Highlights
-
•
Glioblastoma multiforme is one of the most aggresive tumours with poor prognosis for patients.
-
•
Standard therapy has still based on a combination therapy of surgical resection, temozolomide and radiotherapy but clinical lack of response is prelevant.
-
•
New formulations of drug carriers such as liposomes, nanoparticles and polymers have been intensively explored to improve clinical outcomes in glioblastoma.
-
•
Targeted therapy based on new drug formulations or advanced carrier drugs based on a thorough understanding of glioblastoma pathology could bring the expected breakthrough in clinical therapy.
Keywords: Glioblastoma, Targeted therapy, Chemoresistance phenotype, Anticancer treatment
Abstract
Glioblastoma multiforme (GBM) is one of the most aggressive and lethal brain tumors, characterized by rapid growth, invasiveness, and resistance to standard therapies, including surgery, chemotherapy, and radiotherapy. Despite advances in treatment, GBM remains highly resistant due to its complex molecular mechanisms, including angiogenesis, invasion, immune modulation, and lipid metabolism dysregulation. This review explores recent breakthroughs in targeted therapies, focusing on innovative drug carriers such as nanoparticles and liposomes, and their potential to overcome GBM's chemo- and radioresistant phenotypes. We also discuss the molecular pathways involved in GBM progression and the latest therapeutic strategies, including immunotherapy and precision medicine approaches, which hold promise for improving clinical outcomes. The review highlights the importance of understanding GBM's genetic and molecular heterogeneity to develop more effective, personalized treatment protocols aimed at increasing survival rates and enhancing the quality of life for GBM patients.
Graphical abstract
Introduction
Glioblastoma multiforme (GBM), a grade IV tumor according to the WHO classification, is the most aggressive subtype, known for its rapid proliferation, invasiveness, and poor prognosis despite available treatment strategies [1]. The annual incidence of GBM is about 2–3 new cases per 100,000 people, representing 15–20 % of all primary brain tumors, and primarily affects older adults, with a median age of 64 years at the time of diagnosis [2]. Despite multimodal treatment, including surgery, radiotherapy, and chemotherapy, the median survival time for patients is only 12 to 15 months [3].
The classification of GBM into molecular subtypes—classical, proneural, and mesenchymal—further complicates the disease's landscape [4]. For example, the classical subtype is associated with EGFR amplification, while proneural gliomas are usually linked to mutations in the IDH1 and PDGFRA genes [5]. The mesenchymal subtype, characterized by NF1 mutations and activation of the NF-κB pathway, has the worst prognosis [5]. Understanding these molecular differences is crucial for developing personalized therapies [6].
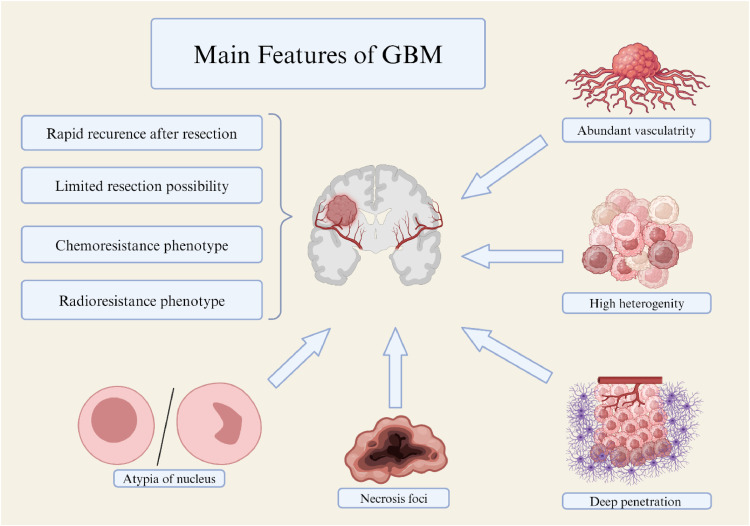
From a genetic perspective, key mutations in genes such as PTEN, TP53, and EGFR drive the progression of GBM [7]. Specific changes, such as MGMT promoter methylation, influence treatment response, particularly to alkylating agents like temozolomide (TMZ) [8]. Additionally, mutations in the IDH1 gene, common in lower-grade gliomas but also found in GBM, are associated with better treatment outcomes [9]. Typical morphological features include an increasing vascular network resembling glomeruli, necrotic foci, nuclear atypia and pleomorphism, abnormal mitotic figures, variable GFAP expression, and the presence of giant cells characteristic of giant cell glioma (Fig. 1).
Fig. 1.
Characteristic features of glioblastoma multiforme (GBM) biology linked with treatment failure. Created with BioRender.com.
Current treatment options for GBM are limited and include surgical resection, radiotherapy, and chemotherapy, with TMZ as the standard chemotherapeutic agent [10]. However, GBM's well-documented resistance to therapy, along with its heterogeneity and invasive nature, leads to frequent relapses and poor long-term outcomes [11]. New therapies, such as targeted treatments and immunotherapies, are being intensely studied, offering hope for future breakthroughs [12].
Despite these advances, the complexity of GBM, its genetic diversity, and treatment resistance underscore the need for the development of novel therapeutic strategies [13]. Future research must focus on improving the understanding of the molecular mechanisms driving GBM progression and overcoming barriers such as drug resistance and the challenges of crossing the blood-brain barrier [14].
Genetic features
Specific genetic variants frequently associated with GBM, such as activating mutations in the EGFR gene, its amplifications and point mutations (e.g. EGFRvIII), are observed in a significant number of patients [6,7,15]. In addition, mutations in tumor suppressor genes such as PTEN and TP53 are widespread and affect 30–40 % of patients [16,17]. Mutations in IDH1 (isocitrate dehydrogenase), which are more common in low-grade astrocytic gliomas may be also present in GBM, which influence patient prognosis and response to treatment, where mutation in IDH1 give more favorable overall survival prognosis for patient compare to IDH wild type [6,9,15].
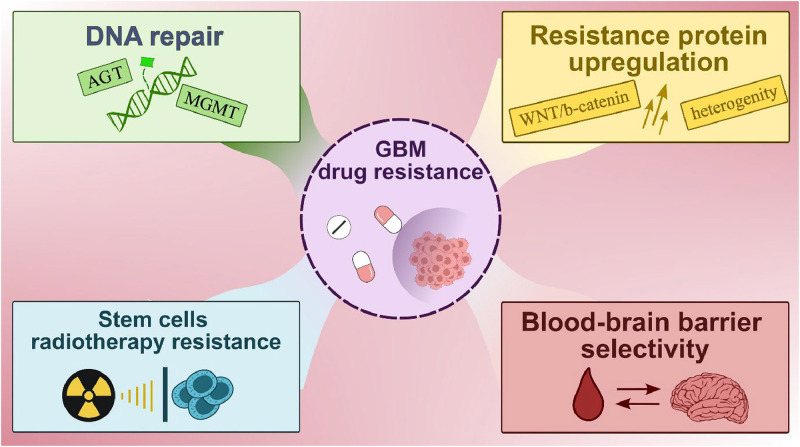
Expression of MGMT (6 -methylguanine-DNA methyltransferase) gene, which is involved in DNA repair, affects treatment sensitivity, with methylation of its promoter region mostly increasing sensitivity to alkylating agents, such as TMZ [8,10]. Other gene mutations, including PIK3CA, RB1, NF1 and CDKN2A/B, may also affect disease progression by activating signaling pathways such as PI3K/Akt/mTOR or disrupting cell cycle regulation [[18], [19], [20]]. Loss of function of tumor suppressor genes such as RB1 and NF1 can lead to uncontrolled cell division and faster disease progression [20,21]. Understanding the underlying genetic mutations and molecular pathways that drive tumor growth is critical to developing effective therapeutic strategies and improving patient outcomes. Recent literature which focuses on GBM therapy has emphasized the importance of personalized medicine approaches, targeted therapies, immunotherapies, and molecular profiling in tailoring treatment regimens for individual patients [12,22,23]. Advances in genome sequencing technology have enabled the identification of new therapeutic targets and the development of precision medicine approaches for GBM treatment, for example non-coding mutations with regulatory potential (like in the genes DLX5, DLX6, FOXA1, ISL1), and also epigenetic changes like DNA methylation (in SPINT2, GATA6, NEFM, CCNA1) [15,24]. In addition, ongoing research is focused on overcoming challenges such as drug resistance, blood-brain barrier (BBB) penetration, and tumor heterogeneity to improve treatment efficacy and patient outcomes (Fig. 2) [14,25,26].
Fig. 2.
A key challenge in the treatment of GBM which may lead to clinical impairment in patients undergoing therapy. Created with BioRender.com.
Methods and concepts
This manuscript is a narrative review, aimed at summarizing current insights into the genetic landscape, treatment resistance, and potential therapeutic targets for glioblastoma multiforme (GBM). Our objective was to provide a comprehensive overview of the key pathways and molecular mechanisms involved in GBM, as well as to highlight new treatment avenues and challenges in overcoming resistance.
To select relevant literature for this review, we conducted a broad search across various databases, including PubMed, Scopus, and Web of Science, focusing on studies published between 2000 and 2023. Keywords used in our search strategy included "glioblastoma," "genetic mutations," "targeted therapy," "chemoresistance," and "treatment resistance, “temozolomide in glioblastoma", “radiotherapy in glioblastoma We also included recent studies discussing novel drug formulations, nanomedicine, and immunotherapies for GBM.
We applied inclusion criteria, which required studies to focus on genetic pathways, molecular targets, and new treatment strategies for glioblastoma. Exclusion criteria involved non-English studies and articles lacking relevance to GBM treatment.
Current treatment for GBM
Radiotherapy is the standard adjuvant treatment after surgery or in cases where surgery is not possible. Its aim is to destroy remaining cancer cells and delay tumor growth [27,28]. Radiotherapy can be used as the primary form of therapy or in combination with chemotherapy. Chemotherapy plays a decisive role both in the initial treatment and in the treatment of recurrences [29,30]. The pharmacokinetics of chemotherapeutic agents current used in the treatment of GBM are described in Table 1.
Table 1.
The pharmacokinetics of chemotherapeutic agents used in the treatment of GBM in terms of LADME (liberation, absorption, distribution, metabolism, excretion/elimination processes).
| Temozolomide (TMZ) | Lomustine (CCNU) | Procarbazine | Carmustine (BCNU) | Vincristine | References | |
|---|---|---|---|---|---|---|
| Administration | oral (28-day cycles: 5 days drug and 23 day break) | oral administration (6-8 weeks cycles) | oral administration | intravenously | Intravenously | [[115], [116], [117], [118], [119]] |
| Absorption and distribution |
|
|
|
can cross the BBB, however its distribution in tumor tissue may vary and tumor concentrations may often be lower than in other tissues. | it is less effective at penetrating the BBB due to its chemical properties, which may limit its availability in tumor tissue in the brain; used in the treatment of GBM due to its antimitotic effect |
[58,[120], [121], [122], [123], [124], [125], [126]] |
| Half-life | approximately 1–2 h | 1–6 h (metabolites 16–48 h) | approximately 10 min | approximately 15–30 min | initial – 5 min, middle – 2.3 h, terminal – 85 h | [119,123, [127], [128], [129]] |
| Metabolism | metabolized by enzymes from the cytochrome P450 (CYP) | metabolized in the liver by microsomal enzymes | metabolized in the liver by oxidation into various active metabolites | metabolized in the liver by microsomal enzymes | metabolized in the liver by microsomal enzymes | [[130], [131], [132], [133], [134], [135], [136]] |
| Elimination | in the urine | mainly in the urine | in the urine | rapid elimination, primarily through excretion in the urine. | primarily through bile, with a minor proportion being excreted in urine | [119,123,127,128,133] |
In recent years, immunotherapies have emerged as promising approaches that aim to harness the patient's immune system to combat neoplasm, although they are still in the clinical trial phase [31,32]. These treatment regimens may represent a new path for the treatment of GBM.
Supportive therapies, including symptomatic treatment, rehabilitation, palliative care and psychological support, are integral components of comprehensive care for neuro-oncological practice aimed at optimizing patient's quality of life [29,[33], [34], [35]]. Individualized treatment approaches are of paramount importance, taking into account factors such as the patient's age, general health, tumor location and stage and other relevant considerations [[36], [37], [38]].
Alkylating agents
In the field of chemotherapy destined for GBM and other gliomas, several drugs play a crucial role in treatment regimens. TMZ, imidazotetrazole derivative of decarbazine, an orally administered DNA alkylating agent, is one of the most commonly used drugs. It disrupts the DNA double strands, inhibits their growth and triggers apoptosis. TMZ is often used as first-line therapy or in combination with radiotherapy and is a cornerstone in the treatment of newly diagnosed GBM. [[39], [40], [41], [42]]. TMZ is renowned for its enhanced RT, which is attributed to the rising incidence of DNA double-stranded breaking during the RT process. This synergy effect is particularly pronounced in tumours with MGMT-negative status, where the repair processes are diminished [43,44].
TMZ induces cell cycle arrest in the G2/M phase or apoptosis [40]. This compound can cross the blood-brain barrier and is spontaneously activated in acidic pH to monomethyltriazene 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MITC), which then undergoes a series of chemical transformations to form 5-aminoimidazole-4-carboxamide (AIC) and finally the methyl diazonium cation. This cation is involved in the methylation of DNA at the N7 and O6 positions of the guanines and also at the N3 regions of the adenines. This leads to cytotoxic effects due to the unrepaired O6 methylation of guanine (O6mG), which leads to breakage of the DNA double strands. For patients undergoing treatment, TMZ therapy is a standard regimen followed by radiotherapy, usually with a dose of 2 Gy per fraction administered on a patient-dependent schedule per day until the total dose reaches 60 Gy. With this standard regimen, clinical results can be achieved in 55 % of patients with glioblastoma (GBM). However, due to the DNA repair system methyl-guanine-methyl-transferase (MGMT), GBM can revert to a TMZ-resistant phenotype [10,40]. The second mechanism that counteracts the effect of TMZ in MMR (mismatch repair); both are described later in this article
Lomustine (CCNU) is another DNA-alkylating agent used in the treatment of various brain tumors, including GBM. By forming bonds with DNA through the transfer of alkyl groups, lomustine causes DNA damage and inhibits replication, ultimately leading to apoptosis of cancer cells [45,46].
Carmustine (BCNU), alkylating agents acts as an inhibitor of monoamine dehydrogenase, is also used to treat GBM. It inhibits DNA and protein synthesis and thus contributes to the inhibition of cancer cell growth. BCNU is administered by intravenous injection, either as monotherapy or in combination with other drugs [47,48]. BCNU is also available in the form of Gliadel wafers, implanted directly into the brain during surgical removal of the tumor [49,50]. Procarbazine, a monoamine oxidase inhibitor, is frequently used in complex chemotherapy protocols for GBM treatment, often in combination with drugs such as CCNU and vincristine. This combination therapy is particularly effective in the treatment of anaplastic gliomas [[51], [52], [53]].
Mitosis inhibition
Vincristine presents a particular challenge due to its chemical properties, which may compromise its ability to effectively penetrate the BBB and distribute throughout brain tumor tissue. Due to its relatively high molecular weight and hydrophilic nature, vincristine has difficulty penetrating the BBB, which is primarily composed of lipid cell membranes [54,55]. Despite its hydrophilic nature, vincristine also exhibits some lipophilicity, which impairs its distribution. Excessive lipophilicity may lead to nonspecific distribution of the drug in tissues, which could compromise its efficacy against brain tumors [56,57]. Nevertheless, its antimitotic effect makes it suitable for inclusion in GBM therapeutic regimens in certain cases [58]. Vincristine, an agent that interferes with cancer cell division by inhibiting microtubules, is occasionally used in chemotherapy regimens for GBM. By destabilizing microtubules and disrupting mitotic function, vincristine triggers apoptosis of cancer cells [51,59]. Irinotecan, a topoisomerase I inhibitor, is sometimes used in the treatment of GBM, particularly in cases of relapse or treatment failure. By inhibiting topoisomerase I, irinotecan induces DNA damage and apoptosis of tumor cells [60,61]. Common combinations of chemotherapeutic agents used in the treatment of gliomas include TMZ with CCNU and vincristine, procarbazine with CCNU and vincristine, and TMZ with bevacizumab (BVC) [51,53,62,63].
Antiangiogenic therapy
Bevacizumab (BVC) is a monoclonal antibody directed against vascular endothelial growth factor A (VEGF-A). Administration of BVC against VEGF-A has been shown to inhibit angiogenesis and consequently reduce vascular permeability [4]. However, the benefits of BVC administration in clinical tumor therapy vary. In GBM therapy, BVC is most commonly used as an additional treatment alongside standard chemotherapy with TMZ and radiotherapy [63,65,66].
In some GBM patients, treatment with BVC may lead to a temporary improvement in symptoms, but this effect is often short-lived as the disease subsequently relapses. While some clinical trials have shown benefits in symptom relief and disease stabilization, the overall survival of GBM patients has not been significantly prolonged by treatment with BVC [66,67]. Nevertheless, BVC continues to be used in selected GBM cases to improve patients' quality of life by reducing brain edema and alleviating neurological symptoms [64,66,68].
Mutation status and clinical response to treatment
The choice of chemotherapy for glioblastoma multiforme (GBM) is increasingly influenced by molecular mutations, as new research on the molecular subtypes of GBM suggests that certain mutations may influence the efficacy of certain drugs [4,43,69,70]. For example, glioma patients with MGMT promoter methylation show increased sensitivity to TMZ compared to patients without MGMT methylation [[71], [72], [73], [74]].
The activity of MGMT has been shown to facilitate the repair of DNA lesions under certain physiological conditions. This is achieved by converting the DNA lesion O6-methylguanine to guanine and the methyl group to cysteine. This mechanism is present in the DNA damage caused by TMZ therapy, leading to a reduction in the efficacy of this treatment [75]. Consequently, the combination of MGMT inhibitors and TMZ has the potential to improve clinical outcomes. The effects of the novel MGMT inhibitor AA-CW236 have recently been investigated in a number of studies demonstrating its potential [75].
In addition, the level of MGMT expression may serve as a prognostic factor for the efficacy of TMZ treatment, with tumors with higher MGMT levels exhibiting lower sensitivity to alkylating agents [36]. In addition, the methylation status of the MGMT promoter, which influences MGMT activity, is a prognostic factor for a more effective response to TMZ.
Another mechanism that counteracts the activity of TMZ is MMR (mismatch repair). The MMR pathway is activated as a consequence of the formation of base pairs resulting from the methylation of guanine during TMZ action. In general, MMR fixes a second strand of DNA when one of the strands is methylated, acting through the proteins MSH2, MSH6, MLH1 and PMS2. These proteins bind to guanine residues that are in an unequal position [40]. In contrast to the methylated guanine (O6mG), the thymine is in the leading position, and the repetition of this mechanism is known asthe futile cycle of MMR [76].
This leads to the initiation of the DNA repair phase in the cell cycle. However, the differentiation between the parental and daughter DNA strands leads to futile repair, DNA damage and consequent elimination of the cell. If there is insufficient MMR activity, the mutations accumulate in the cells, which can increase the risk of cancer development [40].
To overcome the TMZ resistance generated by the MGMT mechanism, a dose-dense response (DDR) was performed in conjunction with TMZ treatment. However, this strategy did not improve patient outcomes [40]. During TMZ treatment, resistance and a side effect in the form of TMZ-induced mutation occurred regardless of the specific TMZ treatment regimen. Hypermutated glioblastoma tumors are associated with a poorer prognosis for patient survival. It is interesting to note that low-grade tumors are more frequently hypermutated than high-grade tumors [40].
The differences in MGMT and MMR play a role in the treatment of patients with TMZ. From a clinical perspective, glioblastoma may be TMZ-sensitive in the absence of MGMT expression, with MMR increasing the efficacy of eliminating cancer cells in a futile cycle. Conversely, TMZ-resistant GBM tumors express MGMT or have no MMR expression. This peculiarity of GBM biology can be exploited to increase the efficacy of TMZ through the use of various inhibitors [77].
Although MGMT is known to be the most important desentizing factor in GBM for TMZ-RT treatment, recent studies suggest that there are other options, such as retinoblastoma binding protein 4 (RBBP4), which may be involved in treatment resistance regardless of MGMT status [78].
Patients diagnosed with gliomas with mutations in the IDH1 or IDH2 gene generally have a more favorable prognosis and are more likely to be classified as lower grade glioma (e.g., grade II or III glioma). These patients may respond better to chemotherapy, including drugs used to treat low grade gliomas [[79], [80], [81], [82], [83]].
The enzymes of isocitrate dehydrogenase (IDH), especially IDH1 and IDH2, play a central role in metabolic processes, particularly in the Krebs cycle, where they enable the oxidative decarboxylation of isocitrate. They are also involved in glutamine metabolism, lipogenesis and redox regulation. IDH mutations have been observed in a number of cancers, including GBM, acute leukemia and chondrosarcoma. In GBM grade IV, the IDH mutation is observed in over 70 % of cases, while in the lowest grades it is present in over 80 % of cases.
The IDH mutation is associated with a more favorable prognosis for the patient compared to the IDH wild-type phenotype. It is also associated with the highest sensitivity to radiotherapy and chemotherapy [9,80].
The consequence of the mutation in the IDH structure is the replacement of arginine at position 132 (in IDH1) or 140 or 172 (in IDH2), which is crucial for isocitrate binding, with amino acids that have a lower affinity for isocitrate: histidine, lysine and cysteine. these amino acids lead to a reduction in the affinity of isocitrate, while the affinity for NADPH is increased. This is due to the biochemical inactivation of the enzyme. This leads to further changes in metabolic processes, such as the utilization of glucose and glutamine in the absence of the Krebs cycle. In gliomas, glutaminolysis plays a compensatory role in this context. The affinity of mutant IDH for NAPDH is associated with the accumulation of ROS and oxidative damage. In addition, the IDH mutation has been observed to hinder the DNA repair process, with the formation of d-2-HG (D-2-hydroxyglutarate) playing a role in the Krebs cycle. d-2-HG has been identified as an inhibitor of DNA repair enzymes, including ALKBH2/3 (alpha-ketoglutarate-dependent dioxygenase alkB homolog 2). It is noteworthy that IDH-mutated gliomas exhibit a distinct metabolic profile characterized by reduced glycolysis, which is a common metabolic pathway in tumors [9]. It is noteworthy that the IDH mutation may prove useful for the targeted therapy of GBM. The IDH1 and IDH2 mutation inhibitor vorasidenib has recently demonstrated its potential against GBM, as described by Mellinghoff [83].
EGFR amplification is characteristic of GBM, most commonly associated with the classical subtype, and is detected in almost 60 % of primary GBM patients and 8 % of patients with secondary GBM tumors [70]. EGFR is a member of the ERBB family, which consists of the receptors ERBB1-ERBB4 (also known as HER1-HER4). Mechanistically, phosphorylation of EGFR leads to dimerization and activation of the GRB2 (growth factor receptor bound protein 2), PI3k/Akt, c-Src/FAK, RAS/MAPK/ERK and JAK/STAT-3 signaling pathways, which play an important role in processes that promote tumor progression, such as invasion and metastasis, proliferation, apoptosis inhibition and glioma stem cells [70,84].
Mutations of EGFR lead to an activation of this receptor and thus to a constitutive activation of downstream signaling pathways in GBM cells. Some EGFR mutations are specific for different types of cancer and can serve as biomarkers. In glioblastoma, the most common mutation is the EGFRvIII mutation, which is described as a loss of amino acids 6–273 and association with a new glycine residue between 5 and 274 amino acids compared to wild-type EGFR. As a result, a tumor-specific and immunogenic epitope is created by this novel linkage to the extracellular domain of EGFR [85].
Other EGRF mutations are also observed in EGFRvI (N-terminal deletion), EGFRvII (deletion of exons 14–15), EGFRvIII (deletion of exons 2–7), EGFRvIV (deletion of exons 25–27), EGFRvV (deletion of exons 25–28) as well as EGFRvII and EGFRvIII. A typical mutation in non-small cell lung cancer, but not common in GBM, is L858R in exon 21 and an in-frame deletion in exon 19 [70]. In addition, point mutations in the EGF receptor occurred in 24 % of GBMs: R108 K, A289V/D/T, G598D.
The activation of EGFR III is typical for tumor cells and could therefore have potential for targeted therapies with EGRF inhibitors. Hovewer, its expression in GBM is heterogeneous, and furthermore, EGFRIII amplicons may not be available for EGFR inhibitors because EGFRIII expression in cells changes dynamically during and after treatment and inhibitors are only present in low levels in tumor tissue. Resistance to EGFR inhibitors therefore remains a challenge as there are relatively few successful clinical results [70,86,87]. Current clinical trials, including new EGFR inhibitors, are described below in this article. Of note,the selection of EGFR-amplified tumors is critical to verify EGFR or variant III (EGFRvIII) expression status for more precise therapy, and there is no consensus in this area [86]. Recently, studies by French et al. have shown that FISH together with RT-qPCR methods can be a useful tool to select patients with EGFR amplification [86].
Patients carrying these mutations may show an increased response to EGFR-targeted drugs such as tyrosine kinase inhibitors (e.g. gefitinib, erlotinib). However, the efficacy of these drugs may vary depending on the specific EGFR mutations and the presence of associated molecular aberrations [70,[88], [89], [90]].
Numerous other molecular mutations, such as in the PTEN, TP53 and PDGFRA genes, may also be important in GBM treatment. Analysis of the molecular profile of the tumor can help in tailoring treatment and selecting an appropriate therapy, including chemotherapy [25,[91], [92], [93]].
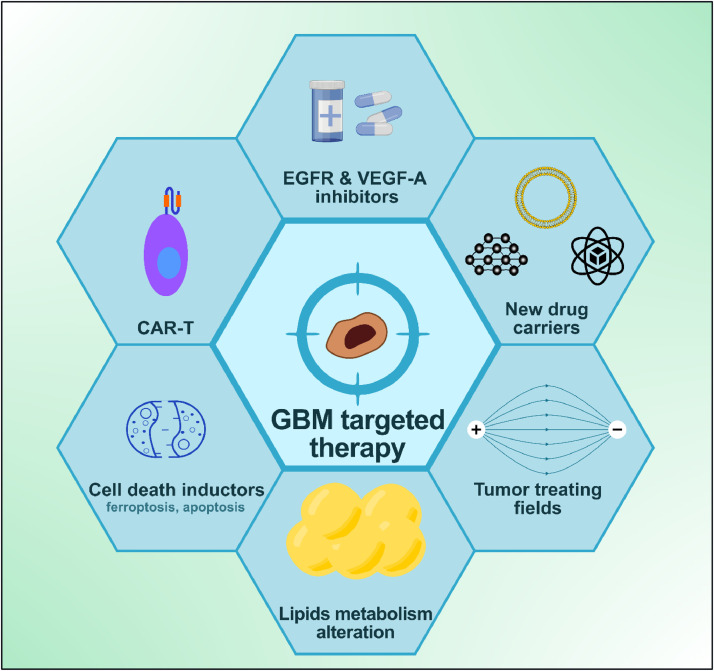
Despite progress, there are still gaps in the understanding of GBM, emphasizing the need for continued research and collaboration between stakeholders. Molecularly targeted therapies aim to target specific molecules or signaling pathways that are critical for cancer cell proliferation and survival [43,69,94]. Key points for targeted therapy was presented on Fig 3.
Fig. 3.
New avenues for targeted therapy design in GBM are currently under investigation and appear promising. Created with BioRender.com.
Examples include tyrosine kinase inhibitors such as EGFR or VEGF inhibitors, which may be indicated for certain glioma subtypes. Molecular profiling is crucial for tailoring therapeutic approaches for GBM [70,95].
The choice of therapy often depends on the molecular mutations of the tumor. Understanding the molecular makeup of the tumor helps predict the propensity for relapse and response to treatment [[96], [97], [98]]. Patients with EGFR mutations or HER2 amplifications may benefit from targeted therapy with tyrosine kinase inhibitors such as erlotinib or lapatinib [70,[99], [100], [101]].
MGMT promoter methylation status determines sensitivity to alkylating agents [70,102,103].
IDH1 mutations describe a different biology and prognosis and influence the choice of treatment [79,104]. TMZ-based chemotherapy may be more effective in patients with IDH1 mutations [105]. Gene expression profiling facilitates the classification of molecular subtypes and aids in treatment prognosis and survival prediction [[106], [107], [108]]. Tumors with pathway mutations can be targeted with pathway inhibitors. Molecular techniques such as whole genome sequencing and gene expression profiling enable the identification of unique tumor characteristics and thus personalized therapy [97,109].
In clinical practice, molecular mutation analysis is increasingly being integrated into the management of GBM patients. The knowledge gained from these procedures allows physicians to better profiling neoplasms and tailor treatment to individual's needs [96,110]. However, ongoing research is focused on optimizing the use of this information to improve therapeutic outcomes in GBM patients [4,94,96]. Table 2 presents the current clinical trials in GBM, which also include the use of new inhibitors.
Table 2.
Ongoing GBM clinical trials according to the clinicaltrials.gov database provided by the National Institutes of Health (NIH).
| Treatment | Target | Status | Enrollment | Primary outcome measures | NCT number |
|---|---|---|---|---|---|
| Rindopepimut (CDX-110) with GM-CSF in combination with Bevacizumab | EGFRvIII | Completed, phase II | 127 | Progression-free survival (PFS), Objective Response Rate (ORR) | NCT01498328 |
| Rindopepimut (CDX-110) with GM-CSF in combination with Temozolomide | EGFRvIII | Completed, phase III | 745 | Overall Survival (OS) | NCT01480479 |
| SurVaxM Vaccine with Temozolomide | cells that express survivin | Active, not recruiting, phase II | 66 | PFS | NCT02455557 |
| SurVaxM Vaccine with Temozolomide | cells that express survivin | Active, not recruiting, phase II | 247 | OS | NCT05163080 |
| Anti-LAG-3 + Anti-PD-1 | LAG-3, PD-1 | Completed, phase I | 63 | Maximum tolerated dose (MTD) | NCT02658981 |
| HER.CAR CMV-specific CTLs | HER2 | Completed, phase I | 16 | Number of subjects with dose limiting toxicity after CTL infusion | NCT01109095 |
| Epidermal growth factor receptor(EGFRv)III Chimeric antigen receptor (CAR) transduced PBL | EGFRvIII | Completed, phase I phase II |
18 | Number of treatment related adverse events, PFS | NCT01454596 |
| IL13Ralpha2-CAR T cells | IL13Ralpha2 | Active, not recruiting, phase I | 82 | Incidence of grade 3 toxicity, dose limiting toxicity (DLT) | NCT02208362 |
| IL13Ralpha2-CAR T cells | IL13Ralpha2 | Recruiting, phase I | 30 | Incidence of adverse events, OS | NCT04661384 |
| B7-H3-targeting CAR-T cells | B7-H3 (CD276) | Recruiting, phase I | 30 | Incidence and severity of adverse events, OS | NCT05241392 |
| B7-H3-targeting CAR-T cells | B7-H3 (CD276) | Recruiting, phase I phase II |
40 | Incidence and type of adverse events, MTD, PFS | NCT04077866 |
| NK-92/5.28.z + Ezabenlimab | HER2 | Active, not recruiting, phase I |
42 | Treatment-related adverse events, MTD, period of detectability of NK-92/5.28.z cells in blood and cerebrospinal fluid (CSF), cytokine profile in the blood and the CSF | NCT03383978 |
| C225-ILs-dox | EGFR | Completed, phase I | 9 | Ratio of C225-ILs-dox concentration | NCT03603379 |
| SGT-53 | Delivery of the p53 cDNA to the tumor cells | Terminated, phase II | 1 | Tumor response | NCT02340156 |
| Liposomal Curcumin (LC) | Rb, p53, MAPK, P13K/Akt, JAK/STAT, Shh, and NF-κB pathways | Recruiting, phase I phase II |
30 | The number of observed Dose Limiting Toxicity (DLTs) | NCT05768919 |
| Verteporfin | mutant or amplified EGFR | Recruiting, phase I phase II |
24 | Incidence of adverse events (phase I), PFS, OS, response rate (RR) (phase II) | NCT04590664 |
| NovoTTF-100A | antimitotic effect of tumor treating fields (TTF) | Completed, phase III | 700 | PFS | NCT00916409 |
| ASC40 + Bevacizumab | fatty acid synthase (FASN) | Recruiting, phase III | 180 | PFS, OS | NCT05118776 |
| ERAS-801 | EGFR/ERBB1 | Active, not recruiting, phase I | 52 | DLT, MTD, recommended dose (RD), adverse events | NCT05222802 |
| Erlotinib | EGFR | Completed, phase I | 22 | Clinical Benefit Rate (either radiographic response or at least 6 months of progression-free survival) | NCT01257594 |
| Epitinib Succinate (HMPL-813) | EGFR | Unknown, phase I | 29 | Objective Response Rate (ORR) | NCT03231501 |
| Afatinib (BIBW 2992) | EGFR | Completed, phase I | 36 | DLT, MTD | NCT00977431 |
| CART-EGFRvIII + Pembrolizumab | EGFRvIII | Completed, phase I | 7 | Number of subjects with treatment-related adverse events | NCT03726515 |
| Peposertib | DNA-PK | Recruiting, phase I | 29 | MTD (stage I), ability of Peposertib (M3814) to cross the blood brain barrier (stage II) | NCT04555577 |
| WP1066 | STAT3 pathway | Recruiting, phase II | 39 | PFS, tumor microenvironment activation and cluster interactions | NCT05879250 |
| BMS-986,205 | IDO1 | Active, not recruiting, phase I | 18 | Incidence of adverse events (AEs) | NCT04047706 |
| Paxalisib (GDC-0084) | PI3K/mTOR | Completed, phase II | 30 | Dose limiting toxicities (DLTs) | NCT03522298 |
| Selinexor | XPO1 | Recruiting, phase I phase II |
97 | Recommended phase 2 dose (RP2D) (phase I), PFS | NCT05432804 |
| Veliparib (ABT-888) | PARP-1, PARP-2 | Active, not recruiting, phase II phase III |
447 | OS | NCT02152982 |
| BGB-290 | PARP-1, PARP-2 | Recruiting, phase I | 78 | Proportion of participants with Dose Limiting Toxicities (DLTs) | NCT03749187 |
| metformin (Glucophage) | Complex I in tumor mitochondria | Recruiting, phase II | 640 | PFS | NCT04945148 |
Resistance to chemotherapy
The pharmacokinetics of chemotherapeutic agents used in GBM treatment are summarized in Table 1, where each drug is subject to LADME processes (Liberation, Absorption, Distribution, Metabolism, Excretion/elimination) that may vary depending on individual patient characteristics, disease state and drug dosage. Despite current therapeutic options, CNS tumors can develop resistance to the chemotherapeutic drugs commonly used in GBM treatment, such as TMZ, through various alterations in cell signaling pathways, including changes in DNA repair mechanisms [109,111,112]. Although these drugs can cross the BBB, their penetration may be limited, which may compromise treatment efficacy [25]. In addition, rapid metabolism and elimination from the body may reduce their therapeutic effect [113]. Given the great heterogeneity of GBM and its diverse cellular composition, the response to chemotherapeutic agents may vary from tumor region to tumor region, with some areas showing increased resistance [25,43,114].
Therapy for GBM in clinical trials
The search for new treatment options for malignant brain tumors is necessary due to the low efficacy of the currently used methods, which include surgical resection, radiotherapy and chemotherapy. An important trend in the development of new GBM therapies is the use of immunotherapy, which recently appears to be the most promising therapeutic strategy in the oncology. Ongoing clinical trials on GBM with a focus on different targets are listed in Table 2.
To illustrate, CAR-T therapy has been approved for the treatment of patients with lymphoma and leukaemia (193). LAG-3 represents a promising target in melanoma and colorectal cancer (190). Monoclonal antibodies directed against PD-1 (pembolizumab, pembrolizumab, nivolumab) have demonstrated efficacy in the treatment of non-small cell lung carcinoma (NSCLC), small cell lung carcinoma (SCLC), oesophageal cancer and cervical cancer. The targeted PD-L1 (atezolizumab) demonstrated efficacy in TNBC (triple negative breast cancer) [137,138]. The therapy, which is targeted anti-PD-1/PD-L1 antibodies, has been approved as an immunotherapy for metastatic non-small cell lung cancer (NSCLC) patients [138].
The advantage of this form of therapy is that it targets exclusively neoplasm without damaging normal cells. It is currently being used in clinical trials focusing on anti-cancer vaccines, antibodies that block immune system checkpoints, T cells with chimeric antigen receptors (CAR-T) and natural killer (NK) cells.
Cancer vaccines
Peptide vaccines against cancer acting by presenting tumor-related antigens for antigen-presenting cells, such as dendritic cells, to trigger an immune response against tumor. Recognition of the antigens as foreign by cytotoxic T lymphocytes leads to activation of these cells, resulting in elimination of the cancer cells. Amplification of the EGFR gene is frequently observed in solid tumors, including GBM. In tumors with EGFR gene amplification, a rearrangement often occurs, leading to the most common variant of the mutated extracellular domain, EGFRvIII. This variant lacks exons 2–7 and has a glycine residue at the junction of exons 1 and 8 [85]. This variant is not found in normal brain tissue, which is crucial for the development of a targeted therapy for this type of glioma. Currently, tumor-associated antigens or antigen-based vaccines are not used in the standard treatment of GBM. However, clinical trials are being conducted to investigate their safety and efficacy in the treatment of GBM.
The rindopepimut vaccine, also known as CDX-110, targets the surface antigen EGFRvIII. The ReACT phase II clinical trial investigated the effect of this vaccine in combination with the monoclonal antibody BVC (NCT01498328), which is currently used to treat GBM. This study analyzed a group of 73 patients (36 received rindopepimut, 37 were in the control group) and found that 80 % of patients treated with rindopepimut achieved high anti-EGFRvIII titers. These titers were associated with longer overall survival (OS) [139]. The efficacy of rindopepimut therapy was further validated in the phase III clinical trial ACT IV (NCT01480479). However, an interim analysis showed no significant clinical benefit of the vaccine, which led to the study being discontinued. The discrepancy between the results of ReACT and ACT IV may be due to the loss of EGFRvIII expression in recurrent tumors, which occurs in approximately 60 % of patients. This is a significant issue that limits the efficacy of immunotherapy in the treatment of GBM [140].
A phase II clinical trial was conducted with the vaccine SurVaxM, which consists of synthetic peptide antigens targeting the protein survivin expressed in GBM (NCT02455557). The translation results are 1652 out of 5000. Survivin, an apoptosis inhibitor, is overexpressed in cancer cells compared to normal cells and is involved in numerous signaling pathways related to tumor progression. These properties make survivin an attractive target for cancer therapies [141].The aim of the study was to investigate the safety, immunological effects and survival of patients with newly diagnosed GBM who received SurVaxM together with adjuvant TMZ after surgery and chemoradiotherapy. The study group comprised 64 patients, including 38 men and 26 women aged 20–82 years. Patients were administered four doses of SurVaxM subcutaneously, at a dose of 500 μg once every 2 weeks. The results of the study showed that the vaccine was safe and well tolerated by the patients. It also had a positive effect on progression-free survival (PFS) and OS by boosting the immune system's response. Six months after diagnosis, 60 % of all study participants were progression-free. The study found that the median progression-free survival (PFS) was 11.4 months and the median overall survival (OS) was 25.9 months after the first dose of SurVaxM [142]. A Phase II clinical trial (NCT05163080) is currently underway to determine whether the addition of SurVaxM to standard TMZ chemotherapy is more effective than treatment with TMZ alone in patients with newly diagnosed glioma.
Antibodies that block checkpoints of the immune system
Antibodies that inhibit the checkpoints of the immune system constitute a growing trend in the development of cancer therapies. These antibodies are designed to prevent the formation of an immunosuppressive environment around the tumor and thus increase the effectiveness of the immune system in anticancer response. T lymphocytes have co-stimulatory molecules on their surface that have both activating and suppressive effects. These molecules act as checkpoints for the immune system. Ligands for these receptors are located on the surface of cancer cells. This allows the cancer cells to block the cytotoxic effects of T lymphocytes, which in turn allows the tumor to evade immune surveillance [143]. Inhibition of T lymphocyte activity is primarily achieved through activation of lymphocyte gene 3 (LAG-3), programmed death receptor 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) [144]. Antibody-mediated blockade of these receptors should restore function and increase proliferation of cytotoxic T lymphocytes, thereby enhancing the immune response in immunosuppressed diseases. Therefore, cancer immunotherapy that blocks immune checkpoints appears to be a promising direction for the development of cancer therapies. Current clinical trials are focused on determining the appropriate doses and administration regimens to maximize the efficacy of the therapy in treating cancer. The development of therapies based on antibodies that block checkpoints involves the combination with other therapeutics and the use of antibodies that block multiple checkpoints of the immune system.
A preclinical study in mice showed that blocking LAG-3 alone or in combination with an anti-PD-1 antibody led to a regression of gliomas [145]. The study also showed that LAG-3 is expressed in human gliomas, which served as the basis for subsequent clinical trials. A Phase I study was conducted in a group of patients with recurrent glioblastoma to evaluate the safety and effective dose of the anti-LAG-3 monoclonal antibody alone and in combination with the anti-PD1 antibody (nivolumab) (NCT02658981). Three doses of anti-LAG-3 were tested in the study: 80 mg, 160 mg and 800 mg. Anti-PD-1 was administered at a dose of 240 mg in combination with anti-LAG-3 at doses of 80 mg and 160 mg. It was found that the highest safe dose for anti-LAG-3 alone is 800 mg, and in combination with anti-PD-1, the doses are 160 mg for anti-LAG-3 and 240 mg for anti-PD-1 [146].
CAR-T
Another promising approach in GBM therapy is adoptive cell therapy (ACT), a form of immunotherapy in which immune cells are in use. In ACT, cells are taken from the patient (autologous therapy) or a healthy donor (allogeneic therapy), cultivated ex vivo and then re-administered to the patient. It can be seen as a strategy for improving the immune response to various types of cancer. This therapy includes, among others: CAR-T involves genetically modifying T lymphocytes isolated from the patient's blood and attaching a chimeric antigen receptor (CAR) to their surface. These modified T lymphocytes are then administered to the patient, where the receptors on their surface bind to antigens on the surface of the cancer cells, leading to their elimination. This therapy specifically targets cancer cells and does not harm normal cells. CAR-T therapy is approved for the treatment of lymphoma and leukemia, where it has shown the greatest efficacy [147]. Although research suggests that it could also be used for solid tumors such as glioblastoma, no CAR-T-based therapy has yet been approved for this purpose. The development of this type of therapy is challenging due to the limited number of surface antigens that can be targeted by CAR-T and the high heterogeneity and antigenic variability of the tumor.
In the case of GBM, there is often overexpression of human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor variant III (EGFRvIII) and interleukin-13 receptor alpha 2 (IL13Rα2) [148]. Targeting these receptors has shown promising preclinical results, which led to further evaluation in clinical trials. In a phase I clinical trial (NCT01109095), the administration of autologous HER2 CAR-T cells to patients was found to have no adverse effects. Eight patients showed clinical benefit with a median overall survival of 24.5 months [149]. The administration of CAR-T cells targeting EGFRvIII only showed positive effects in one animal model. However, a pilot study conducted in a group of 10 patients with recurrent GBM (NCT01454596) showed that this therapy neither delayed tumor progression nor prolonged survival [150]. The trial failed, possibly due to the loss of EGFRvIII in recurrent tumors, similar to the ACT IV trial. A phase I clinical trial (NCT02208362) is currently underway to evaluate the safety and optimal dosing of CAR-T immunotherapy targeting the interleukin-13 alpha 2 receptor (IL13Rα2) for the treatment of patients with relapsed or refractory malignant glioma.
Results of clinical studies described a case of 50-year-old patient who underwent standard therapy, including tumor resection, radiotherapy and TMZ. However, after 6 months, magnetic resonance imaging (MRI) and positron emission tomography combined with computed tomography (PET-CT) of the brain showed signs of relapse. The patient received CAR-T cells directed against IL13Rα2, which led to a regression of the tumor. The clinical effect lasted for 7.5 months [151]. However, the recurrent tumor showed low expression of IL13Rα2, and therefore low target protein for CAR-T cells, and this is a major challenge for the use of this therapy in clinical practice. The studies on single-epitope CAR- T-cell therapy have not produced the expected results. However, the development of this therapy can be improved by using multivalent CAR-T cells targeting different glioma antigens. This approach can overcome the problem of antigen variability and tumor escape from the immune system [152]. Combining CAR-T therapy with immunotherapy using monoclonal antibodies can improve the immune system's ability to overcome GBM. Currently, a phase I clinical trial (NCT04003649) is recruiting patients to evaluate the efficacy of IL13Rα2 CAR-T cells alone or in combination with nivolumab and ipilimumab for the treatment of relapsed or refractory GBM.
B7-H3 (CD276) is a checkpoint protein. Its expression was found in tumour cells and immune cells from the tumour microenvironment [153]. B7-H3 is also associated with cancer cell proliferation, metastasis and treatment resistance, and therefore is a promising molecular target for CAR-T cells in GBM [153]. It is expressed in approximately 70 % of GBM patients, with two isoforms present in GBM and only one in healthy brain tissue: 2IgB7-H3 and 4IgB7-H3, respectively. Research has shown that recurrent GBM is characterized by increased expression of 2IgB7-H3, leading to resistance to TMZtreatment. The protein 4IgB7-H3, which is only found in cancer cells, may be a promising candidate for targeted GBM therapies, while 2IgB7-H3 may be associated with tumor resistance to chemotherapy [154]. Two phase I and I/II clinical trials (NCT05241392, NCT04077866) are currently underway to evaluate the safety and efficacy of B7-H3-targeted CAR-T therapy. The studies will also determine the maximum tolerated dose (MTD) and the recommended Phase II dose (RP2D). In the case of a Phase I/II trial, the efficacy of the therapy between TMZ cycles compared to TMZ alone will also be evaluated.
CAR-NK
The hope for a possible breakthrough in GBM therapy also lies with NK cells. Unlike T-cell therapy, NK-cell therapy does not require the presence of specific tumor-associated surface antigens. This overcomes the problem of heterogeneity within and between GBM tumors, which limits the efficacy of CAR-T [155]. Natural killer (NK) cells identify healthy cells by detecting the presence of major histocompatibility complex (MHC) class I molecules. In contrast, cancer cells are identified by their reduced or absent expression of MHC-I. The receptor KIR (killer-cell immunoglobulin-like receptor) on the surface of NK cells binds to MHC-I, inhibits its function and prevents the destruction of healthy cells [156]. The activity of NK cells is regulated by the balance between signals from stimulatory and inhibitory receptors on their surface. These immune receptors enable the cells to identify and eliminate cancer cells [157]. In addition, NK cells play a crucial role in the recruitment of key cells for antitumor immunity, such as conventional dendritic cells type 1 (cDC1) and cytotoxic T lymphocytes. This helps to overcome the immunosuppressive tumor environment [158].
NK cells have properties that make them a promising therapeutic strategy in the medication against treatment-resistant cancers, including GBM. It is frequently observed that the number and activity of NK cells decreases in individuals diagnosed with cancer. In clinical trials of NK cell immunotherapy, the NK-92 cell line isolated from the blood of a 50-year-old man with malignant non-Hodgkin's lymphoma is used [159]. The cells of this line exhibit cytotoxic activity, produce cytokines and have unlimited proliferation potential. In addition, they can be genetically modified to express chimeric antigen receptors, resulting in CAR-NK cells that resemble CAR-T cells [160]. The ongoing phase I clinical trial (NCT03383978) aims to evaluate the safety and tolerability of HER2-specific CAR-NK cells 92/5.28.z in patients with relapsed or refractory HER2-positive glioma. This is due to the fact that elevated levels of this protein have been found in a significant percentage of GBM tumors [161]. The aim of the CAR2BRAIN study was to determine the maximum tolerated dose (MTD) and assess the safety of the therapy. In this study, participants were administered up to twelve injections of NK-92/5.28.z cells. The distribution of the injected cells in the brain, cerebrospinal fluid and blood was examined and their pharmacokinetics and pharmacodynamics were determined. The results suggest that immunotherapy based on NK cells in combination with other regimes of anticancer therapy could be a promising research option and a potential breakthrough in the treatment of GBM.
Liposomes
In addition to immunotherapy, the use of nanotechnology, in particular liposome technology, is also promising for GBM therapy. Liposomes are small vesicles that consist of one or more lipid bilayers and serve as a non-toxic and biodegradable carrier for hydrophobic and hydrophilic drugs. In addition, their phospholipid structure is biocompatible with the BBB [162]. Liposomes have the advantage that they can be targeted by binding ligands for receptors present on the target cells to their surface.
A phase I clinical trial (NCT03603379) investigated the efficacy of immunoliposomes targeting the epidermal growth factor receptor (EGFR) when administering doxorubicin to GBM. The study was conducted in a group of patients with relapsed or refractory glioblastoma multiforme with amplification of the EGFR gene. Immunoliposomes containing doxorubicin (C225-ILs-dox) were administered intravenously to patients at a dose of 50 mg/m2 for up to four treatment cycles of 28 days each. The drug concentration in blood plasma, cerebrospinal fluid (CSF) and tumor tissue was then examined. The results showed that doxorubicin was detectable in the resected tumor, but not in the CSF. It is assumed that C225-ILs-Dox cannot overcome the intact BBB. However, in GBM, the BBB is often disrupted, which may allow the use of liposomes for drug delivery to tumor tissue.
The use of liposomes with ligands for receptors associated with the BBB has already been explored. The BBB is the main obstacle to the delivery of anticancer drugs to central nervous system tumors, including GBM. Key receptors associated with the BBB include the low-density lipoprotein receptor (LDLR), transferrin receptor (TfR) and glucose transporter (GLUT) [163]. In addition, cancer cells are characterized by an increased expression of TfR. This is due to their high demand for iron, which is necessary to maintain a rapid growth and proliferation rate [164]. To eliminate the BBB and achieve precise delivery of macromolecular drugs to the GBM, the researchers developed cationic liposomes to which a fragment of a single-chain anti-TfR antibody was attached. These liposomes also contained a plasmid with the human tumor suppressor gene TP53, which encodes wild-type p53 (SGT-53) [165]. In a phase II clinical trial (NCT02340156), the efficacy of treatment with TMZ in combination with tumor-targeted SGT-53 liposomes was investigated in patients with relapsed GBM. However, due to the small number of patients and the inability to perform a meaningful statistical analysis, SGT-53 with anti-TfR antibody did not pass phase II clinical trials.
An ongoing phase I/II clinical trial aims to determine the maximum tolerated dose/recommended dose of liposomal curcumin in combination with standard radiotherapy and TMZ in patients with newly diagnosed high-grade glioma (HGG) (NCT05768919). Curcumin, a plant polyphenol, exhibits anticancer properties by disrupting metabolic pathways and inhibiting angiogenesis in cancer cells [166]. The use of curcumin in free form in therapy is limited due to its low solubility in water and poor bioavailability. To improve its delivery to cancer cells, a carrier is necessary. In this case, liposomes are used as carriers. The study aims to include a group of about 30 patients receiving weekly intravenous infusions of liposomal curcumin. The efficacy of the treatment will be assessed by overall survival (OS) and progression-free survival (PFS) observed at three different doses of the drug. The duration of treatment for each patient is expected to be up to 34 weeks.
The liposomal form of verteporfin (Visudyne) is being tested in a phase I/II clinical trial for the pharmacologic control of GBM in patients with recurrent EGFR-positive GBM (NCT04590664). Verteporfin is an FDA-approved drug used as a photosensitizer for photodynamic therapy (PDT) of eye diseases. This therapy generates reactive oxygen species (ROS) by activating verteporfin with red light (635 nm) to eliminate abnormal tissue [167]. In vitro results have shown that verteporfin could also be used in the treatment of GBM. PDT with verteporfin as a photosensitizer caused cell death in human glioma stem cells. Verteporfin is a compound that can be internalized in GBM cells. It can be used for PDT and as a fluorescent dye for intraoperative tumor mapping [168]. The current clinical trial consists of two phases: Phase I aims to evaluate the safety and MTD of verteporfin, while Phase II aims to determine its antitumor activity by evaluating OS and PFS. Patients enrolled in the study will receive weekly intravenous infusions of verteporfin over 83 min for 6 weeks in Cycle 1. They will then receive weekly infusions for 5 weeks unless disease progression or significant toxicity occurs. The cycles are repeated every 6 weeks. After completion of therapy, patients are observed for 30 days, followed by observation every 12 weeks.
Biomaterials as drug carriers
In silico models of GBM suggest three main features that could serve as targets for precision chemistry in this type of neoplasm: Angiogenesis, invasiveness and diffusion of GBM cells if they spread in brain tissue [169]. Computational modeling can be useful in predicting the efficacy of therapies in terms of the response of the tissue microenvironment [169]. In addition, in silico modeling is a tool for developing nanomedicine solutions and predicting their efficacy. New drug formulations, such as nanoparticles, have a significant impact on pharmacokinetic parameters and can be used to develop combination therapies with more appropriate patterns of drug concentration changes and BBB permeability properties. For example, drug encapsulation solutions such as delphinidin can improve the efficacy of drug delivery in brain tissue [169].
One of the new strategies for GBM treatment involves the use of nanomaterials and polymer carriers in which active drugs are embedded. For example, Gliadel® wafers (manufactured by Eisai Inc. for Azurity Pharmaceuticals, Inc.) containing carmustine can be implanted into the niche after resection of the GBM tumor. This strategy was approved by the FDA (U.S. Food and Drug Administration Agency) in 2003 as an alternative in GBM treatment, especially in combination with radiotherapy and TMZ, which increased the overall survival of patients by up to 4 months [170]. The main advantage of this approach is the biodegradation of the polymer, which releases carmustine slowly over two weeks [43]. However, clinical studies have shown that complications such as brain swelling, seizures, wound healing disorders or brain infections can occur in some cases, which could be a reason for excluding this treatment option.
New strategies for local or systemic application in GBM currently include polymer carriers such as nanoparticles, polymeric micelles, lipid-based drug delivery systems (liposomes) or hydrogels with the potential to incorporate active macromolecules [171]. It is worth noting that locally implanted treatments can bypass physiological barriers such as the BBB and can act more aggressively and directly on tumor tissue [171].
In addition to chemotherapy for GBM, other treatment options are being investigated, including EGFR inhibitors. One such inhibitor, afatinib, has been shown to extend patient survival in combination with TMZ. New EGFR inhibitors, such as erlotinib and gefitinib, are currently being investigated in clinical trials [172]. In the treatment of GBM, a combination therapy of TMZ and either BVC or afatinib has been shown to improve patient survival. However, the development of resistance to TMZ or radiotherapy remains a major challenge in GBM treatment, leading to poor outcomes with targeted anti-angiogenic or anti-EGFR therapies. BBB permeability is a significant obstacle to targeted drug delivery and the second mechanism of chemoresistance plays a crucial role in treatment failure [173]. New drug formulations such as liposomes, polymers and nanoparticle carriers are being investigated for their improved properties in targeted drug delivery against GBM. An in vitro study showed that the combination of doxorubicin and erlotinib encapsulated in liposomes improved the uptake of the drug by glioblastoma U-78 MG cells, which could be a promising alternative [174].
Studies on the encapsulation of TMZ, the most effective drug in GBM since 2005, have also been presented, although they are limited in number and efficacy has not been fully confirmed. TMZ carriers such as liposomes, solid lipid nanoparticles, polymeric nanoparticles, dendrimers, mesoporous silica nanoparticles, graphene oxide nanoparticles, and magnetic nanoparticles have been investigated to date [175]. These probes are necessary due to the limitations of TMZ, including hydrolysis, poor solubility, non-specific toxicity, and also due to GBM characteristics such as differentiation into a chemoresistant phenotype and the general heterogeneity of this neoplasm, not disregarding the permeability of the BBB [175].
Electrical field
Optune's TTF (tumor treatment fields) devices (manufactured by Novocure) were approved by the FDA in 2015 as the latest innovation for the treatment of newly diagnosed GBM. This method applies electric fields with low intensity (1–3 V/cm) and medium frequency (200 kHz) pulses [176]. Despite the increase in disease-free survival, this method is not standard of care due to the marginal changes in overall survival, high cost and inconvenience to patients (skin toxicity and seizures) [177]. Remarkably, co-therapy with TMZ, RT and TTF has been observed to prolong progression-free survival by up to 56 % at six months in clinical trials conducted (NCT00916409). To date, this is the last FDA-approved therapy for GBM.
Overcoming radiotherapy
Radiotherapy is still one of the standard therapies in the treatment of GBM, in addition to chemotherapy, usually TMZ [178]. However, the restrictions on the use of radiotherapy depend on the patient's general physical condition. The standard regimen involves radiotherapy over a period of 6 weeks, with a total dose of 40–60 Gy, usually in 2 Gy doses [179,180]. However, there is still a need to improve the effectiveness of γ-radiation exposure in the postoperative cavity to protect critical organs at risk such as optic nerves, optic chiasm, eyes, lenses, brain and brainstem with respect to the accepted margin [179]. Cheng et al. (2022) [181], have recently described an improvement in magnetic resonance imaging (MRI) that allows differentiation between neoplasms and normal brain tissue based on the protein VCAM-1 (vascular cell adhesion molecule-1). The higher expression of this protein in tumor endothelial cells as opposed to normal tissue could have the potential to better mark the interface between them. These results suggest a new role for the membrane protein in the treatment of GBM. In addition, the resistance to therapy in the development of GBM could be due to the reduced sensitivity of γ-irradiation. This phenomenon is related to the population of glioma stem cells (GSC) that survive radiotherapy and are involved in the recurrence of the radioresistance phenotype. GSC are thought to be the main reason for GBM tumor recurrence [182]. Mechanistically, increased expression of N-cadherin is involved in radioresistance, which inhibits the Wnt/β-catenin signaling pathway and causes anti-apoptotic effects and decreased neuronal differentiation. The observed increase in N-cadherin levels was caused by IGF-1, which was upregulated by γ-irradiation. Picropodophyllin, a clinically approved IGF-1 inhibitor, may have the potential to reverse the radioresistance phenotype of GBM [183]. In this regard, targeted N-cadherin therapy could be a promising direction to combat this aggressive phenotype.
Alteration of the lipid metabolism
Lipid metabolism is significantly altered in glioblastoma, not only limited in glioma itself, but present in the whole brain. Changes in lipids are frequently observed in neoplasms and also in other neuronal disorders, in autoimmune diseases such as multiple sclerosis, have also been described [[184], [185], [186]]. A typical finding in GBM diagnosis is an increase in choline levels observed on MRI, which reflects an imbalance in lipid metabolism. Changes in lipidomics and fatty acids appear to be a promising area for GBM treatment. Fatty acids are involved in the induction of immune scavenging in GBM, promote cell proliferation and also regulate ferroptosis [187,188].
First, as a way to combat neoplasms by destabilizing metabolism through an imbalance of anabolism and catabolism processes in GBM cells. Secondly, lipid droplets serve as a space for drug delivery and changes in lipid droplet content can attenuate this phenomenon. Oxidation of fatty acids, especially β-oxidation, is a potential target in anti-GBM therapy. However, this is a relatively new area of research and so far only two clinical trials (NCT05118776 and NCT03032484) have been conducted using fatty acid synthase as a target protein, with inhibitors: TVB-2640 and ASC40. The detailed role of fatty acids in GBM has been described in an excellent review by Jason Miska and Navdeep S. Chandel [189]. Recently, CDKN2A was also found to be responsible for the distribution of polyunsaturated fatty acids (PUFA) in lipid droplets. As observed in an in vitro and in vivo model of GBM, the consequences of CDKN2A deletion were lipid peroxidation and ferroptosis, resulting in prolonged survival of xenografts in mice. These findings open up new possibilities for the reorganization of lipid metabolism in GBM and also for the development of glutation peroxidase inhibitors suitable for brain treatment [190].
Ferroptosis
Ferroptosis leads to an accumulation of lipid peroxide and thus to non-apoptotic and non-necrotic cell death due to an iron-dependent mechanism [191]. Lipid metabolism is closely linked to ferroptosis. ROS generated in the Fenton reaction, in which Fe2+ is converted to Fe3+ in the presence of H2O2, are involved in the peroxidation of PUFAs in cell structures such as membranes and alter them, leading to cell death [238]. Recently, the usefulness of ferroptosis in anticancer treatment, especially in GBM, has been the focus of research [192]. Ferroptosis induction, including by disulfiram, resulted in recurrence of radiotherapy sesitivity in vitro studies on glioblastoma cell lines U251 and LN229 [193]. It is worth noting that mutation of p53 determines the response to pro-ferroptosis treatment in GBM, while this response is weaker in p53 wild-type GBM in an in vivo mouse model [194].
One of the latest concepts of ferroptosis useful in targeting GBM is found in the work of Cao and coworkers, in which the authors described the anti-tumor effect of biomimetic nanoparticles consisting of macrophage membrane-coated nanoparticles as carriers for the ferroptosis inducer ALOX15 lipoxygenase in a mouse model of GBM [195]. Induction of ferroptosis could also be triggered by fatostatin and acts mechanistically by inhibiting the AKT/mTORC1/GPX4 pathway in GBM and has anti-tumor effects in vitro and in vivo models [196]. In addition, recent studies have shown that decreased ferroptosis is related to the TMZ-resistant phenotype of GBM, and to date, the Fanconi anemia-associated gene Fanconi anemia complementation group D2 (FANCD2) has been found to play this role [197].
Moreover, ferroptosis is linked to apoptosis and autophagy, and this interplay is observed in GBM, which is considered a potential approach for therapeutic strategies, with many questions still open that deserve a scientific answer [198].
Autophagy
Autophagy is a lysosome-mediated process that is observed at different stages of carcinogenesis as a dual tumor-promoting or tumor-suppressing mechanism, depending on tumor microbiota formation. In general, this process increases cancer cell survival during unfavorable times such as starvation [199]. Autophagy is also observed in GBM as a side effect of TMZ treatment and is responsible for the chemoresistant phenotype of GBM. Some autophagy inhibitors, such as chloroqiunone, cause these neoplasm cells to become resistant to TMZ without significantly increasing the overall survival rate of patients [152]. In contrast, the antitumor role of autophagy, classified as exosomal or secretory autophagy, has been described in GBM and associated with repolarization of macrophage M1 to a tumor-suppressive M1 phenotype, resulting in TMZ sensitization [200]. In more recent studies by Chryplewicz et al. [199], CD8+ and CD4+ T cell efflux was observed as a result of stimulation of autophagy following concomitant treatment with VEGFR inhibitors and the tricyclic antidepressant imipramine. Surprisingly, this stimulation of autophagy also leads to an increase in PDL-1 and together suggests a potential for immunotherapy in GBM in conjunction with autophagy inducers [201]. In addition, autophagy inducers such as loperamide, pimozide and STF-62,247 were found to induce cell death in vitro in MZ-5426,29, LN-229 and U343 GOS-3 glioblastoma cells through large-scale induction of autophagy [202]. Currently, no autophagy inducers are clinically viable, and deeper insights are urgently needed to better understand the potential of autophagy-based therapy in GBM.
Concluding remarks and future challenges
GBM is one of the most vascularized tumors, with a dynamic molecular differentiation in terms of chemoresistance and a -resistant phenotype that emerges during treatment. Targeted therapies have not yet produced successful results, despite the administration of BVC, which was believed to be a milestone for clinical outcomes, but it does not improve overall patient survival alone. To improve clinical outcomes of GBM patients, therapeutic strategies targeting different goals should be considered when developing new treatments. GBM therapy is undoubtedly one of the greatest challenges in the field of oncology. This review presents some current multidisciplinary solutions that could be seen as the beginning of new possibilities in the design of GBM treatment. On closer inspection, the mechanisms involved in GBM development and progression are interconnected and overlapping, and a holistic approach could yield the most clinically meaningful new co-therapy regimens. New drug carriers, such as lisosomes and polymers, are predestined to bypass the BBB and should therefore be considered in the development of therapeutic strategies.
CRediT authorship contribution statement
Agnieszka Rusak: Writing – review & editing, Writing – original draft, Conceptualization. Benita Wiatrak: Writing – review & editing, Writing – original draft. Klaudia Krawczyńska: Writing – review & editing, Writing – original draft. Tomasz Górnicki: Writing – review & editing. Karol Zagórski: Writing – review & editing. Łukasz Zadka: Writing – review & editing. Wojciech Fortuna: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
All data has been included in the manuscript. If required, it can be available from the corresponding author upon reasonable request.
Contributor Information
Agnieszka Rusak, Email: agnieszka.rusak@umw.edu.pl.
Benita Wiatrak, Email: benita.wiatrak@umw.edu.pl.
Klaudia Krawczyńska, Email: klaudia.krawczynska@umw.edu.pl.
Łukasz Zadka, Email: l.zadka@umw.edu.pl.
Wojciech Fortuna, Email: wojciech.fortuna@umw.edu.pl.
References
- 1.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/S00401-016-1545-1/TABLES/5. [DOI] [PubMed] [Google Scholar]
- 2.Pellerino A., Caccese M., Padovan M., Cerretti G., Lombardi G. Epidemiology, risk factors, and prognostic factors of gliomas. Clin. Transl. ImAging. 2022;10:467–475. doi: 10.1007/S40336-022-00489-6/METRICS. [DOI] [Google Scholar]
- 3.Davis M.E. Glioblastoma: overview of disease and treatment. Clin. J. Oncol. Nurs. 2016;20:S2. doi: 10.1188/16.CJON.S1.2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdugo E., Puerto I., Medina M.Á. An update on the molecular biology of glioblastoma, with clinical implications and progress in its treatment. Cancer Commun. 2022;42:1083. doi: 10.1002/CAC2.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behnan J., Finocchiaro G., Hanna G. The landscape of the mesenchymal signature in brain tumours. Brain. 2019;142:847. doi: 10.1093/BRAIN/AWZ044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Khayari A., Bouchmaa N., Taib B., Wei Z., Zeng A., El Fatimy R. Metabolic rewiring in glioblastoma cancer: EGFR, IDH and beyond. Front. Oncol. 2022;12 doi: 10.3389/FONC.2022.901951/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H.J., Park J.W., Lee J.H. Genetic architectures and cell-of-origin in glioblastoma. Front. Oncol. 2021;10 doi: 10.3389/FONC.2020.615400/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zappe K., Pühringer K., Pflug S., Berger D., Weis S., Spiegl-Kreinecker S., Cichna-Markl M. Association of MGMT promoter and enhancer methylation with genetic variants, clinical parameters, and demographic characteristics in glioblastoma. Cancers. (Basel) 2023;15:5777. doi: 10.3390/CANCERS15245777/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han Sue, et al. IDH Mutation in glioma: molecular mechanisms and potential therapeutic targets. Br. J. Cancer. 2020;122(11):1580–1589. doi: 10.1038/s41416-020-0814-x. Springer US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strobel H., Baisch T., Fitzel R., Schilberg K., Siegelin M.D., Karpel-Massler G., Debatin K.M., Westhoff M.-A. TMZe and other alkylating agents in glioblastoma therapy. Biomedicines. 2019;7:69. doi: 10.3390/BIOMEDICINES7030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J.C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021;18:170–186. doi: 10.1038/S41571-020-00447-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rončević A., Koruga N., Soldo Koruga A., Rončević R., Rotim T., Šimundić T., Kretić D., Perić M., Turk T., Štimac D. Personalized treatment of glioblastoma: current state and future perspective. Biomedicines. 2023;11:1579. doi: 10.3390/BIOMEDICINES11061579. 2023, Vol. 11, Page 1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan F., Zhang H., Dai Z., Zhang Y., Xia Z., Cao H., Yang K., Hu S., Guo Y., Ding F., et al. A Comprehensive prognostic signature for glioblastoma patients based on transcriptomics and single cell sequencing. Cellular Oncology. 2021;44:917–935. doi: 10.1007/S13402-021-00612-1/METRICS. [DOI] [PubMed] [Google Scholar]
- 14.Tang L., Feng Y., Gao S., Mu Q., Liu C. Nanotherapeutics overcoming the blood-brain barrier for glioblastoma treatment. Front. Pharmacol. 2021;12 doi: 10.3389/FPHAR.2021.786700/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakthikumar S., Sakthikumar S., Roy A., Haseeb L., Pettersson M.E., Sundström E., Marinescu V.D., Lindblad-Toh K., Lindblad-Toh K., Forsberg-Nilsson K. Whole-genome sequencing of glioblastoma reveals enrichment of non-coding constraint mutations in known and novel genes. Genome Biol. 2020;21:1–22. doi: 10.1186/S13059-020-02035-X/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Yang J., Zheng H., Tomasek G.J., Zhang P., McKeever P.E., Lee E.Y.H.P., Zhu Y. Expression of Mutant P53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 2009;15:514. doi: 10.1016/J.CCR.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serebriiskii I.G., Pavlov V., Tricarico R., Andrianov G., Nicolas E., Parker M.I., Newberg J., Frampton G., Meyer J.E., Golemis E.A. Comprehensive characterization of PTEN mutational profile in a series of 34,129 colorectal cancers. Nat. Commun. 2022;13:1–17. doi: 10.1038/s41467-022-29227-2. 2022 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortin Ensign S.P., Jenkins R.B., Giannini C., Sarkaria J.N., Galanis E., Kizilbash S.H. Translational significance of CDKN2A/B homozygous deletion in isocitrate dehydrogenase-mutant astrocytoma. Neuro Oncol. 2023;25:28–36. doi: 10.1093/NEUONC/NOAC205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaviano A., Foo A.S.C., Lam H.Y., Yap K.C.H., Jacot W., Jones R.H., Eng H., Nair M.G., Makvandi P., Geoerger B., et al. PI3K/AKT/MTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer. 2023;22:1–37. doi: 10.1186/S12943-023-01827-6/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao J., Sun D., Dong L., Zhu H., Hou H. Advancement in research and therapy of NF1 mutant malignant tumors. Cancer Cell Int. 2020;20:1–8. doi: 10.1186/S12935-020-01570-8/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linn P., Kohno S., Sheng J., Kulathunga N., Yu H., Zhang Z., Voon D., Watanabe Y., Takahashi C. Targeting RB1 loss in cancers. Cancers. (Basel) 2021;13:3737. doi: 10.3390/CANCERS13153737. 2021, Vol. 13, Page 3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karagiannakos A., Adamaki M., Tsintarakis A., Vojtesek B., Fåhraeus R., Zoumpourlis V., Karakostis K. Targeting oncogenic pathways in the era of personalized oncology: a systemic analysis reveals highly mutated signaling pathways in cancer patients and potential therapeutic targets. Cancers. (Basel) 2022;14:664. doi: 10.3390/CANCERS14030664/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hersh A.M., Gaitsch H., Alomari S., Lubelski D., Tyler B.M., Hersh A.M., Gaitsch H., Alomari S., Lubelski D., Tyler B.M. Molecular pathways and genomic landscape of glioblastoma stem cells: opportunities for targeted therapy. Cancers. (Basel) 2022;14:3743. doi: 10.3390/CANCERS14153743. 2022, Vol. 14, Page 3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeitouni D., Catalino M.P., Wise J., McCabe S., Pietrosimone K., Rashid N., Khagi S. Clinical application of next-generation sequencing in recurrent glioblastoma. Onco (Basel) 2021;1:38–48. doi: 10.3390/onco1010005. [DOI] [Google Scholar]
- 25.Yalamarty S.S.K., Filipczak N., Li X., Subhan M.A., Parveen F., Ataide J.A., Rajmalani B.A., Torchilin V.P. Mechanisms of resistance and current treatment options for glioblastoma multiforme (GBM) Cancers. (Basel) 2023;15:2116. doi: 10.3390/CANCERS15072116. 2023, Vol. 15, Page 2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D., Dai X., Tao Z., Zhou H., Hong W., Qian H., Cheng H., Wang X. Advances in blood–brain barrier-crossing nanomedicine for anti-glioma. Cancer Nanotechnol. 2023;14:1–32. doi: 10.1186/S12645-023-00211-9. 2023 14:1. [DOI] [Google Scholar]
- 27.Ryan J.T., Nakayama M., Gleeson I., Mannion L., Geso M., Kelly J., Ng S.P., Hardcastle N. Functional brain imaging interventions for radiation therapy planning in patients with glioblastoma: a systematic review. Radiation Oncology. 2022;17:1–20. doi: 10.1186/S13014-022-02146-8/TABLES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Liu R., Zhang Q., Dong M., Wang D., Chen J., Ou Y., Luo H., Yang K., Wang X. Charged particle therapy for high-grade gliomas in adults: a systematic review. Radiation Oncology. 2023;18:1–17. doi: 10.1186/S13014-022-02187-Z/TABLES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong L., Li N., Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J. Exper. Clin. Cancer Res. 2022;41:1–18. doi: 10.1186/S13046-022-02349-7. 2022 41:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon D.J., Gleeson J.P., O'Reilly S., Bambury R.M. Management of newly diagnosed glioblastoma multiforme: current state of the art and emerging therapeutic approaches. Med. Oncology. 2022;39:1–12. doi: 10.1007/S12032-022-01708-W/METRICS. [DOI] [PubMed] [Google Scholar]
- 31.Yasinjan F., Xing Y., Geng H., Guo R., Yang L., Liu Z., Wang H. Immunotherapy: a promising approach for glioma treatment. Front. Immunol. 2023;14 doi: 10.3389/FIMMU.2023.1255611/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das S., Dash B.S., Premji T.P., Chen J.P. Immunotherapeutic Approaches for the Treatment of Glioblastoma Multiforme: mechanism and Clinical Applications. Int. J. Mol. Sci. 2023;24:10546. doi: 10.3390/IJMS241310546. 2023, Vol. 24, Page 10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koekkoek J.A.F., van der Meer P.B., Pace A., Hertler C., Harrison R., Leeper H.E., Forst D.A., Jalali R., Oliver K., Philip J., et al. Palliative care and end-of-life care in adults with malignant brain tumors. Neuro Oncol. 2023;25:447–456. doi: 10.1093/NEUONC/NOAC216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergo E., Lombardi G., Pambuku A., Della Puppa A., Bellu L., D'avella D., Zagonel V. Cognitive rehabilitation in patients with gliomas and other brain tumors: state of the art. Biomed. Res. Int. 2016 doi: 10.1155/2016/3041824. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young J.S., Al-Adli N., Sibih Y.E., Scotford K.L., Casey M., James S., Berger M.S. Recognizing the psychological impact of a glioma diagnosis on mental and behavioral health: what neurosurgeons need to know. J. Neurosurg. 2023;139:11. doi: 10.3171/2022.9.JNS221139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoud A.B., Ajina R., Aref S., Darwish M., Alsayb M., Taher M., AlSharif S.A., Hashem A.M., Alkayyal A.A. Advances in immunotherapy for glioblastoma multiforme. Front. Immunol. 2022;13 doi: 10.3389/FIMMU.2022.944452/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mowforth O.D., Brannigan J., El Khoury M., Sarathi C.I.P., Bestwick H., Bhatti F., Mair R. Personalised therapeutic approaches to glioblastoma: a systematic review. Front. Med. (Lausanne) 2023;10 doi: 10.3389/FMED.2023.1166104/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aldoghachi A.F., Aldoghachi A.F., Breyne K., Ling K.H., Cheah P.S. Recent advances in the therapeutic strategies of glioblastoma multiforme. Neuroscience. 2022;491:240–270. doi: 10.1016/J.NEUROSCIENCE.2022.03.030. [DOI] [PubMed] [Google Scholar]
- 39.Karachi A., Dastmalchi F., Mitchell D.A., Rahman M. TMZe for immunomodulation in the treatment of glioblastoma. Neuro Oncol. 2018;20:1566. doi: 10.1093/NEUONC/NOY072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hotchkiss K.M., Sampson J.H. TMZe treatment outcomes and immunotherapy efficacy in brain tumor. J. Neurooncol. 2021;151:55. doi: 10.1007/S11060-020-03598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagasawa D.T., Chow F., Yew A., Kim W., Cremer N., Yang I. TMZe and other potential agents for the treatment of glioblastoma multiforme. Neurosurg. Clin. N. Am. 2012;23:307–322. doi: 10.1016/J.NEC.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Lee C.Y. Strategies of TMZe in future glioblastoma treatment. Onco Targets. Ther. 2017;10:265–270. doi: 10.2147/OTT.S120662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu W., Klockow J.L., Zhang M., Lafortune F., Chang E., Jin L., Wu Y., Daldrup-Link H.E. Glioblastoma Multiforme (GBM): an overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021:171. doi: 10.1016/J.PHRS.2021.105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakravarti Arnab, Erkkinen Michael G., Nestler Ulf, Stupp Roger, Mehta Minesh, Aldape Ken, Gilbert Mark R., Black Peter McL., Loeffler Jay S. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin. Cancer Res. 2006;12(15):4738–4746. doi: 10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- 45.Weller M., Le Rhun E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat. Rev. 2020;87 doi: 10.1016/J.CTRV.2020.102029. [DOI] [PubMed] [Google Scholar]
- 46.Vaz-Salgado M.A., Villamayor M., Albarrán V., Alía V., Sotoca P., Chamorro J., Rosero D., Barrill A.M., Martín M., Fernandez E., et al. Recurrent glioblastoma: a review of the treatment options. Cancers. (Basel) 2023;15:4279. doi: 10.3390/CANCERS15174279. 2023, Vol. 15, Page 4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y.D., Dai R.Y., Chen Z., Zhang Y.H., He X.Z., Zhou J. Efficacy and safety of carmustine wafers in the treatment of glioblastoma multiforme: a systematic review. Turk. Neurosurg. 2014;24:639–645. doi: 10.5137/1019-5149.JTN.8878-13.1. [DOI] [PubMed] [Google Scholar]
- 48.Xiao Z.Z., Wang Z.F., Lan T., Huang W.H., Zhao Y.H., Ma C., Li Z.Q. Carmustine as a supplementary therapeutic option for glioblastoma: a systematic review and meta-analysis. Front. Neurol. 2020;11:1036. doi: 10.3389/FNEUR.2020.01036/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry J., Chambers A., Spithoff K., Laperriere N. Gliadel wafers in the treatment of malignant glioma: a systematic review. Current Oncology. 2007;14:189. doi: 10.3747/CO.2007.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Bonis P., Anile C., Pompucci A., Fiorentino A., Balducci M., Chiesa S., Maira G., Mangiola A. Safety and efficacy of gliadel wafers for newly diagnosed and recurrent glioblastoma. acta neurochir. (wien) 2012;154:1371–1378. doi: 10.1007/S00701-012-1413-2/METRICS. [DOI] [PubMed] [Google Scholar]
- 51.Parasramka S., Talari G., Villano J.L., Rosenfeld M., Guo J. Procarbazine, lomustine and vincristine for recurrent high-grade glioma. Cochrane Database Syst. Rev. 2017;2017:1–34. doi: 10.1002/14651858.CD011773.PUB2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim S.H., Yoo H., Chang J.H., Kim C.Y., Chung D.S., Kim S.H., Park S.H., Lee Y.S., Yang S.H. Procarbazine and CCNU Chemotherapy for Recurrent Glioblastoma with MGMT Promoter Methylation. J. Korean Med. Sci. 2018;33 doi: 10.3346/JKMS.2018.33.E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solimando D.A., Waddell J.A. Procarbazine, Lomustine, and Vincristine (PCV) Regimen for Central Nervous System Tumors. Hosp. Pharm. 2017;52:98. doi: 10.1310/HPJ5202-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He Q., Liu J., Liang J., Liu X., Li W., Liu Z., Ding Z., Tuo D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells. 2018:7. doi: 10.3390/CELLS7040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mojarad-Jabali S., Farshbaf M., Hemmati S., Sarfraz M., Motasadizadeh H., Shahbazi Mojarrad J., Atyabi F., Zakeri-Milani P., Valizadeh H. Comparison of three synthetic transferrin mimetic small peptides to promote the blood-brain barrier penetration of vincristine liposomes for improved glioma targeted therapy. Int. J. Pharm. 2022:613. doi: 10.1016/J.IJPHARM.2021.121395. [DOI] [PubMed] [Google Scholar]
- 56.Shukla R., Singh A., Singh K.K. Vincristine-Based Nanoformulations: a Preclinical and Clinical Studies Overview. Drug Deliv. Transl. Res. 2024;14:1–16. doi: 10.1007/S13346-023-01389-6/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee C.M., Zane N.R., Veal G., Thakker D.R. Physiologically based pharmacokinetic models for adults and children reveal a role of intracellular tubulin binding in vincristine disposition. CPT. Pharmacometrics. Syst. Pharmacol. 2019;8:759. doi: 10.1002/PSP4.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Škubník J., Pavlíčková V.S., Ruml T., Rimpelová S. Vincristine in combination therapy of cancer: emerging trends in clinics. Biology. (Basel) 2021:10. doi: 10.3390/BIOLOGY10090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aydin B., Patil M., Bekele N., Wolff J.E.A. Vincristine in High-Grade Glioma. Anticancer Res. 2010;30:2303–2310. [PubMed] [Google Scholar]
- 60.Abdel-Rahman O., Fouad M. Irinotecan-based regimens for recurrent glioblastoma multiforme: [corrected] a systematic review. Expert. Rev. NeurOther. 2015;15:1255–1270. doi: 10.1586/14737175.2015.1101346. [DOI] [PubMed] [Google Scholar]
- 61.Vredenburgh J.J., Desjardins A., Reardon D.A., Friedman H.S. Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol. 2009;11:80. doi: 10.1215/15228517-2008-075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrlinger U., Tzaridis T., Mack F., Steinbach J.P., Schlegel U., Sabel M., Hau P., Kortmann R.D., Krex D., Grauer O., et al. Lomustine-TMZe combination therapy versus standard TMZe therapy in patients with newly diagnosed glioblastoma with methylated mgmt promoter (CeTeG/NOA–09): a Randomised, Open-Label, Phase 3 Trial. Lancet. 2019;393:678–688. doi: 10.1016/S0140-6736(18)31791-4. [DOI] [PubMed] [Google Scholar]
- 63.Linde M.E., Verhoeff J.J.C., Richel D.J., Furth W.R., Reijneveld J.C., Verheul H.M.W., Stalpers L.J.A. Bevacizumab in combination with radiotherapy and TMZe for patients with newly diagnosed glioblastoma multiforme. Oncologist. 2015;20:107–108. doi: 10.1634/THEONCOLOGIST.2014-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gil-Gil M.J., Mesia C., Rey M., Bruna J. Bevacizumab for the Treatment of Glioblastoma. Clin. Med. Insights. Oncol. 2013;7:123. doi: 10.4137/CMO.S8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diaz R.J., Ali S., Qadir M.G., De La Fuente M.I., Ivan M.E., Komotar R.J. The role of bevacizumab in the treatment of glioblastoma. J. Neurooncol. 2017;133:455–467. doi: 10.1007/S11060-017-2477-X. [DOI] [PubMed] [Google Scholar]
- 66.Fu M., Zhou Z., Huang X., Chen Z., Zhang L., Zhang J., Hua W., Mao Y. Use of bevacizumab in recurrent glioblastoma: a scoping review and evidence map. BMC. Cancer. 2023;23:1–23. doi: 10.1186/S12885-023-11043-6/TABLES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melhem J.M., Tahir A., Calabrese E., Granovskaya I., Atenafu E.G., Sahgal A., Lim-Fat M.J., Perry J.R. Dose-dependent efficacy of bevacizumab in recurrent glioblastoma. J. Neurooncol. 2023;161:633–641. doi: 10.1007/S11060-023-04248-Z. [DOI] [PubMed] [Google Scholar]
- 68.Nagpal S., Harsh G., Recht L. Bevacizumab improves quality of life in patients with recurrent glioblastoma. ChemOther Res. Pract. 2011;2011:1–6. doi: 10.1155/2011/602812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Begagić E., Pugonja R., Bečulić H., Čeliković A., Tandir Lihić L., Kadić Vukas S., Čejvan L., Skomorac R., Selimović E., Jaganjac B.;., et al. Molecular Targeted therapies in glioblastoma multiforme: a systematic overview of global trends and findings. Brain Sci. 2023:13. doi: 10.3390/BRAINSCI13111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.An Z., Aksoy O., Zheng T., Fan Q.W., Weiss W.A. Epidermal Growth Factor Receptor (EGFR) and EGFRvIII in Glioblastoma (GBM): signaling Pathways and Targeted Therapies. Oncogene. 2018;37:1561. doi: 10.1038/S41388-017-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Do D.T., Yang M.R., Lam L.H.T., Le N.Q.K., Wu Y.W. Improving MGMT Methylation Status Prediction of Glioblastoma through optimizing radiomics features using genetic algorithm-based machine learning approach. Sci. Rep. 2022;12:1–12. doi: 10.1038/s41598-022-17707-w. 2022 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kitange G.J., Carlson B.L., Mladek A.C., Decker P.A., Schroeder M.A., Wu W., Grogan P.T., Giannini C., Ballman K.V., Buckner J.C., et al. Evaluation of MGMT promoter methylation status and correlation with TMZe response in orthotopic glioblastoma xenograft model. J. Neurooncol. 2009;92:23–31. doi: 10.1007/S11060-008-9737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donson A.M., Addo-Yobo S.O., Handler M.H., Gore L., Foreman N.K. MGMT promoter methylation correlates with survival benefit and sensitivity to tmze in pediatric glioblastoma. Pediatr. Blood Cancer. 2007;48:403–407. doi: 10.1002/PBC.20803. [DOI] [PubMed] [Google Scholar]
- 74.Hegi M.E., Diserens A.-C., Gorlia T., Hamou M.-F., de Tribolet N., Weller M., Kros J.M., Hainfellner J.A., Mason W., Mariani L., et al. MGMT gene silencing and benefit from TMZe in glioblastoma. New England J. Med. 2005;352:997–1003. doi: 10.1056/NEJMOA043331. [DOI] [PubMed] [Google Scholar]
- 75.Lee C.Y. Strategies of TMZe in Future Glioblastoma Treatment. Onco Targets. Ther. 2017;10:265–270. doi: 10.2147/OTT.S120662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fuchs R.P., Isogawa A., Paulo J.A., Onizuka K., Takahashi T., Amunugama R., Duxin J.P., Fujii S. Crosstalk between repair pathways elicits double-strand breaks in alkylated DNA and implications for the action of temozolomide. Elife. 2021;10:e69544. doi: 10.7554/eLife.69544. PMID: 34236314; PMCID: PMC8289412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miramova A., Gartner A., Ivanov D. How to sensitize glioblastomas to temozolomide chemotherapy: a gap-centered view. Front. Cell Dev. Biol. 2024;12 doi: 10.3389/fcell.2024.1436563. PMID: 39011394; PMCID: PMC11246897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J., Song C., Gu J., Li C., Zang W., Shi L., Chen L., Zhu L., Zhou M., Wang T., Li H., Qi S., Lu Y. RBBP4 regulates the expression of the Mre11-Rad50-NBS1 (MRN) complex and promotes DNA double-strand break repair to mediate glioblastoma chemoradiotherapy resistance. Cancer Lett. 2023;557 doi: 10.1016/j.canlet.2023.216078. Epub 2023 Feb 2. PMID: 36736531. [DOI] [PubMed] [Google Scholar]
- 79.Han S., Liu Y., Cai S.J., Qian M., Ding J., Larion M., Gilbert M.R., Yang C. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br. J. Cancer. 2020;122:1580–1589. doi: 10.1038/s41416-020-0814-x. 2020 122:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen A.L., Holmen S.L., Colman H. IDH1 and IDH2 mutations in gliomas. Curr. Neurol. Neurosci. Rep. 2013;13:345. doi: 10.1007/S11910-013-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan H., Parsons D.W., Jin G., McLendon R., Rasheed B.A., Yuan W., Kos I., Batinic-Haberle I., Jones S., Riggins G.J., et al. IDH1 and IDH2 mutations in gliomas. New England J. Med. 2009;360:765–773. doi: 10.1056/NEJMOA0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koh J., Cho H., Kim H., Kim S.I., Yun S., Park C.K., Lee S.H., Choi S.H., Park S.H. IDH2 Mutation in gliomas including novel mutation. Neuropathology. 2015;35:236–244. doi: 10.1111/NEUP.12187. [DOI] [PubMed] [Google Scholar]
- 83.Mellinghoff I.K., van den Bent M.J., Blumenthal D.T., Touat M., Peters K.B., Clarke J., Mendez J., Yust-Katz S., Welsh L., Mason W.P., et al. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. New England J. Med. 2023;389:589–601. doi: 10.1056/NEJMOA2304194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li J., Feng L., Lu Y. Glioblastoma multiforme: diagnosis, treatment, and invasion. J. Biomed. Res. 2023;37(1):47–58. doi: 10.7555/JBR.36.20220156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson L.A., Scholler J., Ohkuri T., Kosaka A., Patel P.R., McGettigan S.E., Nace A.K., Dentchev T., Thekkat P., Loew A., Boesteanu A.C., Cogdill A.P., Chen T., Fraietta J.A., Kloss C.C., Posey A.D., Engels B., Singh R., Ezell T.…Maus M.V. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci. Transl. Med. 2015;7(275):1–30. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.French P.J., Eoli M., Sepulveda J.M., de Heer I., Kros J.M., Walenkamp A., Frenel J.S., Franceschi E., Clement P.M., Weller M., Ansell P., Looman J., Bain E., Morfouace M., Gorlia T., van den Bent M. Defining EGFR amplification status for clinical trial inclusion. Neuro Oncol. 2019;21(10):1263–1272. doi: 10.1093/neuonc/noz096. PMID: 31125418; PMCID: PMC6784284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nathanson D.A., Gini B., Mottahedeh J., Visnyei K., Koga T., Gomez G., Eskin A., Hwang K., Wang J., Masui K., Paucar A., Yang H., Ohashi M., Zhu S., Wykosky J., Reed R., Nelson S.F., Cloughesy T.F., James C.D., Rao P.N., Kornblum H.I., Heath J.R., Cavenee W.K., Furnari F.B., Mischel P.S. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science (1979) 2014;343(6166):72–76. doi: 10.1126/science.1241328. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4049335/ Epub 2013 Dec 5. PMID: 24310612; PMCID: PMC4049335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shaban N., Kamashev D., Emelianova A., Buzdin A. Targeted inhibitors of EGFR: structure, biology, biomarkers, and clinical applications. Cells. 2023;13:47. doi: 10.3390/CELLS13010047. 2024, Vol. 13, Page 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan P.C., Magge R.S. Mechanisms of EGFR Resistance in Glioblastoma. Int. J. Mol. Sci. 2020;21:1–21. doi: 10.3390/IJMS21228471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oprita A., Baloi S.C., Staicu G.A., Alexandru O., Tache D.E., Danoiu S., Micu E.S., Sevastre A.S. Updated insights on EGFR signaling pathways in glioma. Int. J. Mol. Sci. 2021;22:1–21. doi: 10.3390/IJMS22020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y., Dube C., Gibert M., Cruickshanks N., Wang B., Coughlan M., Yang Y., Setiady I., Deveau C., Saoud K., et al. The P53 pathway in glioblastoma. Cancers. (Basel) 2018:10. doi: 10.3390/CANCERS10090297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang P., Meng X., Liu L., Li S., Li Y., Ali S., Li S., Xiong J., Liu X., Li S., et al. Identification of the prognostic signatures of glioma with different PTEN status. Front. Oncol. 2021:11. doi: 10.3389/FONC.2021.633357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jankowska S., Lewandowska M., Masztalewicz M., Sagan L., Nowacki P., Urasińska E. Molecular classification of glioblastoma based on immunohistochemical expression of Egfr, Pdgfra, Nf1, Idh1, P53 and Pten proteins. Polish Journal of Pathology. 2021;72:1–10. doi: 10.5114/PJP.2021.106439. [DOI] [PubMed] [Google Scholar]
- 94.El Atat O., Naser R., Abdelkhalek M., Habib R.A., El Sibai M. Molecular targeted therapy: a new avenue in glioblastoma treatment. Oncol. Lett. 2022:25. doi: 10.3892/OL.2022.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weathers S.-P., de Groot J. VEGF manipulation in glioblastoma. Oncology (Williston. Park) 2015;29:720–727. [PubMed] [Google Scholar]
- 96.Zhang P., Xia Q., Liu L., Li S., Dong L. Current Opinion on Molecular Characterization for GBM classification in guiding clinical diagnosis, prognosis, and therapy. Front. Mol. Biosci. 2020;7 doi: 10.3389/FMOLB.2020.562798/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muir M., Gopakumar S., Traylor J., Lee S., Rao G. Glioblastoma multiforme: novel therapeutic targets. Expert. Opin. Ther. Targets. 2020;24:605–614. doi: 10.1080/14728222.2020.1762568. [DOI] [PubMed] [Google Scholar]
- 98.Wang L., Jung J., Babikir H., Shamardani K., Jain S., Feng X., Gupta N., Rosi S., Chang S., Raleigh D., et al. A single-cell atlas of glioblastoma evolution under therapy reveals cell-intrinsic and cell-extrinsic therapeutic targets. Nat. Cancer. 2022;3:1534–1552. doi: 10.1038/s43018-022-00475-x. 2022 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Addeo R., Zappavigna S., Parlato C., Caraglia M. Erlotinib: early clinical development in brain cancer. Expert. Opin. Investig. Drugs. 2014;23:1027–1037. doi: 10.1517/13543784.2014.918950. [DOI] [PubMed] [Google Scholar]
- 100.Yu A., Faiq N., Green S., Lai A., Green R., Hu J., Cloughesy T.F., Mellinghoff I., Nghiemphu P.L. Report of safety of pulse dosing of lapatinib with TMZe and radiation therapy for newly-diagnosed glioblastoma in a pilot phase II study. J. Neurooncol. 2017;134:357–362. doi: 10.1007/S11060-017-2533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dumbrava E.E.I., Balaji K., Raghav K., Hess K., Javle M., Blum-Murphy M., Ajani J., Kopetz S., Broaddus R., Routbort M., et al. Targeting ERBB2 (HER2) amplification identified by next-generation sequencing in patients with advanced or metastatic solid tumors beyond conventional indications. JCo Precis. Oncol. 2019:1–12. doi: 10.1200/PO.18.00345/ASSET/IMAGES/LARGE/PO.18.00345TA1.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Szylberg M., Sokal P., Śledzińska P., Bebyn M., Krajewski S., Szylberg Ł., Szylberg A., Szylberg T., Krystkiewicz K., Birski M., et al. MGMT Promoter methylation as a prognostic factor in primary glioblastoma: a single-institution observational study. Biomedicines. 2022:10. doi: 10.3390/BIOMEDICINES10082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rivera A.L., Pelloski C.E., Gilbert M.R., Colman H., De La Cruz C., Sulman E.P., Bekele B.N., Aldape K.D.MGMT. Promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12:116–121. doi: 10.1093/NEUONC/NOP020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan I., Waqas M., Shamim M.S. Prognostic significance of IDH 1 mutation in patients with glioblastoma multiforme. J. Pak. Med. Assoc. 2017;67:816–817. [PubMed] [Google Scholar]
- 105.Songtao Q., Lei Y., Si G., Yanqing D., Huixia H., Xuelin Z., Lanxiao W., Fei Y. IDH mutations predict longer survival and response to TMZe in secondary glioblastoma. Cancer Sci. 2012;103:269–273. doi: 10.1111/J.1349-7006.2011.02134.X. [DOI] [PubMed] [Google Scholar]
- 106.Tang J., He D., Yang P., He J., Zhang Y. Genome-wide expression profiling of glioblastoma using a large combined cohort. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-33323-z. 2018 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang Y., Diehn M., Watson N., Bollen A.W., Aldape K.D., Nicholas M.K., Lamborn K.R., Berger M.S., Botstein D., Brown P.O., et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc. Natl. Acad. Sci. U S. A. 2005;102:5814–5819. doi: 10.1073/PNAS.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Q., Aishwarya S., Li J.P., Pan D.X., Shi J.P. Gene expression profiling of glioblastoma to recognize potential biomarker candidates. Front. Genet. 2022;13:1. doi: 10.3389/FGENE.2022.832742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khabibov M., Garifullin A., Boumber Y., Khaddour K., Fernandez M., Khamitov F., Khalikova L., Kuznetsova N., Kit O., Kharin L. Signaling pathways and therapeutic approaches in glioblastoma multiforme (Review) Int. J. Oncol. 2022:60. doi: 10.3892/IJO.2022.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oh Y.T., Cho H.J., Kim J., Lee J.H., Rho K., Seo Y.J., Choi Y.S., Jung H.J., Song H.S., Kong D.S., et al. Translational validation of personalized treatment strategy based on genetic characteristics of glioblastoma. PLoS. One. 2014:9. doi: 10.1371/JOURNAL.PONE.0103327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh N., Miner A., Hennis L., Mittal S. Mechanisms of TMZe resistance in glioblastoma - a comprehensive review. Cancer Drug Resistance. 2021;4:17. doi: 10.20517/CDR.2020.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pearson J.R.D., Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal. Transduct. Target. Ther. 2017;2:1–11. doi: 10.1038/sigtrans.2017.40. 2017 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jackson C.M., Choi J., Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat. Immunol. 2019;20:1100–1109. doi: 10.1038/S41590-019-0433-Y. [DOI] [PubMed] [Google Scholar]
- 114.Dymova M.A., Kuligina E.V., Richter V.A. Molecular Mechanisms of Drug Resistance in glioblastoma. Int. J. Mol. Sci. 2021;22 doi: 10.3390/IJMS22126385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trinh V.A., Patel S.P., Hwu W.J. The safety of temozolomide in the treatment of malignancies. Expert. Opin. Drug Saf. 2009;8(4):493–499. doi: 10.1517/14740330902918281. PMID: 19435405. [DOI] [PubMed] [Google Scholar]
- 116.Lomustine [Internet]. Cancer information | Cancer Research UK. Available from: https://www.cancerresearchuk.org/about-cancer/treatment/drugs/lomustine-ccnu.
- 117.Procarbazine Monograph for Professionals [Internet]. Drugs.com. Available from: https://www.drugs.com/monograph/procarbazine.html.
- 118.Carmustine medac (previously Carmustine Obvius) | European Medicines Agency [Internet]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/carmustine-medac-previously-carmustine-obvius.
- 119.PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 5978, Vincristine; [cited 2024 May 7]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Vincristine.
- 120.Petrenko D., Chubarev V., Syzrantsev N., Ismail N., Merkulov V., Sologova S., Grigorevskikh E., Smolyarchuk E., Alyautdin R. Temozolomide efficacy and metabolism: the implicit relevance of nanoscale delivery systems. Molecules. 2022;27(11):3507. doi: 10.3390/molecules27113507. PMID: 35684445; PMCID: PMC9181940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Y.S., Wang W., Chen N., Wang L.C., Huang J.B. Research progress of anti-glioma chemotherapeutic drugs (Review) Oncol. Rep. 2022;47(5):101. doi: 10.3892/or.2022.8312. Epub 2022 Apr 1. PMID: 35362540; PMCID: PMC8990335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim Y.J., Choe J.H., Park J.H., Hong Y.K. Efficacy of procarbazine, lomustine, and vincristine chemotherapy for recurrent primary central nervous system lymphomas. Brain Tumor. Res. Treat. 2015 Oct;3(2):75–80. doi: 10.14791/btrt.2015.3.2.75. Epub 2015 Oct 30. PMID: 26605261; PMCID: PMC4656899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.PubChem [Internet] Lomustine; 2004. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information.https://pubchem.ncbi.nlm.nih.gov/compound/Lomustine PubChem Compound Summary for CID 3950[cited 2024 May 7]. Available from: [Google Scholar]
- 124.Alcaniz J., Winkler L., Dahlmann M., Becker M., Orthmann A., Haybaeck J., Krassnig S., Skofler C., Kratzsch T., Kuhn S.A., Jödicke A., Linnebacher M., Fichtner I., Walther W., Hoffmann J. Clinically relevant glioblastoma patient-derived xenograft models to guide drug development and identify molecular signatures. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1129627. PMID: 37114125; PMCID: PMC10126369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rani V., Venkatesan J., Prabhu A. Carmustine-loaded liposomal delivery effectively targets malignant glioma cells and seizes endothelial sprouting in vitro [internet] J. Clust. Sci. 2024 https://link.springer.com/article/10.1007/s10876-023-02511-x Available from: [Google Scholar]
- 126.Warren K.E. Beyond the blood:brain barrier: the importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors, including diffuse intrinsic pontine glioma. Front. Oncol. 2018;8:239. doi: 10.3389/fonc.2018.00239. PMID: 30018882; PMCID: PMC6037693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.PubChem [Internet] Temozolomide; 2004. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information.https://pubchem.ncbi.nlm.nih.gov/compound/Temozolomide PubChem Compound Summary for CID 5394[cited 2024 May 7]. Available from: [Google Scholar]
- 128.PubChem [Internet] Procarbazine; 2004. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information.https://pubchem.ncbi.nlm.nih.gov/compound/Procarbazine PubChem Compound Summary for CID 4915[cited 2024 May 7]. Available from: [Google Scholar]
- 129.PubChem [Internet] Carmustine; 2004. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information.https://pubchem.ncbi.nlm.nih.gov/compound/Carmustine PubChem Compound Summary for CID 2578[cited 2024 May 7]. Available from: [Google Scholar]
- 130.Ariano A., Attinà G., Triarico S., Coccia P., Capozza M.A., Mastrangelo S., Maurizi P., Ruggiero A. Efficacy of Temozolomide in Children with Solid Tumors. Biomed Pharmacol J. 2020;13(1) [Google Scholar]
- 131.Dobson Jane M, Hohenhaus Ann E, Peaston Anne E. W.B. Saunders; 2008. Chapter 15 - Cancer chemotherapy, Editor(s): JILL E Maddison, Stephen W Page, David B Church, Small Animal Clinical Pharmacology (Second Edition) pp. 330–366.https://www.sciencedirect.com/science/article/pii/B9780702028588500178 ISBN 9780702028588, [DOI] [Google Scholar]
- 132.Singh Ranjit, Malhotra Anjleena, Bansal Ranju. Medicinal Chemistry of Chemotherapeutic Agents. Academic Press; 2023. Chapter 15 - Synthetic cytotoxic drugs as cancer chemotherapeutic agents; pp. 499–537.https://www.sciencedirect.com/science/article/pii/B9780323905756000107 Editor(s): Pratap Chandra Acharya, Michio Kurosu. ISBN 9780323905756, [DOI] [Google Scholar]
- 133.Gerson Stanton L., Caimi Paolo F., William Basem M., Creger Richard J. 7th Edition. 9780323357623. Elsevier; 2018. Chapter 57 - Pharmacology and Molecular Mechanisms of Antineoplastic Agents for Hematologic Malignancies; pp. 849–912.https://www.sciencedirect.com/science/article/pii/B9780323357623000573 (Hematology). Editor(s): Ronald Hoffman, Edward J. Benz, Leslie E. Silberstein, Helen E. Heslop, Jeffrey I. Weitz, John Anastasi, Mohamed E. Salama, Syed Ali Abutalib. ISBN. [DOI] [Google Scholar]
- 134.BEN SPRANGERS, LAURA COSMAI, CAMILLO PORTA, 16 - Conventional chemotherapy, Editor(s): Kevin W. Finkel, Mark A. Perazella, Eric P. Cohen, Onco-Nephrology, Elsevier, 2020, 127-153.e11, ISBN 9780323549455, 10.1016/B978-0-323-54945-5.00025-4. (https://www.sciencedirect.com/science/article/pii/B9780323549455000254). [DOI]
- 135.Dennison J.B., Jones D.R., Renbarger J.L., Hall S.D. Effect of CYP3A5 expression on vincristine metabolism with human liver microsomes. J. Pharmacol. Exp. Ther. 2007;321(2):553–563. doi: 10.1124/jpet.106.118471. Epub 2007 Feb 1. PMID: 17272675. [DOI] [PubMed] [Google Scholar]
- 136.Marin J.J.G., Cives-Losada C., Asensio M., Lozano E., Briz O., Macias R.I.R. Mechanisms of anticancer drug resistance in hepatoblastoma. cancers (basel). 2019 ;11(3):407. doi: 10.3390/cancers11030407. PMID: 30909445; PMCID: PMC6468761. [DOI] [PMC free article] [PubMed]
- 137.Munari E., Mariotti F.R., Quatrini L., Bertoglio P., Tumino N., Vacca P., Eccher A., Ciompi F., Brunelli M., Martignoni G., et al. PD-1/PD-L1 in cancer: pathophysiological, diagnostic and therapeutic aspects. Int. J. Mol. Sci. 2021;22:5123. doi: 10.3390/ijms22105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghiringhelli F., Bibeau F., Greillier L., Fumet J.-D., Ilie A., Monville F., Laugé C., Catteau A., Boquet I., Majdi A., et al. Immunoscore immune checkpoint using spatial quantitative analysis of CD8 and PD-L1 markers is predictive of the efficacy of anti- PD1/PD-L1 immunotherapy in non-small cell lung cancer. EBioMedicine. 2023:92. doi: 10.1016/j.ebiom.2023.104633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reardon D.A., Desjardins A., Vredenburgh J.J., O'Rourke D.M., Tran D.D., Fink K.L., Nabors L.B., Li G., Bota D.A., Lukas R.V., Ashby L.S., Paul Duic J., Mrugala M.M., Cruickshank S., Vitale L., He Y., Green J.A., Yellin M.J., Turner C.D.…Sampson J.H. Rindopepimut with bevacizumab for patients with relapsed EGFRvIII-expressing glioblastoma (REACT): results of a double-blind randomized phase II trial. Clin. Cancer Res. 2020;26(7):1586–1594. doi: 10.1158/1078-0432.CCR-18-1140. [DOI] [PubMed] [Google Scholar]
- 140.Xiong Z., Raphael I., Olin M., Okada H., Li X., Kohanbash G. Review glioblastoma vaccines: past, present and opportunities. EBioMedicine. 2024;100 doi: 10.1016/j.ebiom.2023.104963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Warrier N.M., Agarwal P., Kumar P. Emerging importance of survivin in stem cells and cancer: the development of new cancer therapeutics. Stem Cell Rev. Rep. 2020;16(5):828–852. doi: 10.1007/s12015-020-09995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ahluwalia M.S., Reardon D.A., Abad A.P., Curry W.T., Wong E.T., Figel S.A., Mechtler L.L., Peereboom D.M., Hutson A.D., Withers H.G., Liu S., Belal A.N., Qiu J., Mogensen K.M., Dharma S.S., Dhawan A., Birkemeier M.T., Casucci D.M., Ciesielski M.J., Fenstermaker R.A. Phase IIa study of SurVaxM Plus adjuvant TMZe for newly diagnosed glioblastoma. J. Clin. Oncol. 2023;41(7):1453–1465. doi: 10.1200/JCO.22.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zheng X., Tang Q., Ren L., Liu J., Li W., Fu W., Wang J., Du G. A narrative review of research progress on drug therapies for glioblastoma multiforme. Ann. Transl. Med. 2021;9(11):943. doi: 10.21037/atm-20-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chocarro L., Blanco E., Arasanz H., Fernández-Rubio L., Bocanegra A., Echaide M., Garnica M., Ramos P., Fernández-Hinojal G., Vera R., Kochan G., Escors D. Clinical landscape of LAG-3-targeted therapy. Immuno-Oncol. Technol. 2022;14(C) doi: 10.1016/j.iotech.2022.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harris-Bookman S., Mathios D., Martin A.M., Xia Y., Kim E., Xu H., Belcaid Z., Polanczyk M., Barberi T., Theodros D., Kim J., Taube J.M., Burger P.C., Selby M., Taitt C., Korman A., Ye X., Drake C.G., Brem H.…Lim M. Expression of LAG-3 and efficacy of combination treatment with anti-LAG-3 and anti-PD-1 monoclonal antibodies in glioblastoma. Int. J. Cancer. 2018;143(12):3201–3208. doi: 10.1002/ijc.31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lim M., Ye X., Piotrowski A.F., Desai A.S., Ahluwalia M.S., Walbert T., Fisher J.D., Desideri S., Nabors L.B., Wen P.Y., Grossman S.A. Updated safety phase I trial of anti-LAG-3 alone and in combination with anti-PD-1 in patients with recurrent GBM. J. Clin. Oncol. 2020;38(15_suppl):2512. doi: 10.1200/JCO.2020.38.15_suppl.2512. [DOI] [Google Scholar]
- 147.Yip A., Webster R.M. The market for chimeric antigen receptor T cell therapies. Nature Rev. Drug Discovery. 2018;17(3):161–162. doi: 10.1038/nrd.2017.266. [DOI] [PubMed] [Google Scholar]
- 148.Land C.A., Musich P.R., Haydar D., Krenciute G., Xie Q. Chimeric antigen receptor T-cell therapy in glioblastoma: charging the T cells to fight. J. Transl. Med. 2020;18(1):1–13. doi: 10.1186/s12967-020-02598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ahmed N., Brawley V., Hegde M., Bielamowicz K., Kalra M., Landi D., Robertson C., Gray T.L., Diouf O., Wakefield A., Ghazi A., Gerken C., Yi Z., Ashoori A., Wu M.-F., Liu H., Rooney C., Dotti G., Gee A.…Gottschalk S. HER2-Specific chimeric antigen receptor–modified virus-specific t cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMa Oncol. 2017;3(8):1094–1101. doi: 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Goff S.L., Morgan R.A., Yang J.C., Sherry R.M., Robbins P.F., Restifo N.P., Feldman S.A., Lu Y.-C., Lu L., Zheng Z., Xi L., Epstein M., McIntyre L.S., Malekzadeh P., Raffeld M., Fine H.A., Rosenberg S.A. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced t cells targeting EGFRvIII in patients with glioblastoma. J. Immunotherapy (Hagerstown, Md. : 1997) 2019;42(4):126–135. doi: 10.1097/CJI.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brown C.E., Wagner J.R., Naranjo A., Ostberg J.R., Ph D., Blanchard M.S., Ph D., Wang X., Ph D., Harshbarger T.L., Apuzzo M.D. Regression of glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2017;375(26):2561–2569. doi: 10.1056/NEJMoa1610497.Regression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bielamowicz K., Fousek K., Byrd T.T., Samaha H., Mukherjee M., Aware N., Wu M.F., Orange J.S., Sumazin P., Man T.K., Joseph S.K., Hegde M., Ahmed N. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20(4):506–518. doi: 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Getu A.A., Tigabu A., Zhou M., Lu J., Fodstad Ø., Tan M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol. Cancer. 2023;22(1):1–15. doi: 10.1186/s12943-023-01751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Digregorio M., Coppieters N., Lombard A., Lumapat P.N., Scholtes F., Rogister B. The expression of B7-H3 isoforms in newly diagnosed glioblastoma and recurrence and their functional role. Acta Neuropathol. Commun. 2021;9(1):1–13. doi: 10.1186/s40478-021-01167-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bodduluru L.N., Kasala E.R., Madhana R.M.R., Sriram C.S. Natural killer cells: the journey from puzzles in biology to treatment of cancer. Cancer Lett. 2015;357(2):454–467. doi: 10.1016/j.canlet.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 156.Morimoto T., Nakazawa T., Maeoka R., Nakagawa I., Tsujimura T., Matsuda R. Natural killer Cell-based immunotherapy against glioblastoma. Int. J. Mol. Sci. 2023;24(3):1–23. doi: 10.3390/ijms24032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morvan M.G., Lanier L.L. NK cells and cancer: you can teach innate cells new tricks. Nature Rev. Cancer. 2016;16(1):7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 158.Böttcher J.P., Bonavita E., Chakravarty P., Blees H., Cabeza-Cabrerizo M., Sammicheli S., Rogers N.C., Sahai E., Zelenay S., Reis e Sousa C. NK Cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5) doi: 10.1016/j.cell.2018.01.004. 1022-1037.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kotzur R., Duev-Cohen A., Kol I., Reches A., Mandelboim O., Stein N. NK-92 cells retain vitality and functionality when grown in standard cell culture conditions. PLoS. One. 2022;17(3 March):1–13. doi: 10.1371/journal.pone.0264897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X., Yang X., Yuan X., Wang W., Wang Y. Chimeric antigen receptor-engineered NK cells: new weapons of cancer immunotherapy with great potential. Exper. Hematol. Oncol. 2022;11(1):1–19. doi: 10.1186/s40164-022-00341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burger M.C., Forster M.T., Romanski A., Straßheimer F., Macas J., Zeiner P.S., Steidl E., Herkt S., Weber K.J., Schupp J., Lun J.H., Strecker M.I., Wlotzka K., Cakmak P., Opitz C., George R., Mildenberger I.C., Nowakowska P., Zhang C.…Wels W.S. Intracranial injection of natural killer cells engineered with a HER2-targeted chimeric antigen receptor in patients with recurrent glioblastoma. Neuro Oncol. 2023;25(11):2058–2071. doi: 10.1093/neuonc/noad087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Agrawal M., Ajazuddin, Tripathi D.K., Saraf S., Saraf S., Antimisiaris S.G., Mourtas S., Hammarlund-Udenaes M., Alexander A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer's disease. J. Controlled Release. 2017;260:61–77. doi: 10.1016/J.JCONREL.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 163.Xie J., Shen Z., Anraku Y., Kataoka K., Chen X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224(June) doi: 10.1016/j.biomaterials.2019.119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kawak P., Sawaftah N.M.A., Pitt W.G., Husseini G.A. Transferrin-targeted liposomes in glioblastoma therapy: a review. Int. J. Mol. Sci. 2023;24(17) doi: 10.3390/ijms241713262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pandey N., Anastasiadis P., Carney C.P., Kanvinde P.P., Woodworth G.F., Winkles J.A., Kim A.J. Nanotherapeutic treatment of the invasive glioblastoma tumor microenvironment. Adv. Drug Deliv. Rev. 2022;188 doi: 10.1016/j.addr.2022.114415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tomeh M.A., Hadianamrei R., Zhao X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019;20(5) doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Read, R.D. (2022). Repurposing the drug verteporfin as anti-neoplastic therapy for glioblastoma. 24(January), 708–710. [DOI] [PMC free article] [PubMed]
- 168.Jeising S., Geerling G., Guthoff R., Hänggi D., Sabel M., Rapp M., Nickel A.C. In-vitro use of verteporfin for photodynamic therapy in glioblastoma. Photodiagnosis. Photodyn. Ther. 2022;40(July) doi: 10.1016/j.pdpdt.2022.103049. [DOI] [PubMed] [Google Scholar]
- 169.Ozdemir-kaynak, E., Qutub, A.A. and Yesil-celiktas, O. (2018) ‘Advances in glioblastoma multiforme treatment: new models for nanoparticle therapy’, 9(March), pp. 1–14. doi: 10.3389/fphys.2018.00170. [DOI] [PMC free article] [PubMed]
- 170.Ashby Lynn S., Smith Kris A., Stea Baldassarre. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J. Surg. Oncol. 2016 doi: 10.1186/s12957-016-0975-5. BioMed Central Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Belousov, A. et al. (2019) ‘The extracellular matrix and biocompatible materials in glioblastoma treatment’, 7(November). doi: 10.3389/fbioe.2019.00341. [DOI] [PMC free article] [PubMed]
- 172.Li J., Feng L., Lu Y. Glioblastoma multiforme: diagnosis, treatment, and invasion. J. Biomed. Res. 2023;37(1):47–58. doi: 10.7555/JBR.36.20220156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alphandéry E. Nano-therapies for glioblastoma treatment. Cancers. MDPI AG. 2020 doi: 10.3390/cancers12010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lakkadwala S., Singh J. Vol. 173. Elsevier; 2019. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model; pp. 27–35. (Colloids and Surfaces B: Biointerfaces). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Iturrioz-Rodríguez Nerea, Sampron Nicolas, Matheu Ander. Current advances in TMZe encapsulation for the enhancement of glioblastoma treatment’. Theranostics. 2023;13(9):2734–2756. doi: 10.7150/thno.82005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stupp R., Taillibert S., Kanner A.A., et al. Maintenance therapy with tumor-treating fields plus TMZe vs TMZe alone for glioblastoma: a randomized clinical trial. JAMa. 2015;314(23):2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 177.Fisher Jacob P., Adamson David C. Current FDA-approved therapies for high-grade malignant gliomas. Biomedicines. MDPI AG. 2021 doi: 10.3390/biomedicines9030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ciechomska I.A., et al. Exploring novel therapeutic opportunities for glioblastoma using patient-derived cell cultures. Cancers. (Basel) 2023;15(5):1–25. doi: 10.3390/cancers15051562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Niyazi M., et al. ESTRO-EANO guideline on target delineation and radiotherapy details for glioblastoma. Radiotherapy Oncol.. Authors. 2023;184 doi: 10.1016/j.radonc.2023.109663. [DOI] [PubMed] [Google Scholar]
- 180.Cabrera A.R., et al. Radiation therapy for glioblastoma: executive summary of an american society for radiation oncology evidence-based clinical practice guideline. Pract. Radiation Oncol.. Am. Soc. Radiation Oncol. 2016;6(4):217–225. doi: 10.1016/j.prro.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 181.Cheng V.W.T., et al. VCAM-1-targeted MRI improves detection of the tumor-brain interface. Clinical Cancer Research. 2022;28(11):2385–2396. doi: 10.1158/1078-0432.CCR-21-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gomez-Roman, Natividad, Ming Y Chong, Sandeep K Chahal, Seamus P Caragher, Mark R Jackson, Katrina H Stevenson, Sidhartha A Dongre, and Anthony J Chalmers. 2020. “Radiation responses of 2D and 3D glioblastoma cells : a novel, 3D-speci fi c radioprotective role of VEGF /Akt signaling through functional activation of NHEJ,” 575–89. 10.1158/1535-7163.MCT-18-1320. [DOI] [PubMed]
- 183.Osuka S., et al. N-cadherin upregulation mediates adaptive radioresistance in glioblastoma. J. Clin. Invest. 2021;131(6):1–15. doi: 10.1172/JCI136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Podbielska Maria, Szulc Zdzisław M., Kurowska Ewa, Hogan Edward L., Bielawski Jacek, Bielawska Alicja, Bhat Narayan R. Cytokine-induced release of ceramide-enriched exosomes as a mediator of cell death signaling in an oligodendroglioma cell line. J. Lipid Res. 2016;57(11):2028–2039. doi: 10.1194/jlr.M070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Podbielska Maria, Szulc Zdzislaw M., Ariga Toshio, Pokryszko-Dragan Anna, Fortuna Wojciech, Bilinska Małgorzata, Podemski Ryszard, et al. Distinctive Sphingolipid patterns in chronic multiple sclerosis lesions. J. Lipid Res. 2020;61(11):1464–1479. doi: 10.1194/jlr.RA120001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Podbielska Maria, Macala Jozefa, Jakubiak-Augustyn Anna, Szulc Zdzislaw M., Fortuna Wojciech, Budrewicz Slawomir, Jaskiewicz Ewa, Bilinska Malgorzata, Hogan Edward L., Pokryszko-Dragan Anna. Ceramide is implicated in humoral peripheral and intrathecal autoimmune response in MS patients. Mult. Scler. Relat. Disord. 2023;71(November 2022) doi: 10.1016/j.msard.2023.104565. [DOI] [PubMed] [Google Scholar]
- 187.Kou Yongjun, Geng Feng, Guo Deliang. Lipid metabolism in glioblastoma: from de novo synthesis to storage. Biomedicines. 2022;10(8):1–25. doi: 10.3390/biomedicines10081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.He Chuan, Hua Chun Geun, Lee Charles S., DelaCruz Chang, Lee Min, Zhou Yang, Ahangari Farida, Ma Bing, et al. Chitinase 3-like 1 regulates cellular and tissue responses via il-13 receptor ??2’. Cell Rep. 2013;4(4):830–841. doi: 10.1016/j.celrep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Miska Jason, Chandel Navdeep S. Targeting fatty acid metabolism in glioblastoma. J. Clin. Invest. 2023;133(1):1–10. doi: 10.1172/JCI163448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Minami Jenna K., Morrow Danielle, Bayley Nicholas A., Fernandez Elizabeth G., Salinas Jennifer J., Tse Christopher, Zhu Henan, et al. CDKN2A deletion remodels lipid metabolism to prime glioblastoma for ferroptosis. Cancer Cell. 2023;41(6) doi: 10.1016/j.ccell.2023.05.001. 1048-1060.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dixon Scott J., Lemberg Kathryn M., Lamprecht Michael R., Skouta Rachid, Zaitsev Eleina M., Gleason Caroline E., Patel Darpan N., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang He, Liu Yingfeng, Che Shusheng, Li Xiangjun, Tang Dongxue, Lv Shaojing, Zhao Hai. Deciphering the link: ferroptosis and its role in glioma. Front. Immunol. 2024;15(January):1–14. doi: 10.3389/fimmu.2024.1346585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Qiu Chen, Zhang Xin, Huang Bin, Wang Shuai, Zhou Wenjing, Li Chao, Li Xingang, Wang Jian, Yang Ning. Disulfiram, a ferroptosis inducer, triggers lysosomal membrane permeabilization by up-regulating ros in glioblastoma. Onco Targets. Ther. 2020;13:10631–10640. doi: 10.2147/OTT.S272312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yuan Fanen, Sun Qian, Zhang Si, Ye Liguo, Xu Yang, Deng Gang, Xu Zhou, Zhang Shenqi, Liu Baohui, Chen Qianxue. The dual role of P62 in ferroptosis of glioblastoma according to P53 status. Cell Biosci. 2022;12(1):1–19. doi: 10.1186/s13578-022-00764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cao Zhengcong, Liu Xiao, Zhang Wangqian, Zhang Keying, Pan Luxiang, Zhu Maorong, Qin Haozhe, et al. Biomimetic macrophage membrane-camouflaged nanoparticles induce ferroptosis by promoting mitochondrial damage in glioblastoma. ACS. Nano. 2023;17(23):23746–23760. doi: 10.1021/acsnano.3c07555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cai Jiayang, Ye Zhang, Hu Yuanyuan, Ye Liguo, Gao Lun, Wang Yixuan, sun Qian, et al. Fatostatin induces ferroptosis through inhibition of the AKT/MTORC1/GPX4 signaling pathway in glioblastoma. Cell Death Disease. 2023;14(3) doi: 10.1038/s41419-023-05738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Song Liying, Wu Jiali, Fu Hua, Wu Cuifang, Tong Xiaopei, Zhang Mingyu. Abnormally expressed ferroptosis-associated FANCD2 in mediating the TMZe resistance and immune response in glioblastoma. Front. Pharmacol. 2022;13(June):1–12. doi: 10.3389/fphar.2022.921963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wan Sicheng, Zhang Guanghui, Liu Ruochen, Nadeem Abbas Muhammad, Cui Hongjuan. Pyroptosis, ferroptosis, and autophagy cross-talk in glioblastoma opens up new avenues for glioblastoma treatment. Cell Commun. Signaling. 2023;21(1):1–19. doi: 10.1186/s12964-023-01108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jawhari Soha, Hélène Ratinaud Marie, Verdier Mireille. Cell Death and Disease. Nature Publishing Group; 2016. Glioblastoma, hypoxia and autophagy: a survival-prone “ménage-à-trois. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li Zhuang, Fu Wen Juan, Qing Chen Xiao, Wang Shuai, Deng Ru Song, Tang Xiao Peng, Di Yang Kai, et al. Autophagy-based unconventional secretion of HMGB1 in glioblastoma promotes chemosensitivity to TMZe through macrophage M1-like polarization. J. Exper. Clin. Cancer Res. 2022;41(1) doi: 10.1186/s13046-022-02291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chryplewicz Agnieszka, Scotton Julie, Tichet Mélanie, Zomer Anoek, Shchors Ksenya, Joyce Johanna A., Homicsko Krisztian, Hanahan Douglas. Cancer cell autophagy, reprogrammed macrophages, and remodeled vasculature in glioblastoma triggers tumor immunity. Cancer Cell. 2022;40(10) doi: 10.1016/j.ccell.2022.08.014. 1111-1127.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zielke Svenja, Meyer Nina, Mari Muriel, Abou-El-Ardat Khalil, Reggiori Fulvio, Wijk Sjoerd J.L.van, Kögel Donat, Fulda Simone. Loperamide, pimozide, and STF-62247 trigger autophagy-dependent cell death in glioblastoma cells. Cell Death Disease. 2018;9(10):1–16. doi: 10.1038/s41419-018-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]