Highlights
-
•
Chronic infections represent a high burden in the migrant population.
-
•
Regional and country-specific prevalence estimations enable a more accurate risk identification.
-
•
Parasitic infections affect migrants from most tropical and sub-tropical areas.
Keywords: Migrants, Prevalence, Screening, Infectious diseases, Imported diseases, Travel-related illnesses
Abstract
Background
Migrants are disproportionately affected by several infectious diseases differing the risk within migrant groups. This study aimed to estimate the prevalence of six chronic infections in asymptomatic migrants attended at primary care or specialized units where health assessments are offered.
Methods
A multicentric cross-sectional study was conducted to estimate the prevalence of human immunodeficiency virus (HIV), hepatitis B and C virus, Strongyloides stercoralis, Schistosoma spp., and Trypanosoma cruzi infections in the migrant population who participated in a screening programme implemented at six primary health care centres, and two infectious diseases outpatient clinics in Catalonia, Spain.
Results
Of the 314 recruited migrants, 284 (90.4 %) were tested for at least one infection. The prevalence of the infections was as follows: 1.8 % for HIV, 1.8 % for chronic hepatitis B virus, 14.1 % for previous exposure to a hepatitis B virus infection, 0.4 % for hepatitis C virus infection, 2.6 % for S.stercoralis infection, and 7.0 % for T.cruzi infection. No cases infected with Schistosoma spp. were reported. A higher prevalence of HIV, hepatitis B and C virus was observed in Sub-Saharan Africans, whereas a higher prevalence of S.stercoralis and T.cruzi infections was reported in migrants from Latin-America and the Caribbean.
Conclusion
Findings suggest a high burden of the studied infections, including parasitic infections, in the migrant population with variations within migrant groups based on areas of birth.
1. Introduction
International migration is an increasing and challenging phenomenon. The latest estimates from the International Organization for Migration indicate that in 2020, 3.6 % (281 million) of the global population were migrants (International Organization for Migration 2021). Europe was the largest migrant destination, hosting 87 million international migrants (International Organization for Migration 2021), and Spain is among the top tenth receiving countries with 11.3 % of foreign-born population (International Organization for Migration 2021).
Migrants are disproportionately affected by infectious diseases, differing the risk among migrant groups (Sequeira-Aymar et al., 2021). They present a high burden of undiagnosed human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) infections and tend to have a higher mortality rate when compared to the host population (Schousboe and Wejse, 2021; Lazarus et al., 2019). Some migrant groups present a high prevalence of non-endemic chronic infections such as Chagas disease, strongyloidiasis, and schistosomiasis (Navarro et al., 2022; Asundi et al., 2019). Migrants' vulnerability to infections might be explained by the different levels of exposure and the social determinants of health (SDH) that migrants face in their country of birth, during the migration route, and in the host country (European Centre for Disease Prevention and Control (ECDC) 2018).
Early detection and treatment of these infections can reduce the related morbidity and mortality and control the transmission in the host country (Sequeira-Aymar et al., 2021). In this regard, the feasibility (Gonçalves et al., 2022) and health impact (Sequeira-Aymar et al., 2021) of a multi-infection screening programme targeting migrant populations have been demonstrated in a primary care-based study (Gonçalves et al., 2022).
Nonetheless, most prevalence studies for infectious diseases in migrants are hospital-based studies, which may include symptomatic individuals, and target some vulnerable groups within the migrant population, such as undocumented migrants, asylum seekers, and refugees (Norman et al., 2021; Bocanegra et al., 2014; Vignier et al., 2022; Klok et al., 2021). These approaches hold possible selection bias in the results. Furthermore, systematic reviews and meta-analyses may include studies with varying methodologies, which can introduce other types of biases (Schousboe and Wejse, 2021; Lazarus et al., 2019; Navarro et al., 2022; Asundi et al., 2019; Salvador et al., 2020; Requena-Méndez et al., 2015). Therefore, prevalence studies targeting asymptomatic migrants are fundamental to implement effective screening strategies and immunization programmes by accurately classifying their risk.
This study aimed to estimate the prevalence of six chronic infectious diseases in asymptomatic migrants attended at six primary care centres (PCCs) and two hospitals in Catalonia, Spain and to identify potential associations.
2. Material and methods
2.1. Study design and participants
This multicentric cross-sectional study was part of the INPREMI project, which implemented a systematic screening programme for multiple infections in migrant populations (Aguilar et al., 2024). The screening programme was carried out at six PCCs and two hospitals in Catalonia, Spain, from January 2018 to July 2020 and included six infections (HIV, HBV, HCV, Strongyloides stercoralis, Schistosoma spp., and Trypanosoma cruzi infections). These diseases were included because they fulfil the Wilson and Jungner criteria for screening (Wilson and Jungner, 1968) and because all of them had a validated serological test to diagnose the condition. Migrants ≥18 years old who attended the participating centres for any reason were invited to participate. Migrants attended at the hospitals were only recruited if they were referred from non-profit organizations or coming from shelters to perform a health assessment, being therefore asymptomatic individuals or individuals with undifferentiated symptoms. Migrants born in Western Europe, North America, Australia, and New Zealand were excluded from the study (Annex 1) since, for the studied infections, their countries of birth either had a very low prevalence (HIV and viral hepatitis) or were not endemic (T.cruzi, S.stercoralis and Schistosoma spp. infections).
2.2. Study procedures
After signing the corresponding informed consent, a case report form (CRF) with socio-demographic and epidemiological data and blood samples were collected from each participant. HIV, HBV, and HCV serological tests were performed on all recruited individuals, whereas parasitic serological tests were requested according to the individual epidemiological risk of acquisition, determined by the endemicity of the infection at the country of birth, following a systematic review and meta-analysis (Requena-Méndez et al., 2015) and the recommendations from the European Centre for Disease Prevention and Control (ECDC) (European Centre for Disease Prevention and Control (ECDC) 2018). An extra blood sample was collected from each participant for the development of a multiple serological test. These findings have been published elsewhere (Aguilar et al., 2024).
HIV antibodies, the Australian antigen (HBsAg) to identify chronic HBV, the antibody to hepatitis B core antigen (anti-HBc) for exposure to an HBV infection, and HCV total antibodies (anti-HCV) were processed using the Atellica™ IM 1600 System of the Siemens Healthineers commercial house. S.stercoralis serology was handled with a commercial Enzyme-Linked Immunosorbent Assay (ELISA) test based on the In Vitro Diagnostic (IVD) S.stercoralis crude antigen from SciMedx®. Schistosoma spp. serology was processed with a commercial indirect hemagglutination (IHA) test manufactured by Fumouze®. T.cruzi serology was performed using the Chemiluminescent Microparticle Immunoassay test (CMIA) from Architect Abbot® and it was confirmed with a commercial ELISA with recombinant antigens (BioELISA Chagas, Biokit®, Barcelona, Spain). Results for all serology tests were considered positive according to the reference values of Hospital Clínic of Barcelona (Annex 2). Individuals with positive results in any of the serology tests were referred to the corresponding medical specialist.
2.3. Data collection and variables
The CRF included: socio-demographic characteristics (country of birth, sex, age, educational level, professional category); living conditions in the country of birth (area-urban/rural, contact with fresh water bodies, contact with animals); factors concerning the migration journey (in transit countries, suffered violence); time of residence in Spain, behavioural factors (sexual practices, drug use); and medical history (comorbidities, immunosuppression, symptoms, and medication) (Annex 3).
Countries of birth were aggregated into areas of birth adapting the international classification of Geo-Sentinel (Field et al., 2010), as follows: Latin-America and the Caribbean (LA), Sub-Saharan Africa (SSA), Northern Africa (NA), and other areas of birth (Annex 1). Age was categorized as young adulthood (18–39 years) and middle-old adulthood (≥40 years). Educational level was defined as lower studies for those with primary studies or illiteracy, secondary studies, and higher studies for individuals with a higher education than secondary school. For professional categories, participants were defined as unemployed (students and unemployed individuals), and employed. The time of residence in Spain was categorized into newly arrived (<5 years) and long-term residing migrants (≥5 years), following the ECDC's definitions (European Centre for Disease Prevention and Control (ECDC) 2018). Finally, a distinction between cannabis use and hard drug use (which included cocaine, heroin, and other recreational drugs) was made.
2.4. Data analysis
The primary outcome was the prevalence for each infection defined as the number of diagnoses identified by serological tests among the people who were tested. Data collected on serologies and the results were used to estimate the prevalence for each infection with its respective 95 % confidence interval (95 %CI).
Categorical variables were expressed as relative and absolute frequencies and normally distributed continuous variables were described as means and standard deviations (SD). Pearson's chi-square tests or Fisher's exact tests were performed to evaluate the associations between categorical variables. Associations with the continuous variables were evaluated using the one-way analysis of variance (ANOVA) test. Firth´s regression models were executed to check any possible associations between the outcome variables (e.g., exposure to an HBV infection) and predictor variables (area of birth and other factors). Odds Ratio (OR), adjusted OR (aOR), and 95 %CI were reported. The statistical significance was established at the 5 % level. Stata-IC.16.0 (Stata-16, College-Station, TX, USA) was used as the statistical analysis software for this study.
2.5. Ethical considerations
The study was approved by the Ethics Committee of the Hospital Clinic in Barcelona (HCB/2017/0847) and the respective ethic committees of all participating centres. An informed consent was obtained from participants, and the clinical research followed the ethical standards of the Declaration of Helsinki. The study was reported using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Annex 4).
3. Results
A total of 314 participants were included in the study, yet only 284 (90.4 %) were tested for at least one infection (Fig. 1). The highest proportion of migrants were from LA (227/314, 72.3 %), followed by migrants from SSA (44/314, 14.0 %) (Table 1). There were 196/314 (62.4 %) females and the mean age of the study population was 39.4 (SD 0.7), being 168/314 (53.5 %) young adults. Most of the individuals completed secondary studies (153/307, 49.8 %) and were employed (250/309, 80.9 %). Half of the cohort were newly arrived migrants (156/309, 50.5 %). See Table 1 for details of the characteristics of participants by areas of birth.
Fig. 1.
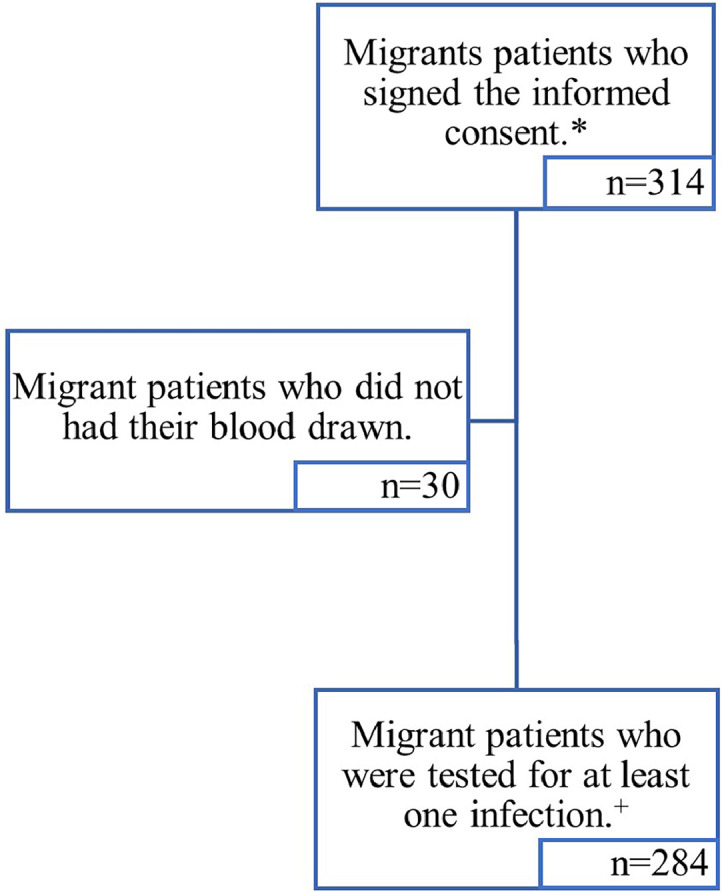
Flow chart of the study population.
*Study population; +Population used for the prevalence data estimations
Table 1.
Characteristics of the sample.
| Total (N = 314) n/N ( %) |
LA (N = 227) n/N ( %) |
SSA (N = 44) n/N ( %) |
NA (N = 11) n/N ( %) |
Other areas of birth (N = 32) n/N ( %) | p-value* | |
|---|---|---|---|---|---|---|
| Total | 314 | 227 (72.3) | 44 (14.0) | 11 (3.5) | 32 (10.2) | |
| Sex (Female) | 196/314 (62.4) | 169/227 (74.5) | 10/44 (22.7) | 3/11 (27.3) | 14/32 (43.8) | <0.001 |
| Age (mean, SD) (n = 314) | 39.4 (0.7) | 39.7 (12.9) | 39.7 (10.3) | 37.0 (9.1) | 38.3 (13.2) | 0.169^ |
| Age categories | ||||||
| 18–39 years | 168/314 (53.5) | 123/227 (54.2) | 20/44 (45.5) | 7/11 (63.6) | 18/32 (56.3) | 0.628 |
| ≥40 years | 146/314 (46.5) | 104/227 (45.8) | 24/44 (54.5) | 4/11 (36.4) | 14/32 (43.8) | |
| Education level | ||||||
| Primary studies or illiteracy | 48/307 (15.6) | 26/225 (11.6) | 18/41 (43.9) | 3/11 (27.3) | 1/30 (3.3) | <0.001 |
| Secondary studies | 153/307 (49.8) | 117/225 (52.0) | 20/41 (48.8) | 3/11 (27.3) | 13/30 (43.3) | |
| Higher studies | 106/307 (34.5) | 82/225 (36.4) | 3/41 (7.3) | 5/11 (45.5) | 16/30 (53.3) | |
| Professional categories | ||||||
| Unemployed | 59/309 (19.1) | 43/226 (19.0) | 6/42 (14.3) | 3/11 (27.3) | 7/30 (23.3) | 0.639 |
| Employed | 250/309 (80.9) | 183/226 (81.0) | 36/42 (85.7) | 8/11 (72.7) | 23/30 (76.7) | |
| Living area in country of birth | ||||||
| Urban | 180/308 (58.4) | 132/226 (58.4) | 21/41 (51.2) | 7/11 (63.6) | 20/30 (66.7) | 0.048 |
| Rural | 57/308 (18.5) | 34/226 (15.0) | 14/41 (34.2) | 2/11 (18.2) | 7/30 (23.3) | |
| Both | 71/ 308 (23.1) | 60/226 (26.6) | 6/41 (14.6) | 2/11 (18.2) | 3/30 (10.0) | |
| Contact with lakes or rivers in country of birth | 178/308 (57.8) | 132/226 (58.4) | 25/41 (61.0) | 5/11 (45.5) | 16/30 (53.3) | 0.769 |
| Contact with animals in country of birth | 208/297 (70.0) | 160/216 (74.1) | 28/41 (68.3) | 5/11 (45.5) | 15/29 (51.7) | 0.023 |
| Transportation | ||||||
| Earth | 6/309 (1.9) | 0/227 (0.0) | 2/41 (4.9) | 2/11 (18.2) | 2/30 (6.7) | <0.001 |
| Air | 275/309 (89.0) | 225/227 (99.1) | 21/41 (51.2) | 3/11 (27.3) | 26/30 (86.7) | |
| Sea | 11/309 (3.6) | 0/227 (0.0) | 7/41 (17.1) | 4/11 (36.4) | 0/30 (0.0) | |
| Earth and Air | 6/309 (1.9) | 1/227 (0.4) | 2/41 (4.9) | 1/11 (9.1) | 2/30 (6.7) | |
| Earth and Sea | 6/309 (1.9) | 0/227 (0.0) | 5/41 (12.2) | 1/11 (9.1) | 0/30 (0.0) | |
| Air and Sea | 2/309 (0.7) | 1/227 (0.4) | 1/41 (2.4) | 0/11 (0.0) | 0/30 (0.0) | |
| Earth, Air and Sea | 3/309 (1.0) | 0/227 (0.0) | 3/41 (7.3) | 0/11 (0.0) | 0/30 (0.0) | |
| In transit countries | ||||||
| None | 148/304 (14.7) | 120/224 (53.6) | 13/39 (33.3) | 6/11 (54.6) | 9/30 (30.0) | 0.004 |
| 1 country | 102/304 (33.5) | 74/224 (33.0) | 11/39 (28.2) | 3/11 (27.3) | 14/30 (46.7) | |
| ≥2 countries | 54/304 (17.8) | 30/224 (13.4) | 15/39 (38.5) | 2/11 (18.2) | 7/30 (23.3) | |
| Suffered violence† | 39/298 (13.1) | 36/218 (16.5) | 2/39 (5.1) | 0/11 (0.0) | 1/30 (3.3) | 0.045 |
| Sexual violence | 5/298 (1.7) | 4/218 (1.8) | 1/39 (1.6) | - | 0/30 (0.0) | |
| Physical violence | 21/298 (7.1) | 18/218 (8.3) | 2/39 (5.1) | - | 1/30 (3.3) | |
| Human trafficking | 1/298 (0.3) | 1/218 (0.5) | 0/39 (0.0) | - | 0/30 (0.0) | |
| Other types of violence | 17/298 (5.7) | 17/218 (7.8) | 0/39 (0.0) | - | 0/30 (0.0) | |
| Unknown | 1/298 (0.3) | 1/218 (0.5) | 0/39 (0.0) | - | 0/30 (0.0) | |
| Time residing in Spain in years | ||||||
| <5 years | 156/309 (50.5) | 130/226 (57.5) | 8/41 (19.5) | 4/11 (36.4) | 14/31 (45.2) | <0.001 |
| ≥5 years | 153/309 (49.5) | 96/226 (42.5) | 33/41 (80.5) | 7/11 (63.6) | 17/31 (54.8) | |
| Unsafe sexual practices | 54/308 (17.5) | 37/226 (16.4) | 11/41 (26.8) | 1/11 (9.1) | 5/30 (16.7) | 0.406 |
| Hard drug users+ | 7/307 (2.3) | 6/224 (2.7) | 1/41 (2.4) | 0/11 (0.0) | 0/31 (0.0) | 1.000 |
| Cannabis users | 15/307 (4.9) | 12/224 (5.4) | 2/41 (4.9) | 1/11 (9.1) | 0/31 (0.0) | 0.441 |
| Alcohol consumers | 117/307 (38.1) | 100/225 (44.4) | 6/40 (15.0) | 1/11 (9.1) | 10/31 (32.3) | <0.001 |
| Tobacco use | ||||||
| Non-smoker | 255/307 (83.1) | 194/224 (86.6) | 26/41 (63.4) | 9/11 (81.8) | 26/31 (83.9) | 0.013 |
| Smoker | 38/307 (12.4) | 21/224 (9.4) | 10/41 (24.4) | 2/11 (18.2) | 5/31 (16.1) | |
| Ex-smoker | 14/307 (4.6) | 9/224 (4.0) | 5/41 (12.2) | 0/11 (0.0) | 0/31 (0.0) | |
| Comorbidities† | ||||||
| Any comorbidity | 84/308 (27.3) | 67/226 (29.7) | 10/41 (24.4) | 1/11 (9.1) | 6/30 (20.0) | 0.381 |
| Diabetes mellitus | 13/308 (4.2) | 10/226 (4.4) | 3/41 (7.3) | 0/11 (0.0) | 0/30 (0.0) | |
| Chronic neoplasm | 2/308 (0.7) | 2/226 (0.9) | 0/41 (0.0) | 0/11 (0.0) | 0/30 (0.0) | |
| HIV | 2/308 (0.7) | 1/226 (0.4) | 1/41 (2.4) | 0/11 (0.0) | 0/30 (0.0) | |
| Chronic lung disease | 2/308 (0.7) | 2/226 (0.9) | 0/41 (0.0) | 0/11 (0.0) | 0/30 (0.0) | |
| Inflammatory bowel disease | 1/308 (0.3) | 1/226 (0.4) | 0/41 (0.0) | 0/11 (0.0) | 0/30 (0.0) | |
| Chronic liver disease | 3/308 (1.0) | 3/226 (1.3) | 0/41 (0.0) | 0/11 (0.0) | 0/30 (0.0) | |
| Chronic nephropathy | 3/308 (1.0) | 2/226 (0.9) | 1/41 (2.4) | 0/11 (0.0) | 0/30 (0.0) | |
| Other chronic diseases | 68/308 (22.1) | 53/226 (23.5) | 8/41 (19.5) | 1/11 (9.1) | 6/30 (20.0) | |
| Unknown | 1/308 (0.3) | 1/226 (0.4) | 0/41 (0.0) | 0/11 (0.0) | 0/30 (0.0) | |
| Previous TB episode | 7/294 (2.4) | 5/218 (2.3) | 1/35 (2.9) | 0/11 (0.0) | 1/30 (3.3) | 0.622 |
| Immunosuppression status | 6/308 (2.0) | 5/226 (2.2) | 1/41 (2.4) | 0/11 (0.0) | 0/30 (0.0) | 1.000 |
| Transplant | 1/308 (0.3) | 1/226 (0.4) | 0/41 (0.0) | - | - | |
| Oncological/hematological neoplasm | 1/308 (0.3) | 1/226 (0.4) | 0/41 (0.0) | - | - | |
| Autoimmune diseases | 2/308 (0.7) | 2/226 (0.9) | 0/41 (0.0) | - | - | |
| HIV Immunodeficiency | 2/308 (0.7) | 1/226 (0.4) | 1/41 (2.4) | - | - | |
| Symptoms† | 89/308 (28.9) | 64/226 (28.3) | 12/41 (29.3) | 4/11 (36.4) | 9/30 (30.0) | 0.931 |
| Respiratory | 17/308 (5.5) | 10/226 (4.4) | 2/41 (4.8) | 2/11 (18.2) | 3/30 (10.0) | |
| Cutaneous | 11/308 (3.6) | 7/226 (3.1) | 3/41 (7.3) | 1/11 (9.1) | 0/30 (0.0) | |
| Digestive | 49/308 (15.9) | 36/226 (15.9) | 8/41 (19.5) | 0/11 (0.0) | 5/30 (15.7) | |
| Systemic | 5/308 (1.6) | 3/226 (1.3) | 1/41 (2.4) | 0/11 (0.0) | 1/30 (3.3) | |
| Neurological | 5/308 (1.6) | 5/226 (2.2) | 0/41 (0.0) | 0/11 (0.0) | 0/30 (0.0) | |
| Others | 10/308 (3.3) | 7/226 (3.1) | 2/41 (4.9) | 1/11 (9.1) | 0/30 (0.0) | |
| Current medication† | 103/298 (34.6) | 78/218 (35.8) | 14/40 (35.0) | 3/10 (30.0) | 8/30 (26.7) | 0.823 |
| Antibiotics | 22/298 (7.4) | 16/218 (7.3) | 4/40 (10.0) | 2/10 (20.0) | 0/30 (0.0) | |
| Corticosteroids | 8/298 (2.7) | 7/218 (3.2) | 0/40 (0.0) | 0/10 (0.0) | 1/30 (3.3) | |
| Immunosuppressants | 1/298 (0.3) | 1/218 (0.5) | 0/40 (0.0) | 0/10 (0.0) | 0/30 (0.0) | |
| Proton-pump inhibitor | 8/298 (2.7) | 4/218 (1.8) | 2/40 (5.0) | 2/10 (20.0) | 0/30 (0.0) | |
| Statins | 7/298 (2.4) | 5/218 (2.3) | 1/40 (2.5) | 0/10 (0.0) | 1/30 (3.3) | |
| Antihypertensives | 32/298 (10.7) | 21/218 (9.6) | 6/40 (15.0) | 1/10 (10.0) | 4/30 (13.3) | |
| Others | 54/298 (18.1) | 44/218 (20.2) | 7/40 (17.5) | 1/10 (10.0) | 2/30 (6.7) | |
| Unknown | 1/298 (0.3) | 0/218 (0.0) | 0/40 (0.0) | 0/10 (0.0) | 1/30 (3.3) | |
LA: Latin America and the Caribbean, SSA: Sub-Saharan Africa, NA: Northern Africa, HIV: Human immunodeficiency virus, TB: Tuberculosis;.
Not all questionnaires were fully responded during the interviews explaining why the denominators of the variables vary.
Categories are not mutually exclusive.
Hard drug users include cocaine and other recreational drugs users.
p-values of the bivariate analysis with Pearson's chi-square test or Fisher's exact test.
p-value was estimated with the one-way ANOVA test.
3.1. Prevalence
3.1.1. Parasitic infections
The highest prevalence of the parasitic infections was reported for T.cruzi (14/192, 7.3 % [95 %CI 3.6–11.0]) (Table 2). Of the 14 T.cruzi infected cases, 13 (92.9 %) were migrants from Bolivia and one (7.1 %) from Honduras. There were 7/271 S.stercoralis cases resulting in a prevalence of 2.6 % [95 %CI 0.7–4.5]. Six of the S.stercoralis infected migrants were from LA (6/198, 3.0 % [95 %CI 0.6–5.4]), and one from SSA (1/38, 2.6 % [95 %CI 0.0–8.0]) (Table 2). Nonetheless, we found no cases infected with Schistosoma spp. within the 88 tested individuals, 35 migrants were from LA, 38 were from SSA, three were from NA, 10 were from Eastern and Southeast Asia, and two were from Southern Asia and Middle East.
Table 2.
Prevalence of the infections.
| Total n/N (%, 95 % CI) |
LA n/N (%, 95 % CI) |
SSA n/N (%, 95 % CI) |
NA n/N (%, 95 % CI) |
Other areas of birth n/N (%, 95 % CI) |
p-value* | |
|---|---|---|---|---|---|---|
| T.cruzi infection+ | 14/192 (7.3, 3.6–11.0) | 14/192 (7.3, 3.6–11.0) | – | – | – | – |
| S.stercoralis infection | 7/271 (2.6, 0.7–4.5) | 6/198 (3.0, 0.6–5.4) | 1/38 (2.6, 0.0–8.0) | 0/9 | 0/26 | 1.000 |
| – | – | |||||
| HIV | 5/281 (1.8, 0.2–3.3) | 2/204 (1.0, 0.0–2.3) | 2/40 (5.0, 0.0–12.1) | 0/9 | 1/28 (3.6, 0.0–10.9) | 0.157 |
| – | ||||||
| Chronic HBV (HBsAg +, HBc +) | 5/281 (1.8, 0.2–3.3) | 0/205 | 4/39 (10.3, 0.3–20.2) | 0/9 | 1/28 (3.6, 0.0–10.9) | 0.001 |
| – | ||||||
| – | ||||||
| Exposure to HBV (HBsAg ±, HBc +) | 40/284 (14.1, 10.0–18.2) | 4/207 (1.9, 0.0–3.8) | 30/41 (73.2, 59.0–87.3) | 0/8 | 6/28 (21.4, 5.2–37.6) | <0.001 |
| – | ||||||
| HCV | 1/284 (0.4, 0.0–1.0) | 0/207 | 1/41 (2.4, 0.0–7.4) | 0/9 | 0/27 | 0.271 |
| – | – | – |
LA: Latin America and the Caribbean, SSA: Sub-Saharan Africa, NA: Northern Africa, HIV: Human immunodeficiency virus, HBV: Hepatitis B virus, HBsAg: Australian antigen, HCV: Hepatitis C virus;.
Schistosomiasis was excluded due to the absence of identified cases.
Only people from endemic areas of Latin-America were tested for T.cruzi.
p-values of the bivariate analysis with Pearson's chi-square test or Fisher's exact test.
3.1.2. HIV and viral hepatitis
Five of the 281 migrants tested for HIV were positive presenting a prevalence of 1.8 % [95 %CI 0.2–3.3], two were born in LA (2/204, 1.0 % [95 %CI 0.0–2.3]), two in SSA (2/40, 5.0 % [95 %CI 0.0–12.1]), and one in Southern Asia and the Middle East (Table 2). For viral hepatitis, 1/284 (0.4 % [95 %CI 0.0–1.0]) migrants were infected with HCV, 5/28 (1.8 % [95 %CI 0.2–3.3]) presented a chronic HBV infection, and 40/284 (14.1 % [95 %CI 10.0–18.2]) were previously exposed to HBV. Among the HBsAg positive cases, four were migrants from SSA (4/39, 10.3 % [95 %CI 0.3–20.2]), and the other case was a migrant from other areas of birth (1/28, 3.6 % [95 %CI 0.0–10.9]), specifically from Southeast Asia (p-value=0.001). SSA migrants (30/41, 73.2 % [95 %CI 59.0–87.3]) reported the highest prevalence of exposure to HBV, followed by migrants from other areas of birth (6/28, 21.4 % [95 %CI 5.2–37.6]) (Table 2).
3.2. Factors associated with chronic infections
3.2.1. Parasitic infections
A higher proportion of T.cruzi infections were observed in males (7/52, 13.5 %; p = 0.050), and migrants with primary studies or illiteracy (3/20, 15.0 %; p = 0.028) (Table 3). Most of the diagnosed individuals lived in rural areas (4/29, 13.8 %; p = 0.013) in their country of birth and were considered long-term residing migrants (10/87, 11.5 %; p = 0.041). Moreover, the majority of the migrants infected with S.stercoralis had primary studies or illiteracy (4/39, 10.3 %; p = 0.008), and lived in both urban and rural areas in their country of birth (4/64, 6.3 %; p = 0.029) (Table 3). All cases were reported in long-term residing migrants (7/137, 5.1 %; p = 0.009).
Table 3.
Associations between the social determinants of health and the infectious diseases.
|
T.cruzi infection^ (N=192) |
S.stercoralis infection (N=271) |
HIV (N=281) |
Chronic HBV (N=281) |
|||||
|---|---|---|---|---|---|---|---|---|
| n/N (%) | p-value* | n/N ( %) | p-value* | n/N ( %) | p-value* | n/N ( %) | p-value* | |
| Area of birth | ||||||||
| LA | 14/192 (7.3) | – | 6/198 (3.0) | 1.000 | 2/204 (1.0) | 0.157 | 0/205 (0.0) | 0.001 |
| SSA | – | 1/38 (2.6) | 2/40 (5.0) | 4/39 (10.3) | ||||
| NA | – | 0/9 (0.0) | 0/9 (0.0) | 0/9 (0.0) | ||||
| Other areas of birth | – | 0/26 (0.0) | 1/28 (3.6) | 1/28 (3.6) | ||||
| Sex | ||||||||
| Male | 7/52 (13.5) | 0.050 | 4/106 (3.8) | 0.271 | 4/108 (3.7) | 0.074 | 5/109 (4.6) | 0.008 |
| Female | 7/140 (5.0) | 3/165 (1.8) | 1/173 (0.6) | 0/172 (0.0) | ||||
| Age categories | ||||||||
| 18–39 years | 6/100 (6.0) | 0.473 | 1/139 (0.7) | 0.061 | 2/145 (1.4) | 0.676 | 1/145 (0.7) | 0.201 |
| ≥40 years | 8/92 (8.7) | 6/132 (4.6) | 3/136 (2.2) | 4/136 (2.4) | ||||
| Education level1 | ||||||||
| Primary studies or illiteracy | 3/20 (15.0) | 0.028 | 4/39 (10.3) | 0.008 | 0/40 (0.0) | 0.812 | 3/39 (7.7) | 0.004 |
| Secondary studies | 10/103 (9.7) | 3/138 (2.2) | 3/138 (2.2) | 0/139 (0.0) | ||||
| Higher studies | 1/67 (1.5) | 0/88 (0.0) | 1/96 (1.0) | 1/96 (1.0) | ||||
| Professional categories2 | ||||||||
| Unemployed | 2/35 (5.7) | 1.000 | 0/49 (0.0) | 0.355 | 1/52 (1.9) | 0.568 | 0/50 (0.0) | 0.589 |
| Employed | 12/156 (7.7) | 7/217 (3.2) | 3/224 (1.3) | 5/226 (2.2) | ||||
| Living area in country of birth3 | ||||||||
| Urban | 3/109 (2.8) | 0.013 | 1/155 (0.7) | 0.029 | 3/161 (1.9) | 1.000 | 2/163 (1.2) | 0.365 |
| Rural | 4/29 (13.8) | 2/47 (4.3) | 0/47 (0.0) | 0/47 (0.0) | ||||
| Both | 7/53 (13.2) | 4/64 (6.3) | 1/67 (1.5) | 2/65 (3.1) | ||||
| Contact with rivers or lakes4 | ||||||||
| No | 5/82 (6.1) | 0.392 | 2/116 (1.7) | 0.342 | – | – | – | – |
| Yes | 9/109 (8.3) | 5/150 (3.3) | ||||||
|
In transit countries5 None |
||||||||
| 1 country | 9/99 (9.1) | 0.361 | 4/127 (3.2) | 1.000 | 2/131 (1.5) | 0.119 | 1/131 (0.8) | 0.503 |
| ≥2 countries | 4/67 (6.0) | 2/94 (2.1) | 0/94 (0.0) | 2/95 (2.1) | ||||
| 0/23 (0.0) | 1/41 (2.4) | 2/46 (4.4) | 1/45 (2.2) | |||||
| Suffered violence6 | ||||||||
| No | 11/151 (7.3) | 0.113 | 5/221 (2.3) | 0.590 | 3/228 (1.3) | 0.454 | 4/229 (1.8) | 0.547 |
| Yes | 0/32 (0.0) | 1/35 (2.9) | 1/37 (2.7) | 0/37 (0.0) | ||||
| Time residing in Spain in years7 | ||||||||
| <5 years | 4/104 (3.9) | 0.041 | 0/130 (0.0) | 0.009 | 1/131 (0.8) | 0.624 | 0/134 (0.0) | 0.069 |
| ≥5 years | 10/87 (11.5) | 7/137 (5.1) | 3/145 (2.1) | 4/142 (2.8) | ||||
| Unsafe sexual practices8 | ||||||||
| No | 13/155 (8.4) | 0.217 | 6/218 (2.8) | 0.631 | 3/226 (1.3) | 0.546 | 4/226 (1.8) | 0.454 |
| Yes | 1/36 (2.8) | 1/48 (2.1) | 1/49 (2.0) | 0/49 (0.0) | ||||
| Hard drug users+,9 | ||||||||
| No | 12/185 (6.5) | 1.000 | 7/260 (2.7) | 1.000 | 4/267 (1.5) | 1.000 | 4/267 (1.5) | 1.000 |
| Yes | 0/4 (0.0) | 0/5 (0.0) | 0/7 (0.0) | 0/7 (0.0) | ||||
| Cannabis users10 | ||||||||
| No | 12/180 (6.7) | 1.000 | 7/254 (2.8) | 1.000 | 3/260 (1.2) | 0.190 | 4/260 (1.5) | 1.000 |
| Yes | 0/9 (0.0) | 0/11 (0.0) | 1/14 (7.1) | 0/14 (0.0) | ||||
| Comorbidities11 | ||||||||
| No | 11/137 (8.0) | 0.425 | 5/198 (2.5) | 0.571 | 2/201 (1.0) | 0.294 | 3/200 (1.5) | 0.700 |
| Yes | 3/54 (5.6) | 2/68 (2.9) | 2/74 (2.7) | 1/75 (1.4) | ||||
| Previous TB episode12 | ||||||||
| No | 12/182 (6.6) | 0.764 | 6/250 (2.4) | 0.887 | 3/256 (1.2) | 0.922 | 4/256 (1.6) | 0.897 |
| Yes | 0/4 (0.0) | 0/5 (0.0) | 0/7 (0.0) | 0/7 (0.0) | ||||
| Immunosuppression status13 | ||||||||
| No | 14/189 (7.4) | 0.858 | 7/264 (2.7) | 0.948 | 2/270 (0.7) | 0.002 | 4/271 (1.5) | 0.943 |
| Yes | 0/2 (0.0) | 0/2 (0.0) | 2/5 (40.0) | 0/4 (0.0) | ||||
| Symptoms14 | ||||||||
| No | 11/136 (8.1) | 0.386 | 5/189 (2.7) | 0.672 | 2/195 (1.0) | 0.332 | 3/197 (1.5) | 0.681 |
| Yes | 3/55 (5.5) | 2/77 (2.6) | 2/80 (2.5) | 1/78 (1.3) | ||||
| Current medication15 | ||||||||
| No | 8/115 (7.0) | 0.520 | 3/166 (1.8) | 0.355 | 3/169 (1.8) | 0.541 | 3/170 (1.8) | 0.548 |
| Yes | 4/68 (5.9) | 3/90 (3.3) | 1/96 (1.0) | 1/95 (1.1) | ||||
HIV: Human immunodeficiency virus, HBV: Hepatitis B virus, HCV: Hepatitis C virus, TB: Tuberculosis;.
Not all questionnaires were fully responded during the interviews explaining why the denominators of the variables vary.
Hard drug users include cocaine and other recreational drugs users.
p-values of the bivariate analysis with Pearson's chi-square test or Fisher's exact test.
Only people from endemic areas of Latin-America were tested for T.cruzi;.
7 missing values.
5 missing values.
6 missing values.
6 missing values.
10 missing values.
16 missing values.
5 missing values.
6 missing values.
7 missing values.
7 missing values.
6 missing values.
20 missing values.
6 missing values.
6 missing values.
16 missing values.
3.2.2. HIV and viral hepatitis
There were 2/5 (40.0 %, p = 0.002) immunosuppressed migrants among those infected with HIV. The only HCV infection diagnosis was observed in a 45-year-old female born in SSA. All five chronic HBV cases were reported in males (5/109, 4.6 %; p = 0.008). Three of the four chronic HBV cases that reported their education level had primary studies or illiteracy (3/39, 7.7 %; p = 0.004) (Table 3). Besides, a higher proportion of males (31/109, 28.4 %; p < 0.001) and migrants in their middle-old adulthood (27/137, 19.7 %; p = 0.009) had been previously exposed to an HBV infection (Table 4). Primary studies or illiteracy (18/40, 45.0 %; p < 0.001) and residing in Spain ≥5 years (33/144, 22.9 %; p < 0.001) were associated with exposure to HBV (Table 4).
Table 4.
Associations between the social determinants of health and exposure to an HBV infection.
| Exposure to an HBV infection (N = 284) |
|||||
|---|---|---|---|---|---|
| Unadjusted model | Adjusted model |
||||
| n/N (%) | p-value* | OR (95 % CI) | aOR (95 % CI) | p-value^ | |
| Area of birth | |||||
| LA | 4/207 (1.9) | <0.001 | reference | reference | <0.001 |
| SSA | 30/41 (73.2) | 119.9 (37.8–380.4) | 87.9 (19.3–401.1) | ||
| NA | 0/8 (0.0) | 2.7 (0.1–53.5) | 0.6 (0.0–16.9) | ||
| Other areas of birth | 6/28 (21.4) | 13.1 (3.6–46.9) | 13.0 (2.9–58.8) | ||
| Sex | |||||
| Male | 31/109 (28.4) | <0.001 | reference | reference | 0.058 |
| Female | 9/175 (5.1) | 0.1 (0.1–0.3) | 0.3 (0.1–1.0) | ||
| Age categories | |||||
| 18–39 years | 13/147 (8.8) | 0.009 | reference | reference | 0.032 |
| ≥40 years | 27/137 (19.7) | 2.5 (1.2–5.0) | 3.9 (1.1–13.6) | ||
| Education level1 | 0.3 | ||||
| Primary studies or illiteracy | 18/40 (45.0) | <0.001 | reference | reference | 0.012 |
| Secondary studies | 13/140 (9.3) | 0.1 (0.1–0.3) | 0.1 (0.0–0.5) | ||
| Higher studies | 6/97 (6.2) | 0.1 (0.0–0.2) | 0.4 (0.1–1.7) | ||
| Professional categories2 | |||||
| Unemployed | 5/52 (9.6) | 0.501 | reference | – | – |
| Employed | 33/227 (14.5) | 1.5 (0.6–3.9) | |||
| In transit countries3 | |||||
| None | 14/132 (10.6) | 0.274 | reference | – | – |
| 1 country | 12/96 (12.5) | 1.2 (0.5–2.7) | |||
| ≥2 countries | 9/46 (19.6) | 2.1 (0.8–5.1) | |||
| Suffered violence4 | |||||
| No | 32/231 (13.9) | 0.190 | reference | – | – |
| Yes | 2/37 (5.4) | 0.4 (0.1–1.6) | |||
| Time residing in Spain in years5 | |||||
| <5 years | 4/135 (3.0) | <0.001 | reference | reference | 0.034 |
| ≥5 years | 33/144 (22.9) | 8.8 (3.2–24.2) | 4.2 (1.1–16.0) | ||
| Unsafe sexual practices6 | |||||
| No | 28/228 (12.3) | 0.356 | reference | – | – |
| Yes | 9/50 (18.0) | 1.6 (0.7–3.6) | |||
| Hard drug users+,7 | |||||
| No | 35/270 (13.0) | 0.627 | reference | – | – |
| Yes | 1/7 (14.3) | 1.5 (0.3–9.4) | |||
| Cannabis users8 | |||||
| No | 35/263 (13.3) | 1.000 | reference | ||
| Yes | 1/14 (7.1) | 0.7 (0.1–4.0) | |||
| Comorbidities9 | |||||
| No | 26/202 (12.9) | 0.697 | reference | – | – |
| Yes | 11/76 (14.5) | 1.2 (0.6–2.5) | |||
| Immunosuppression status10 | |||||
| No | 36/273 (13.2) | 0.513 | reference | – | – |
| Yes | 1/5 (20.0) | 2.2 (0.3–14.2) | |||
HBV: Hepatitis B virus, LA: Latin America and the Caribbean, SSA: Sub-Saharan Africa, NA: Northern Africa, OR: odds ratio, aOR: adjusted odds ratio;.
Not all questionnaires were fully responded during the interviews explaining why the denominators of the variables vary.
Variables not considered in the final model.
The multivariate model was adjusted by area of birth, sex, age, education level and time residing in Spain in years.
Hard drug users include cocaine and other recreational drugs users.
p-value of the bivariate analysis with Pearson's chi-square test or Fisher's exact test.
p-value of the adjusted model;
7 missing values.
5 missing values.
10 missing values.
16 missing values.
5 missing values.
6 missing values.
7 missing values.
7 missing values.
6 missing values.
6 missing values.
In the multivariable model, migrants from SSA (aOR: 87.9 [95 %CI 19.3–401.1]) and other areas of birth (aOR: 13.0 [95 %CI 2.9–58.8]) had higher odds of being exposed to HBV than migrants from LA after adjusting by sex, age, educational level, and time of residence in Spain (p < 0.001) (Table 4). When adjusting the model, females (aOR: 0.3 [95 %CI 0.1–1.0]; p = 0.058) and migrants with secondary studies (aOR: 0.1 [95 %CI 0.0–0.5]; p = 0.012) had lower odds of previous exposure to HBV than males and participants with lower educational studies. Migrants in their middle-old adulthood (aOR: 3.9 [95 %CI 1.1–13.6]; p = 0.032) and long-term residing migrants (aOR: 4.2 [95 %CI 1.1–16.0]; p = 0.034) presented higher odds of previous exposure to HBV than young adults and newly arrived migrants, respectively, in the adjusted model (Table 4).
Due to the low number of observations, regression models were not explored for other infections.
4. Discussion
Overall, we found a high prevalence of chronic infections in the migrant population, especially in T.cruzi and previous exposure to an HBV infection. Migrants born in countries of SSA and LA were more vulnerable to infectious diseases. However, these results should be interpreted with caution since the sample was mostly represented by migrants born in LA (∼75 %), following the trend of the migrant population residing in Barcelona (Spain) (Statistical Institute of Catalonia 2022), where most of the participating centres were located.
Interestingly, we were unable to detect any cases infected with Schistosoma spp. despite that more than half of the participants had contact with freshwater bodies in their country of birth. This fact might be related to the low sample size of migrants born in SSA and Asian endemic countries (European Centre for Disease Prevention and Control (ECDC) 2018). The prevalence of S.stercoralis infection in our study was in line with recent experts’ calculations for the World Health Organization (WHO) European Region (2.8 %) (Buonfrate et al., 2020). However, a meta-analysis of migrants living in Spain indicated a 14 % prevalence where the majority of the included studies were conducted at Tropical Medicine Units (Salvador et al., 2020). Differences in prevalence estimates were observed in comparison to prior studies due to differences in sample composition, having recruited a higher number of Asian and African migrants (Asundi et al., 2019; Salvador et al., 2020). Therefore, future studies should consider the inclusion of migrants from origins that were under represented in our sample to estimate the burden of S.stercoralis better among these populations.
We found a prevalence of T.cruzi infection similar to estimates observed in non-hospital settings (8.7 %) in a recent systematic review and meta-analysis (Requena-Méndez et al., 2015). We also found that Bolivians had the highest T.cruzi infection prevalence, followed by people from Honduras, as previous studies had demonstrated (Navarro et al., 2022; Requena-Méndez et al., 2015). Therefore, testing interventions at strategic points, such as primary care and community level, should focus on migrants born in these high-prevalent countries to control the Chagas disease epidemic in non-endemic areas.
Furthermore, low educational levels and living conditions (living in rural or both rural and urban areas) in the country of birth were associated with both T.cruzi and S.stercoralis infections. Similar results have been observed in studies conducted in endemic areas (Fernandez et al., 2019; Beknazarova et al., 2016) and Spain (Ramos-Sesma et al., 2021; Ramos-Sesma et al., 2020), suggesting that both neglected tropical diseases share socio-economical and geographical risk factors (Salvador et al., 2020; Requena-Méndez et al., 2015). Both infections were more prevalent in long-term residing migrants (≥5 years), implying that infected individuals can be asymptomatic and carry the infection for a long time. Since both infections can develop severe complications under certain circumstances, such as immunosuppression, our findings support the idea that screening strategies in non-endemic settings should target all individuals coming from endemic countries (Navarro et al., 2022).
The HIV prevalence was similar to previous estimates made at primary care in Barcelona, Spain (1.2 %) (Hladun et al., 2014). Moreover, a systematic review of HIV prevalence in migrants concluded that migrants living in Europe and coming from SSA had a high burden of HIV infection (>3.0 %), while a lower prevalence was estimated for LA migrants (0.7 %) (Schousboe and Wejse, 2021), which is also similar to the data reported in our study. The heterogeneity within the migrant population should be considered when planning health policies on HIV control strategies for better use of limited resources. These strategies should target migrants at higher risk of infection instead of including individuals coming from low-endemic areas, as most LA countries are considered (Sequeira-Aymar et al., 2021; Schousboe and Wejse, 2021).
Migration itself is not a risk factor for the acquisition of HIV, however, factors related to migration can make an individual vulnerable to the infection (Schousboe and Wejse, 2021). We did not find any association between HIV and factors (violence during migration journey, unsafe sexual practices, drug use) that have been previously associated with the infection (Klok et al., 2021; Prestileo et al., 2022), although the small sample size may have precluded properly assessing this association. Further, 75.0 % of the HIV cases that reported time of residence in Spain were identified as long-time residing migrants, supporting the idea of an increased risk of HIV acquisition after migrating to European countries (European Centre for Disease Prevention and Control, WHO Regional Office for Europe 2021). Expectedly, most HIV cases in our study were diagnosed in males, as these are often associated with a higher burden of infection [(European Centre for Disease Prevention and Control (ECDC) 2018; Klok et al., 2021)]. Hence, all these factors might be considered to identify migrants at higher risk and to conduct educational and screening interventions focusing on these vulnerable groups.
The HCV prevalence found in our study was lower than in previous studies. These findings must be taken with caution, however, they might suggest that current control strategies to decrease the viral hepatitis burden are working (Lazarus et al., 2019). A considerable difference in HCV prevalence was observed within our results and previous studies, such as a meta-analysis (1.6 %) (Lazarus et al., 2019), hospital-based studies (2.6–4.2 %) (Norman et al., 2021; Bocanegra et al., 2014), and non-hospital settings (1.8–3.3 %) (Hladun et al., 2014; Alarcón-Linares et al., 2019), where the study population had a higher representation of migrants from high-prevalent countries. The highest HCV prevalence has been mostly described in migrants from Europe (mostly Eastern Europeans and Italians) and SSAs, followed by NAs, while migrants coming from LA tend to present a lower prevalence (Lazarus et al., 2019; Bocanegra et al., 2014; Hladun et al., 2014; Alarcón-Linares et al., 2019). The only case in our study was observed in a migrant born in SSA, yet none of the migrants coming from LA were diagnosed. In line with the WHO's global hepatitis strategy to achieve the target of HCV elimination by 2030 (World Health Organization 2022), although our results cannot be conclusive, they can recommend the promotion of HCV screening, particularly in migrants coming from SSA countries.
Further, the chronic HBV prevalence data of our study is comparable with two cross-sectional studies conducted in Spain, 1.4 % in a population-based study (Cuadrado et al., 2020) and 2.6 % in a primary care-based study (Hladun et al., 2014); and with two other studies targeting undocumented migrants, one in France (3.1 %) (Vignier et al., 2022) and the other in the Netherlands (2.5 %) (Klok et al., 2021). Other studies suggested a much higher prevalence, such as a European systematic review (Cuadrado et al., 2020) and two Spanish hospital-based studies (Norman et al., 2021; Bocanegra et al., 2014), which reported an HBsAg prevalence of around 6 %. On the other hand, prevalence rates rise up to 18.0 % when considering exposure to HBV estimated in Spanish non-hospital settings (Hladun et al., 2014; Cuadrado et al., 2020), congruent to our data. Our findings suggest that migrants coming from SSA are significantly affected by chronic HBV and have been previously exposed to the infection, presenting higher odds when compared to other geographical areas. As this tendency has also been observed in different low-endemic countries in Europe, experts agree that HBV screening strategies should target migrant populations, particularly those born in SSA countries (Sequeira-Aymar et al., 2021; Vignier et al., 2022; Klok et al., 2021; Hladun et al., 2014; Prestileo et al., 2022).
In Spain, the incidence of HBV has decreased in the last decades due to the expanded vaccination programmes implemented in 1992 (Arístegui Fernández et al., 2015). Nonetheless, migrants residing in Spain can come from countries where vaccination strategies are not yet largely implemented. They can also be exposed to different risk factors during the migration journey, making them more vulnerable to the infection than the autochthonous population (Stroffolini et al., 2021). This can increase the host country's HBV prevalence and become a public health challenge. Therefore, identifying individuals at risk of infection is essential to prevent the transmission of the infection and control disease progression by vaccinating the nonimmunized and treating those already infected, as well as promoting migrants’ health. In accordance with previous studies (Klok et al., 2021; Cuadrado et al., 2020), we found that males with lower educational levels were associated with presenting both exposure to HBV and chronic HBV infection. Also, we found an association between long residing migrants and exposure to HBV, and therefore, immunization as it has been previously reported in undocumented migrants residing in the Netherlands (Klok et al., 2021). Finally, as Cuadrado and colleagues described (Cuadrado et al., 2020), older migrants were more commonly exposed to HBV than younger adults due to the highest possibility of being vaccinated against the infection in the latter group.
Integrated screening of infectious diseases targeting migrant patients based on an individual assessment has already been proven effective (Sequeira-Aymar et al., 2021). Nonetheless, migrant populations are considered a homogenous group in most of the screening guidelines. Policymakers in the public health sector should consider individualizing the screening decision process and, therefore, take into account the prevalence estimations of infections in migrants to identify and treat individuals at risk at an early stage, optimizing scarce resources and decreasing health-related costs. The area of birth and other factors associated with a higher risk of infection may be good indicators to make a screening decision. For example, the parasitic infection burden is usually attributed to African migrants, however, these infections disproportionally affect migrants coming from other areas in LA and Asia. Conversely, HIV and viral hepatitis infections are more globally distributed. Thus, migrants born in intermediate and high-prevalent countries (European Centre for Disease Prevention and Control (ECDC) 2018) are susceptible to these infections. Also, information on the levels of anti-HBc could support the promotion of vaccination strategies targeting migrant groups with a higher risk of acquiring the infection and, at the same time, exclude individuals already exposed to the infection.
4.1. Limitations
The primary care setting offers a more representative sample of the migrant population than hospital-based studies. In our study, individuals were also recruited in two hospitals (18 %), and although researchers were instructed to recruit only asymptomatic migrants or migrants with nonspecific symptoms, these settings could have introduced potential referral bias. In any case, the purpose of this screening programme was to increase the detection of infectious diseases, irrespective of the symptoms presented by the migrant individuals.
Besides, the sample and the selection of the centres were not randomized, thus the estimated prevalence is only representative of the migrant population attended at the participating centres.
Due to the composition of the sample, under- or over-estimations of the prevalence of the different infections could have been reported. In addition, the small sample size of the study limited the seroprevalence comparison among groups due to the low number of infectious disease cases reported in migrants from different areas of birth. The sample size affected the precision of the seroprevalence estimations with wide 95 %CI intervals for some infections, limiting its possible comparison with other populations, such as the autochthonous population. The low number of events for most infections limited the multivariate analysis, with only one possible model, exposure to HBV. Furthermore, the estimates of this model could be biased due to the few events and small sample size, which has limited the power to detect differences if they exist.
Regarding the number of tests performed, only one HBsAg test was done instead of the two recommended, within at least a six-month difference, to confirm the chronicity of an infected individual. Similarly, polymerase chain reaction tests to confirm HCV chronic infections were not performed. On the other side, even though tuberculosis is prevalent in the migrant population, this infection was not included in the prevalence study due to the limitation of adding it to a multiplex serological test (Aguilar et al., 2024), which was one of the objectives of our research work in order to streamline the screening.
Other limitations were the loss of follow-up of some participants during the Covid-19 pandemic and the possible underreporting of certain behavioural risk factors as a result of the face-to-face interviews.
5. Conclusions
A high prevalence of the studied chronic infectious diseases was observed in the migrant population. The country of birth, in particular, and other factors migrants were exposed to in their country of birth, during their migration journey, and in their country of residence were factors associated with the infections. Therefore, the heterogeneity of the migrant population must be considered when planning and implementing preventive strategies.
Funding
This work was supported by the grant “Fondo de Investigación para la Salud (FIS)” [PI17/02,020] from the Instituto de Salud Carlos III (ISCIII) co-financed by the European Regional Development Fund (FEDER) from the European Union. ISGlobal acknowledges support from the grant [CEX2018–000,806-S] funded by MCIN/AEI/10.13039/501,100,011,033, and support from the Generalitat de Catalunya through the CERCA Programme. ARM is funded by the Strategic Research Programme in Epidemiology at Karolinska Institutet. ACz is funded by ISCIII co-financed by FEDER from the European Union through the “Contratos predoctorales de formación en investigación (PFIS)” [FI22/00,156].
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Data availability
Data may be available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Angeline Cruz: Writing – review & editing, Writing – original draft, Project administration, Formal analysis. Angela Martínez-Perez: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization. Alex Almuedo-Riera: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Conceptualization. Carme Roca Saumell: Writing – review & editing, Investigation. Marina Gigante Lopez: Writing – review & editing, Investigation. Oriol Gasch: Writing – review & editing, Investigation, Funding acquisition, Conceptualization. Gemma Falcó: Writing – review & editing, Investigation. Ana Jiménez-Lozano: Writing – review & editing, Investigation. Consol Sanchez-Collado: Writing – review & editing, Investigation. Julio Alonso-Padilla: Writing – review & editing, Methodology, Conceptualization. Juan Carlos Hurtado: Writing – review & editing, Investigation. Miriam J Álvarez-Martínez: Writing – review & editing, Investigation. Aina Casellas: Writing – review & editing, Formal analysis, Data curation. Ana Requena-Méndez: Writing – review & editing, Writing – original draft, Methodology, Funding acquisition, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to all migrants who participated in this study, the general practitioners, nurses, and other staff from the primary care centres and hospitals who were committed to performing the fieldwork required for the study while maintaining their daily tasks. We want to give special thanks to the primary care staff of CAPSBE, including Ethel Sequeira, Laia Cayuelas, Sandra Estévez, Merce Casasayas, Sira Casablancas, and Xavier Freixa. We also acknowledge the effort to recruit made by the clinical care team of the Hospital Clinic in Barcelona, especially Carme Subirà, Natalia Rodríguez Valero, Daniel Camprubí, Magdalena Muelas, Isabel Vera, Montserrat Roldán, Teresa de Alba, Elisabet Ferrer and José Muñoz. We are also grateful to the Microbiology Service of Hospital Clinic in Barcelona for processing the samples collected during recruitment, expressly many thanks to M. Angeles Marcos, Maria del Mar Mosquera, Mikel J. Martínez, and M. Eugenia Valls.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jmh.2024.100278.
Appendix. Supplementary materials
References
- Aguilar R., Cruz A., Jiménez A., Almuedo A., Saumell C.R., Lopez M.G., et al. Vol. 57. Elsevier Inc.; 2024. Evaluation of the accuracy of a multi-infection screening test based on a multiplex immunoassay targeting imported diseases common in migrant populations. (Travel Med Infect Dis). [DOI] [PubMed] [Google Scholar]
- Alarcón-Linares M.E., Torres-Cantero A., Subirá C., Ramírez O., Crespo J., Lazarus J.V., et al. Geographic analysis and estimation of hepatitis C cases in migrant populations living in Spain: is a country-based screening strategy appropriate? Revista Espanola de Enfermedades Digestivas. 2019;111:615–625. doi: 10.17235/REED.2019.6117/2018w. [DOI] [PubMed] [Google Scholar]
- Arístegui Fernández J., Díez-Domingo J., Marés Bermúdez J., Martinón Torres F. Vaccination against hepatitis B. Impact of vaccination programmes after 20 years of use in Spain. is it time for a change? Enferm. Infecc. Microbiol. Clin. 2015;33:113–118. doi: 10.1016/j.eimc.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Asundi A., Beliavsky A., Liu X.J., Akaberi A., Schwarzer G., Bisoffi Z., et al. Prevalence of strongyloidiasis and schistosomiasis among migrants: a systematic review and meta-analysis. Lancet Glob. Health. 2019;7:e236–e248. doi: 10.1016/S2214-109X(18)30490-X. [DOI] [PubMed] [Google Scholar]
- Beknazarova M., Whiley H., Ross K. Strongyloidiasis: a disease of socioeconomic disadvantage. Int. J. Environ. Res. Public Health. 2016;13 doi: 10.3390/ijerph13050517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra C., Salvador F., Sulleiro E., Sánchez-Montalvá A., Pahissa A., Molina I. Screening for imported diseases in an immigrant population: experience from a teaching hospital in Barcelona, Spain. Am. J. Trop. Med. Hygiene. 2014;91:1277–1281. doi: 10.4269/ajtmh.14-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonfrate D., Bisanzio D., Giorli G., Odermatt P., Fürst T., Greenaway C., et al. The Global Prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9:468. doi: 10.3390/pathogens9060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A., Perelló C., Cabezas J., Llerena S., Llop E., Escudero M.D., et al. Update on epidemiology of hepatitis B in a low-endemic European country: there is still much to do. J. Viral. Hepat. 2020;27:1261–1265. doi: 10.1111/jvh.13350. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). Public health guidance on screening and vaccination for infectious diseases in newly arrived migrants within the EU/EEA. 2018.
- European Centre for Disease Prevention and Control, WHO Regional Office for Europe . ECDC; Stockholm: 2021. HIV/AIDS Surveillance in Europe 2021 - 2020 data. [Google Scholar]
- Fernandez M., del P., Gaspe M.S., Gurtler R.E. Inequalities in the social determinants of Chagas disease in indigenous and creole people in the Argentine Chaco. Parasit. Vectors. 2019;12 doi: 10.1186/s13071-019-3444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field V., Gautret P., Schlagenhauf P., Burchard G.D., Caumes E., Jensenius M., et al. Travel and migration associated infectious diseases morbidity in Europe, 2008. BMC. Infect. Dis. 2010;10:330. doi: 10.1186/1471-2334-10-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A.Q., Sequeira-Aymar E., Aguilar Martín C., Dalmau R.M., Cruz A., Evangelidou S., et al. Usefulness and practicality of a multidisease screening programme targeting migrant patients in primary care in Spain: a qualitative study of general practitioners. BMJ Open. 2022;12:1–9. doi: 10.1136/bmjopen-2022-065645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladun O., Grau A., Esteban E., Jansà J.M. Results from screening immigrants of low-income countries: data from a public primary health care. J. Travel. Med. 2014;21:92–98. doi: 10.1111/jtm.12083. [DOI] [PubMed] [Google Scholar]
- International Organization for Migration. World Migration Report 2022. 2021.
- Klok S., van Dulm E., Boyd A., Generaal E., Eskander S., Joore I.K., et al. Hepatitis B Virus (HBV), Hepatitis C Virus (HCV) and Human Immunodeficiency Virus (HIV) infections among undocumented migrants and uninsured legal residents in the Netherlands: a cross-sectional study, 2018–2019. PLoS. One. 2021;16 doi: 10.1371/journal.pone.0258932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus J.V., Bromberg D.J., del Amo J., Norgaard O., García-Samaniego J., Casellas A., et al. Hepatitis C prevalence among the migrant population in Spain: a systematic review and meta-analysis. Enferm. Infecc. Microbiol. Clin. 2019;37:222–230. doi: 10.1016/j.eimc.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Navarro M., Reguero L., Subir C., Bl A., Requena-m A. Estimating chagas disease prevalence and number of underdiagnosed, and undertreated individuals in Spain 2022;47. 10.1016/j.tmaid.2022.102284. [DOI] [PubMed]
- Norman F.F., Comeche B., Martínez-lacalzada M., Gullón B., Monge-maillo B., Chamorro S., et al. Seroprevalence of vaccine-preventable and non-vaccine-preventable infections in migrants in Spain 2021:1–8. 10.1093/jtm/taab025. [DOI] [PubMed]
- Prestileo T., Di Marco V., Dino O., Sanfilippo A., Tutone M., Milesi M., et al. Effectiveness of a screening program for HBV, HCV, and HIV infections in African migrants to Sicily. Digest. Liver Dis. 2022;54:800–804. doi: 10.1016/j.dld.2021.08.024. [DOI] [PubMed] [Google Scholar]
- Ramos-Sesma V., Navarro M., Llenas-García J., Gil-Anguita C., Torrús-Tendero D., Wikman-Jorgensen P., et al. Asymptomatic strongyloidiasis among latin american migrants in Spain: a community-based approach. Pathogens. 2020;9:1–14. doi: 10.3390/pathogens9060511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Sesma V., Navarro M., Llenas-García J., Gil-Anguita C., Torrus-Tendero D., Wikman-Jorgensen P., et al. Community-based screening of Chagas disease among Latin American migrants in a non-endemic country: an observational study. Infect. Dis. Poverty. 2021;10 doi: 10.1186/s40249-021-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena-Méndez A., Aldasoro E., de Lazzari E., Sicuri E., Brown M., Moore D.A.J., et al. Prevalence of Chagas Disease in Latin-American Migrants Living in Europe: a Systematic Review and Meta-analysis. PLoS. Negl. Trop. Dis. 2015;9:1–15. doi: 10.1371/journal.pntd.0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador F., Treviño B., Bosch-Nicolau P., Serre-Delcor N., Sánchez-Montalvá A., Oliveira I., et al. Strongyloidiasis screening in migrants living in Spain: systematic review and meta-analysis. Trop. Med. Int. Health. 2020;25:281–290. doi: 10.1111/tmi.13352. [DOI] [PubMed] [Google Scholar]
- Schousboe C., Wejse C. HIV prevalence in migrant groups based on country of origin: a systematic review on data obtained between 1993 and 2020. Sustain. (Switzerland) 2021;13:1–18. doi: 10.3390/su132111642. [DOI] [Google Scholar]
- Sequeira-Aymar E., Cruz A., Serra-Burriel M., di Lollo X., Gonçalves A.Q., Camps-Vilà L., et al. Improving the detection of infectious diseases in at-risk migrants with an innovative integrated multi-infection screening digital decision support tool (IS-MiHealth) in primary care: a pilot cluster-randomized controlled trial. J. Travel. Med. 2021:1–11. doi: 10.1093/jtm/taab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistical Institute of Catalonia . Total and Foreign Population Series. 2022. Generalitat of Catalonia Municipal Census of Inhabitants.https://www.idescat.cat/poblacioestrangera/?geo=cat&nac=a&b=0&t=2018 [Google Scholar]
- Stroffolini T., Ciancio A., Furlan C., Vinci M., Niro G.A., Russello M., et al. Chronic hepatitis B virus infection in Italy during the twenty-first century: an updated survey in 2019. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:607–614. doi: 10.1007/s10096-020-04065-6. [DOI] [PubMed] [Google Scholar]
- Vignier N., Moussaoui S., Marsaudon A., Wittwer J., Jusot F., Dourgnon P. Burden of infectious diseases among undocumented migrants in France: results of the Premiers Pas survey. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.934050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J., Jungner G. 1968. Principles and Practice of Screening For Disease. Geneva. [Google Scholar]
- World Health Organization . 2022. Global Health Sector Strategies on, respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections For the Period 2022-2030. Geneva. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be available from the corresponding author upon reasonable request.


