the melatonin content decreased, and the number of intestinal macrophages and lysozyme decreased significantly, which suggests that pinealectomy leads to the damage of intestinal immune mucosa in broiler chickens. Further, the proliferative zone of intestinal cells in control group was in the crypt, suggesting the presence of undifferentiated cells with high proliferative capacity in the intestinal glandular epithelium. The number of PCNA-positive cells in the intestinal glandular epithelium of the pinealectomy group showed a significant decrease, suggesting that the proliferation of intestinal crypt cells was reduced, possibly due to intestinal energy deficiency. The decrease in the proliferation of crypt cells may be due to the poor nutritional status of the intestinal mucosal cells caused by the lack of intestinal energy and insufficient substrates, which affects the biosynthesis of the cells and slows down the renewal of the epithelial cells, resulting in mucosal atrophy and epithelial detachment (González-Magaña and Blanco Human, 2020).
Overall, our results suggest that melatonin can act as a signaling molecule to transmit information to the intestine, which in turn modulates the intestinal mucosal barrier function. Specifically, melatonin improves intestinal microbiota homeostasis, promotes energy metabolism, and up-regulates the proliferation and differentiation of intestinal stem cells, and ultimately coordinates the intestinal homeostasis and promotes the growth and development of broiler chickens. Our findings provide new theoretical support for the future use of melatonin in intestinal development and new ideas about the relationship between brain-gut axis.
Availability of data and materials
The data are available from the corresponding author upon reasonable request.
Conflict of interests
The authors declare no financial or commercial conflict of interest.
Acknowledgments
Credit author statement
Ting Gao, Chao Song: Conceptualization, Methodology, Formal analysis, Writing the paper draft,Visualization; Performing the experiments; Zixu Wang: Supervision, Project administration, Methodology; Yaoxing Chen: Conceptualization, Formal analysis, Validation, Correcting the draft, Project administration, Supervising the experimentators, Funding acquisition.
All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
This work was supported by the Chinese National Natural Science Foundation (32402845, 32172801, 32372954) and Beijing Natural Science Foundation (5244041, 6222019).
References
- Chairakaki A.D., Greece T. The role of intestinal microbial/immune cell interactions in patients with inflammatory bowel disease. Ann. Gastroenterol. 2009;22:239–241. [Google Scholar]
- Cho Y.I., Yoon K.J. An overview of calf diarrhea - infectious etiology, diagnosis, and intervention. J. Vet. Sci. 2014;15:1–17. doi: 10.4142/jvs.2014.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Wang Q., Liu S., Sun R., Zhou Y., Li Y. Age-related variations in intestinal microflora of free-range and caged hens. Front. Microbiol. 2017;8:1310. doi: 10.3389/fmicb.2017.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang X., Lee H., S J., Lee J.H., Song S.M., Lee K.Y., Han K., Kim I.H. Tributyrin and anise mixture supplementation improves growth performance, nutrient digestibility, jejunal villus height, and fecal microbiota in weaned pigs. Front. Vet. Sci. 2023;27 doi: 10.3389/fvets.2023.1107149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Carrasco J.M., Casanova N.A., Fernández Miyakawa M.E. Microbiota, gut health and chicken productivity: What is the connection? Microorganisms. 2019;7:374. doi: 10.3390/microorganisms7100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori R., Doi K., Mochizuki T., Oikawa S., Gondaira S., Iwasaki T., Izumi K. Sodium butyrate administration modulates the ruminal villus height, inflammation-related gene expression, and plasma hormones concentration in dry cows fed a high-fiber diet. Anim. Sci. J. 2022;93:e13791. doi: 10.1111/asj.13791. [DOI] [PubMed] [Google Scholar]
- Gao T., Wang Z., Dong Y., Cao J., Lin R., Wang X., Yu Z., Chen Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J Pineal Res. 2019;67:e12574. doi: 10.1111/jpi.12574. [DOI] [PubMed] [Google Scholar]
- Gao T., Li Y., Wang X., Tao R., Ren F. Bifidobacterium longum 68S mediated gut-skin axis homeostasis improved skin barrier damage in aging mice. Phytomedicine. 2023;120 doi: 10.1016/j.phymed.2023.155051. [DOI] [PubMed] [Google Scholar]
- González-Magaña A., Blanco Human F.J. PCNA Structure, Function and Interactions. Biomolecules. 2020;8:570. doi: 10.3390/biom10040570. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W., Huang C., Tang Y., Zhang D., Wu Z., Chen X. Effect of Bacillus subtilis on Aeromonas hydrophila-induced intestinal mucosal barrier function damage and inflammation in grass carp (Ctenopharyngodon idella) Sci. Rep. 2017;7:1588. doi: 10.1038/s41598-017-01336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzon E., Sturniolo G.C., Puzzolo D., Frisina N., Fries W. Effect of stress on the paracellular barrier in the rat ileum. Gut. 2002;51:507–513. doi: 10.1136/gut.51.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naama M., Telpaz S., Awad A., Ben-Simon S., Harshuk-Shabso S., Modilevsky S., Rubin E., Sawaed J., Zelik L., Zigdon M., Asulin N., Turjeman S., Werbner M., Wongkuna S., Feeney R., Schroeder B.O., Nyska A., Nuriel-Ohayon M., Bel S. Autophagy controls mucus secretion from intestinal goblet cells by alleviating ER stress. Cell Host. Microbe. 2023;31:433–446. doi: 10.1016/j.chom.2023.01.006. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut. Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlato M., Yeretssian G. NOD-like receptors in intestinal homeostasis and epithelial tissue repair. Int. J. Mol. Sci. 2014;15:9594–9627. doi: 10.3390/ijms15069594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan D., Cadwell K. Intrinsic defense mechanisms of the intestinal epithelium. Cell Host. Microbe. 2016;19:434–441. doi: 10.1016/j.chom.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W., Wang P., Yan J., Liu G., Zeng B., Hussain T., Peng C., Yin J., Li T., Wei H., Zhu G., Reiter R.J., Tan B., Yin Y. Melatonin alleviates weanling stress in mice: Involvement of intestinal microbiota. J. Pineal. Res. 2018;64:e12448. doi: 10.1111/jpi.12448. [DOI] [PubMed] [Google Scholar]
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Z. Wang, J. Cao, Y. Dong, and Y. Chen. 2023. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. 31;11:17. [DOI] [PMC free article] [PubMed]
- Yeoman C.J., Chia N., Jeraldo P., Sipos M., Goldenfeld N.D., White B.A. The microbiome of the chicken gastrointestinal tract. Anim. Health Res. Rev. 2012;13:89–99. doi: 10.1017/S1466252312000138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.