Abstract
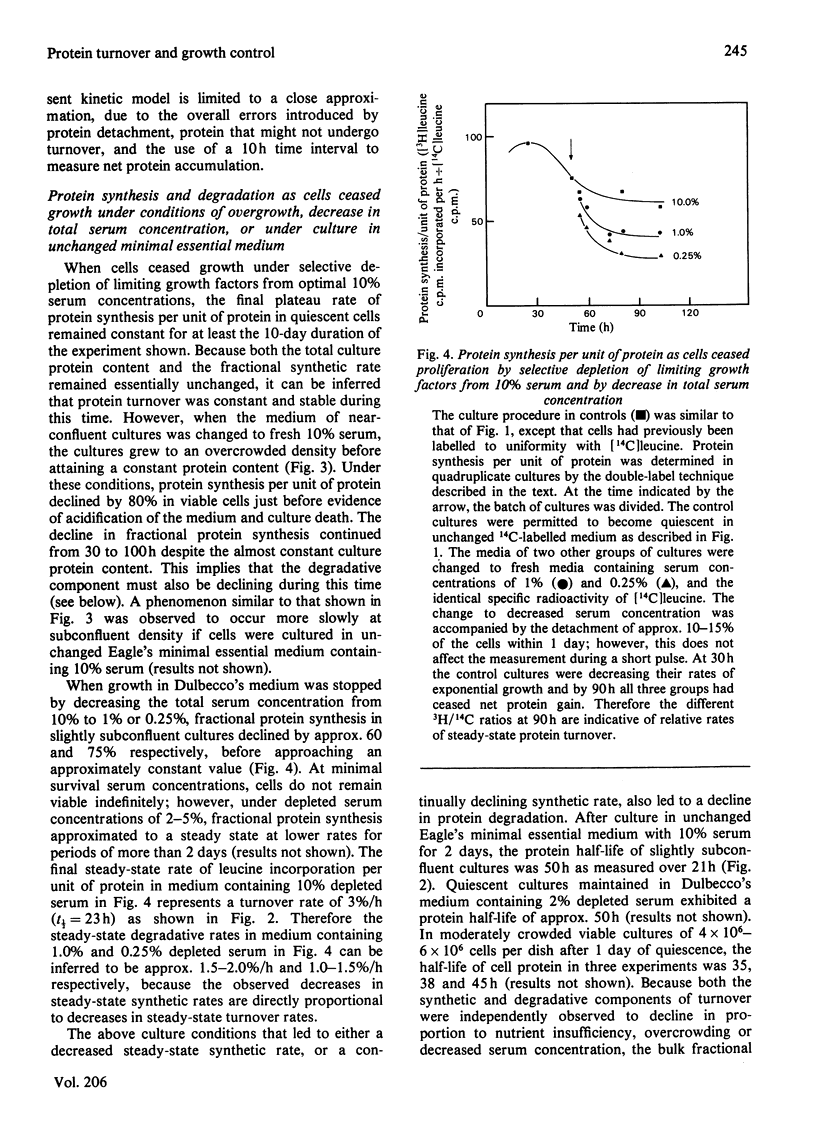
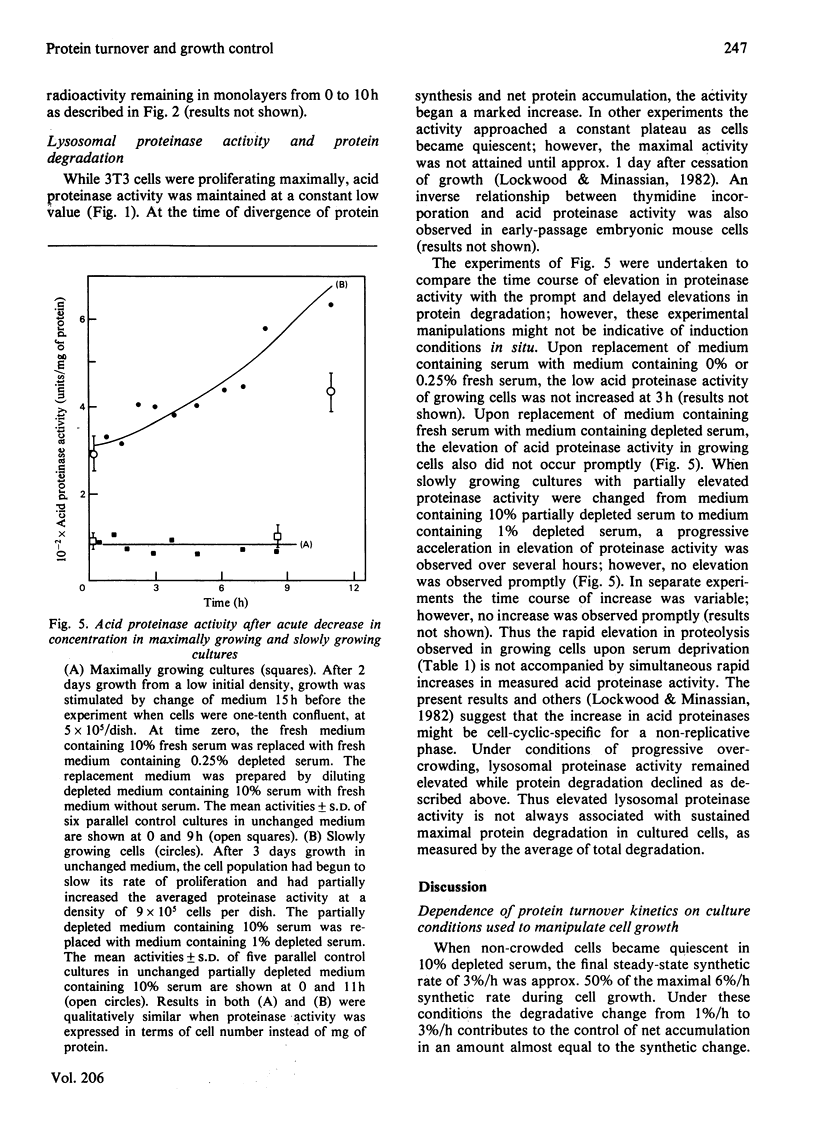
1. At least 95% of the total protein of A31-3T3 cell cultures undergoes turnover. 2. First-order exponential kinetics were used to provide a crude approximation of averaged protein synthesis, Ks, degradation, Kd, and net accumulation, Ka, as cells ceased growth at near-confluent density in unchanged Dulbecco's medium containing 10% serum. The values of the relationship Ka = Ks - Kd were : 5%/h = 6%/h - 1%/h in growing cells, and 0%/h = 3%/h - 3%/h in steady-state resting cells. 3. As determined by comparison of the progress of protein synthesis and net protein accumulation, the time course of increase in protein degradation coincided with the onset of an increase in lysosomal proteinase activity and decrease in thymidine incorporation after approx. 2 days of exponential growth. 4. After acute serum deprivation, rapid increases in protein degradation of less than 1%/h could be superimposed on the prevailing degradation rate in either growing or resting cells. The results indicate that two proteolytic mechanisms can be distinguished on the basis of the kinetics of their alterations. A slow mechanism changes in relation to proliferative status and lysosomal enzyme elevation. A prompt mechanism, previously described by others, changes before changes in cell-cycle distribution or lysosomal proteinase activity. 5. When the serum concentration of growing cultures was decreased to 1% or 0.25%, then cessation of growth was accompanied by a lower steady-state protein turnover rate of 2.0%/h or 1.5%/h respectively. When growth ceased under conditions of overcrowded cultures, or severe nutrient insufficiency, protein turnover did not attain a final steady state, but declined continually into the death of the culture.
Full text
PDF



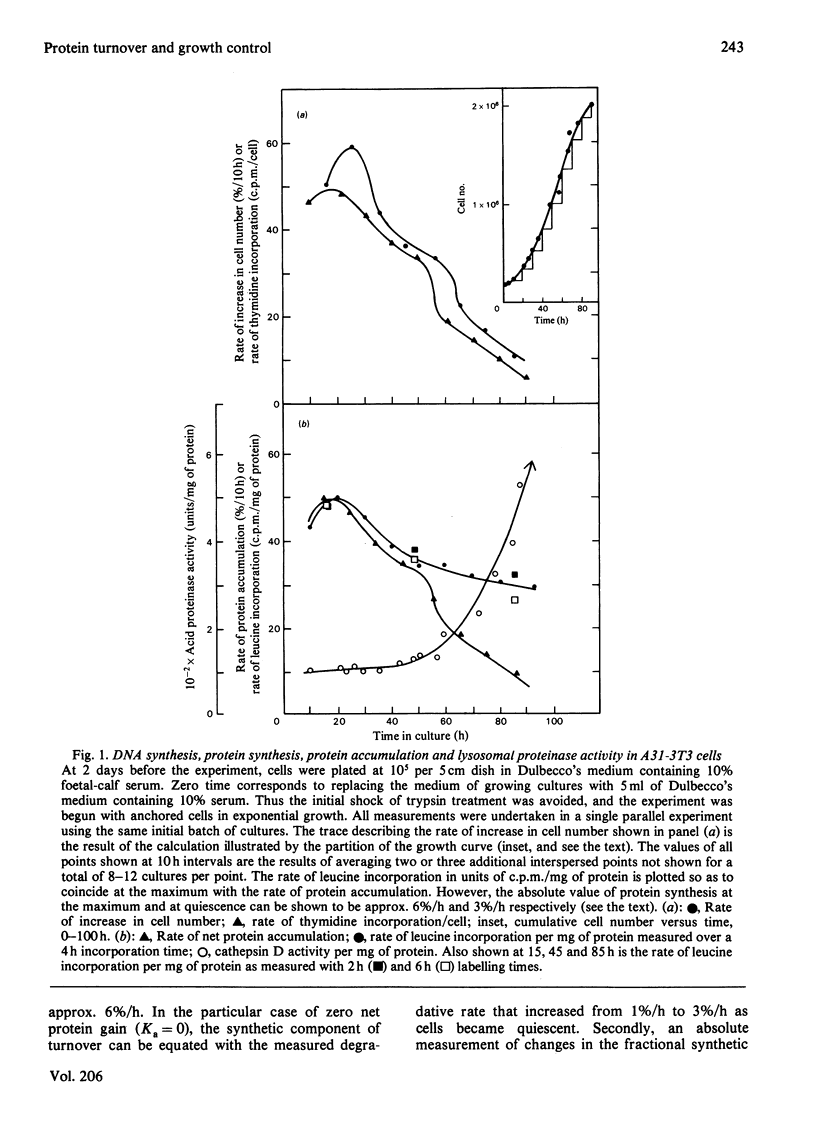
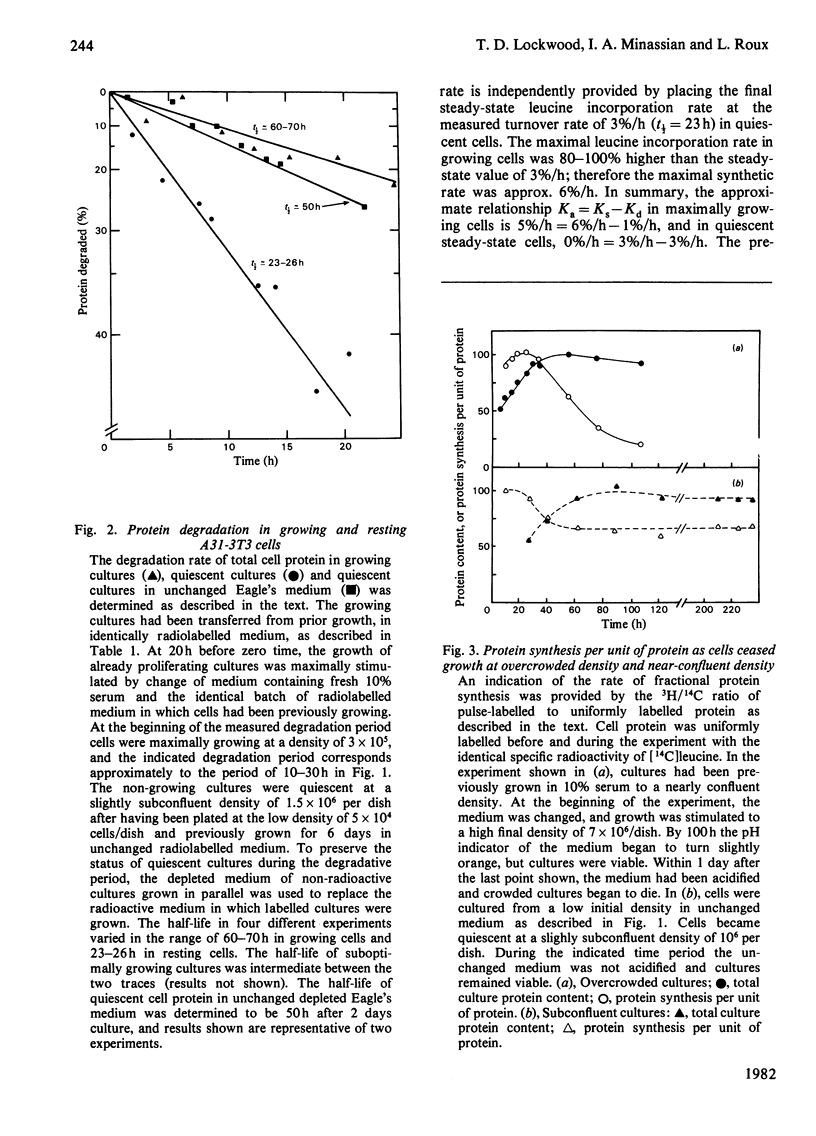



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Brocher S. C. Protein synthesis and degradation in growth regulation in rat embryo fibroblasts: role of fast-turnover and slow-turnover protein. J Cell Physiol. 1980 Oct;105(1):51–61. doi: 10.1002/jcp.1041050108. [DOI] [PubMed] [Google Scholar]
- Bartholomew J. C., Yokota H., Ross P. Effect of serum on the growth of Balb oT3 A31 mouse fibroblasts and an SV40-transformed derivative. J Cell Physiol. 1976 Jul;88(3):277–286. doi: 10.1002/jcp.1040880303. [DOI] [PubMed] [Google Scholar]
- Baxter G. C., Stanners C. P. The effect of protein degradation on cellular growth characteristics. J Cell Physiol. 1978 Aug;96(2):139–145. doi: 10.1002/jcp.1040960202. [DOI] [PubMed] [Google Scholar]
- Bradley M. O., Hayflick L., Schimke R. T. Protein degradation in human fibroblasts (WI-38). Effects of aging, viral transformation, and amino acid analogs. J Biol Chem. 1976 Jun 25;251(12):3521–3529. [PubMed] [Google Scholar]
- Bradley M. O. Regulation of protein degradation in normal and transformed human cells. Effects of growth state, medium composition, and viral transformation. J Biol Chem. 1977 Aug 10;252(15):5310–5315. [PubMed] [Google Scholar]
- Castor L. N. Responses of protein synthesis and degradation in growth control of WI-38 cells. J Cell Physiol. 1977 Sep;92(3):457–467. doi: 10.1002/jcp.1040920313. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Protein degradation in cell cultures: general considerations on mechanisms and regulation. Fed Proc. 1980 Jan;39(1):15–19. [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- Gunn J. M., Clark M. G., Knowles S. E., Hopgood M. F., Ballard F. J. Reduced rates of proteolysis in transformed cells. Nature. 1977 Mar 3;266(5597):58–60. doi: 10.1038/266058a0. [DOI] [PubMed] [Google Scholar]
- Hendil K. B. Intracellular protein degradation in growing, in density-inhibited, and in serum-restricted fibroblast cultures. J Cell Physiol. 1977 Sep;92(3):353–364. doi: 10.1002/jcp.1040920304. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Hille M. B., Barrett A. J., Dingle J. T., Fell H. B. Microassay for cathepsin D shows an unexpected effedt of cycloheximide on limb-bone rudiments in organ culture. Exp Cell Res. 1970 Aug;61(2):470–472. doi: 10.1016/0014-4827(70)90476-3. [DOI] [PubMed] [Google Scholar]
- Hod Y., Hershko A. Relationship of the pool of intracellular valine to protein synthesis and degradation in cultured cells. J Biol Chem. 1976 Jul 25;251(14):4458–4457. [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2942–2945. doi: 10.1073/pnas.71.8.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollwy R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: serum factors. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2908–2911. doi: 10.1073/pnas.71.7.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer H., Heinrich P. C. Control of proteolysis. Annu Rev Biochem. 1980;49:63–91. doi: 10.1146/annurev.bi.49.070180.000431. [DOI] [PubMed] [Google Scholar]
- Kaftory A., Hershko A., Fry M. Protein turnover in senescent cultured chick embryo fibroblasts. J Cell Physiol. 1978 Feb;94(2):147–160. doi: 10.1002/jcp.1040940204. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lockwood T. D., Minassian I. A. Protein turnover and proliferation. Failure of SV-3T3 cells to increase lysosomal proteinases, increase protein degradation and cease net protein accumulation. Biochem J. 1982 Aug 15;206(2):251–258. doi: 10.1042/bj2060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood T. D., Shier W. T. Regulation of acid proteases during growth, quiescence and starvation in normal and transformed cells. Nature. 1977 May 19;267(5608):252–254. doi: 10.1038/267252a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Neff N. T., Ross P. A., Bartholomew J. C., Bissell M. J. Leucine in cultured cells: its metabolism and use as a marker for protein turnover. Exp Cell Res. 1977 Apr;106(1):175–183. doi: 10.1016/0014-4827(77)90254-3. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., McConkey E. H. Evidence for control of protein synthesis in HeLa cells via the elongation rate. J Cell Physiol. 1980 Sep;104(3):269–281. doi: 10.1002/jcp.1041040302. [DOI] [PubMed] [Google Scholar]
- Ochoa S., de Haro C. Regulation of protein synthesis in eukaryotes. Annu Rev Biochem. 1979;48:549–580. doi: 10.1146/annurev.bi.48.070179.003001. [DOI] [PubMed] [Google Scholar]
- Olsen I., Dean M. F., Harris G., Muir H. Direct transfer of a lysosomal enzyme from lymphoid cells to deficient fibroblasts. Nature. 1981 May 21;291(5812):244–247. doi: 10.1038/291244a0. [DOI] [PubMed] [Google Scholar]
- Poole B., Wibo M. Protein degradation in cultured cells. The effect of fresh medium, fluoride, and iodoacetate on the digestion of cellular protein of rat fibroblasts. J Biol Chem. 1973 Sep 10;248(17):6221–6226. [PubMed] [Google Scholar]
- Robinson J. H. The nature of the amino acid pool used for protein synthesis in cultured, androgen-responsive tumour cells. Exp Cell Res. 1977 Apr;106(1):239–246. doi: 10.1016/0014-4827(77)90261-0. [DOI] [PubMed] [Google Scholar]
- Van Venrooij W. J., Moonen H., Van Loon-Klaassen L. Source of amino acids used for protein synthesis in HeLa cells. Eur J Biochem. 1974 Dec 16;50(1):297–304. doi: 10.1111/j.1432-1033.1974.tb03898.x. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Poole B. Effect of medium composition on protein degradation and DNA synthesis in rat embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2427–2431. doi: 10.1073/pnas.74.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]


