Introduction
Pediatric vitiligo affects 1.52% of 4 to 11-year-olds and 2.16% of 12 to 17-year-olds within the United States, with nonsegmental vitiligo (NSV) affecting 65% and 69%, respectively, and the residuum segmental vitiligo (SV).1 The Food and Drug Administration (FDA) has approved topical 1.5% ruxolitinib cream for NSV affecting 10% of body surface area or less for 12 years of age or older. Although the TRuEV-1 and TRuEV-2 trials included children as young as 12 years, the data for the subset of children was not separately analyzed in publication.2 Furthermore, there is no FDA-approved topical agent for SV in any age group.3 We sought to characterize the efficacy of 1.5% ruxolitinib cream for pediatric vitiligo.
Methodology
An IRB-exempted chart review was conducted addressing the usage of 1.5% ruxolitinib cream in children with vitiligo of all types (0-17 years of age) who used topical 1.5% ruxolitinib cream for the treatment of vitiligo. The patients were treated according to standard protocol for NSV of twice a day, however, 2 patients reduced to daily application, and 308-nm laser was recommended concurrently for all segmental cases.
Case series
Twelve children were identified, ages 3 to 16 years (average age: 10.75 years).
Demographics included males (n = 9), females (n = 3). Race/ethnicity data included children who were Black (n = 1), Hispanic/Latin X (n = 4), Indian (n = 1), and White (n = 6).
The vitiligo subtypes included NSV (n = 7), mixed-type vitiligo which is a combination of SV and NSV (n = 1), and isolated SV (n = 4). The summary of these cases is shown in Table I.
Table I.
Summary of 12 children treated for vitiligo with topical ruxolitinib 1.5% cream
| Case | Type of vitiligo | Age (y) | Sex Race Ethnicity |
Location lesions | Time with vitiligo (mo)/frequency therapy | Time until first response (mo) | Add-on therapies | Laboratory tests | Repigmentation, % | Time to final response (mo) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NSV | 3 | Male Indian |
Forehead, perioral | 5/every day | 3 | None | CBC TFT WNL |
100% | 5 |
| 2 | NSV | 9 | Female Black |
Perioral, upper portion of the right arm, right calf | 4/twice a day | 1 | None | CBC CMP TFT WNL |
100% | 9 |
| 3 | NSV | 9 | Male Hispanic |
Bilateral thighs, knees, calves | 4/twice a day | 2 | None | CBC CMP WNL |
100% | 6 |
| 4 | NSV | 10 | Male Hispanic |
Bilateral hands, thighs, knees, calves, ankles | 14/twice a day | 1 | None | None | 100% | 5 |
| 5 | NSV | 12 | Male Hispanic |
Periocular, perioral, bilateral hands, thighs, knees, calves | 1/twice a day | Partial repigmentation at 3 mo/premature discontinuation | None | CBC CMP WNL |
70% | 3 |
| 6 | NSV | 15 | Female White |
Bilateral upper portion of the arms, forearms, hands, thighs, knees, calves | 4/twice a day | 2 | None | None | 100% | 5 |
| 7 | NSV | 15 | Male White |
Face, hands | 5/every day | 3 | None | CBC CMP WNL |
100% | 6 |
| 8 | Mixed | 10 | Female Hispanic |
Knees (NSV) | 12/twice a day | 3 | NB UVB | None | 100% | 12 |
| Lower portion of the abdomen/left leg (SV) | 72/twice a day | No repigmentation | ||||||||
| 9 | SV | 5 | Male White |
Neck | 3/twice a day | No repigmentation/hair color preserved | None | None | 0% | 6 mo trial |
| 10 | SV | 11 | Male White |
Chest, back | 3/twice a day | 6 | NB UVB | None | 70% | 6 mo trial |
| 11 | SV | 14 | Male Male |
Right perioral,right chin | 1/twice a day | 1 | NB UVB Acne meds |
None | 95% (persistent poliosis) | 9 |
| 12 | SV | 16 | Male White |
Scalp, left side of the forehead/left periocular/left cheek | 1/twice a day | 1 | Dexamethasone pulsed therapy and NB UVB | CBC CMP TSH WNL |
99% (persistent poliosis) | 5 |
CBC, Complete blood count; CMP, complete metabolic profile; NB UVB= narrowband ultraviolet B phototherapy; NSV, nonsegmental vitiligo; SV, segmental vitiligo; TFT, thyroid function tests; TSH, thyroid stimulating hormone; UVB, ultraviolet B phototherapy; WNL, within normal limits.
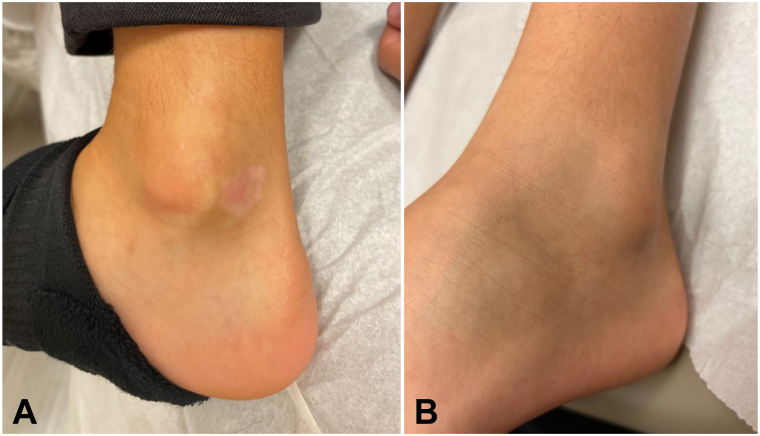
The NSV group and the nonsegmental component of the mixed type included 8 children, average age of 10.4 years (range 3-15 years). Treatment was once daily (n = 2) or twice-daily (n = 6). Average maximum repigmentation occurred at 6.4 months (range 3-12 months), with 6 achieving complete repigmentation and 1 with 70% repigmentation after 3 month trial. NSV was present on average for 6.1 months (1-12 months). Patient 4 is demonstrated in Fig 1 before and after treatment of the ankles. Onset of response was 2.25 months on average (range 1-3 months), starting on the face (n = 4), arms (n = 2), and abdomen (n = 1). No adverse events were reported. Five children had normal complete blood counts while on medication. Four patients previously failed tacrolimus topically, 2 patients failed topical class 2 corticosteroids, and 1 previously failed narrowband ultraviolet B light.
Fig 1.
A, B, Patient 4 before and after therapy.
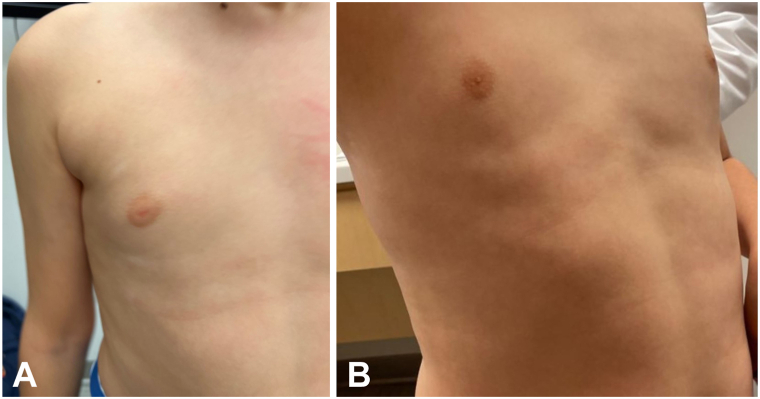
There were 5 patients with SV average age 11.2 years (range 5-16 years) including the segmental component of the mixed disease patient with facial (n = 1), face/neck (n = 1), neck (n = 1), chest/back (n = 1), and lower portion of the abdomen (n = 1). One 5-year-old neck SV failed to repigment after 6 months, but did not develop poliosis. A 10-year-old with segmental disease for 6 years had no response. One patient responded who had failed topical tacrolimus and class 2 topical corticosteroids (4 month trial). Maximal repigmentation required concurrent 308-nm laser for the facial SV (12 treatments), chest/back SV (12 treatments), and 1 NSV had concurrent 308-nm laser (24 treatments) with pulsed dexamethasone, without any notable adverse events. Two teenage males developed acne on sites of application on the face. Patient 10 is demonstrated in Fig 2 before and after treatment of the ankles laboratory screening including complete blood count was normal during treatment for 2 patients evaluated.
Fig 2.
A, B, Patient 10 before and after therapy.
Discussion
There is a growing body of evidence that the topical JAK inhibitor ruxolitinib 1.5% cream may be beneficial in selected cases of pediatric inflammatory skin disease. Data has been published from a recent placebo-controlled clinical trial of topical ruxolitinib 1.5% cream for pediatric atopic dermatitis in children ages 2 to 17 years. The patients had very few, mild adverse events, bone markers were not altered, and blood levels were very low, however, long-term data is lacking for both pediatric atopic dermatitis and vitiligo.3
In pediatric vitiligo, the FDA has approved ruxolitinib 1.5% cream twice a day for vitiligo in 12 years of age and older, for 10% or less body surface area, based on 2 phase 3 clinical trials, the TRuEV-1 and TRuEV-2 trials.2 Notably the trajectory of repigmentation is faster onset and rapid completion in children with NSV in our case series. We believe that part of the reason for the faster and more complete response is that therapy was instituted rapidly in the patients with NSV. Additionally, although only NSV has FDA approval, we note partial repigmentation with rapid institution of topical ruxolitinib 1.5% cream twice-daily and the addition of 308-nm laser treatments. Finally, we noted 2 cases of acne in teenagers, but otherwise no local adverse events and no laboratory abnormalities where applicable. Two patients had excellent response and we believe daily dosing can be considered in children to reduce drug exposure.
Limitations of this study are that the patients were neither randomized nor placebo-controlled, and we did not have blood levels of medication.
Although more systematic, randomized data is needed in children ages 2 to 11 years with vitiligo, it appears that the early institution of topical 1.5% ruxolitinib cream promotes rapid onset of repigmentation and rapid complete response in both NSV, and in the first 6 months of SV. This safety profile appears to be similar to that of recently presented data in children 2 to 11 years with atopic dermatitis, but the sample size is too small to make strong conclusions.4 Vehicle-controlled clinical trials of 1.5% topical ruxolitinib with and without narrowband ultraviolet B light sources for NSV and SV are needed to identify outliers and long-term outcomes.
Conflicts of interest
Dr Silverberg is a consultant, adviser, or speaker for Amryt, Incyte, Sanofi-Regeneron, Novan, and Verrica. Dr Lebwohl is an employee of Mount Sinai and receives research funds from AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara therapeutics, Dermavant Sciences, Eli Lilly, Incyte, Inozyme, Janssen Research & Development, LLC, Ortho Dermatologics, Pfizer, Sanofi-Regeneron, and UCB, Inc, and is a consultant for Almirall, AltruBio Inc, AnaptysBio, Apogee, Arcutis, Inc, AstraZeneca, Atomwise, Avotres Therapeutics, Brickell Biotech, Boehringer Ingelheim, Bristol-Myers Squibb, Castle Biosciences, Celltrion, CorEvitas, Dermavant Sciences, EPI, Evommune, Inc, Facilitation of International Dermatology Education, Forte biosciences, Foundation for Research and Education in Dermatology, Galderma, Genentech, Incyte, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Sanofi-Regeneron, Seanergy, Strata, Takeda, Trevi, and Verrica. Author Meister has no conflicts of interest to declare.
Footnotes
Funding sources: None.
Patient consent: The authors obtained written consent from patients for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available. Patient consent forms were not provided to the journal but are retained by the authors.
IRB approval status: Exempt.
References
- 1.Patel R., Pandya A.G., Sikirica V., et al. Prevalence of vitiligo among children and adolescents in the United States. Dermatology. 2023;239(2):227–234. doi: 10.1159/000528180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ezzedine K., Silverberg N. A practical approach to the diagnosis and treatment of vitiligo in children. Pediatrics. 2016;138(1) doi: 10.1542/peds.2015-4126. [DOI] [PubMed] [Google Scholar]
- 3.Rosmarin D., Passeron T., Pandya A.G., et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387(16):1445–1455. doi: 10.1056/NEJMoa2118828. [DOI] [PubMed] [Google Scholar]
- 4.Leung D.Y.M., Paller A.S., Zaenglein A.L., et al. Safety, pharmacokinetics, and efficacy of ruxolitinib cream in children and adolescents with atopic dermatitis. Ann Allergy Asthma Immunol. 2023;130(4):500–507.e3. doi: 10.1016/j.anai.2022.12.033. [DOI] [PubMed] [Google Scholar]