Abstract
Objective
Enhanced recovery after surgery (ERAS) is a protocol of evidence based practices applied in major surgery. Open aortic aneurysm repair is major surgery in terms of complications and mortality. This study aimed to compare early outcomes of ERAS with a traditional post-operative protocol in patients undergoing elective open aortic surgery.
Methods
This retrospective cohort study was conducted between 2018 – 2022 in two tertiary vascular surgery centres. The ERAS program was routinely implemented in one centre, while the other one used a standard peri-operative protocol. The primary outcome was post-operative length of stay (pLOS). Secondary outcomes were 30 day mortality rate, complications, re-interventions, and re-hospitalisations. Propensity score weighting was used to balance the two groups by comorbidities. Inverse probability of treatment weight (IPTW) was used to estimate the average treatment effect on the treated patients.
Results
A total of 198 patients were enrolled: 128 in the ERAS group (EG) and 70 in the standard group (SG). Mean age was 70.8 ± 6.7 years in EG and 71.1 ± 6.7 in SG (p = 0.39). No significant differences were observed in pre-operative cardiovascular risk factors. The median pLOS was 5 days (IQR 3, 6) in the EG group and 8 days (IQR 6, 11) in the SG group (p < 0.001). No differences in terms of mortality, re-operations, and re-hospitalisations were observed. The IPTW analyses showed a 40% reduction in pLOS and a significant reduction in major complications in EG (OR 0.41, 95% CI 0.26–0.66; p < 0.001). A 45% increase in pLOS in patients with chronic obstructive pulmonary disease was found in both groups.
Conclusion
Enhanced recovery after surgery is safe and feasible for elective open aortic surgery and is associated with earlier hospital discharge without differences in terms of mortality and lower complication rates compared with a standard protocol. Chronic obstructive pulmonary disease is a major risk factor for an increase in pLOS. The ERAS protocol is promising in terms of resource utilisation.
Keywords: ERAS, Enhanced recovery, Open aortic surgery, Post-operative length of stay
Highlights
-
•
No differences in mortality rate were observed when using a fast track protocol.
-
•
Reductions in post-operative length of stay and major complications were observed.
-
•
This protocol might be safe and feasible when applied to abdominal aortic surgery.
-
•
It is also promising in terms of resources utilisation.
Introduction
Enhanced recovery after surgery (ERAS) is a comprehensive peri-operative care pathway that uses evidence based best practices across specialties to achieve early recovery for patients undergoing major surgery.1 Enhanced recovery after surgery pathways have been implemented in different specialties, including cardiac and general surgery, urology, and gynaecology to standardise peri-operative care. Whereas each pathway is specific to its specialty, some fundamental concepts are the same: pre-operative patient counselling for expectation setting and medical and nutritional optimisation, intra-operative goal based fluid strategy, analgesia management, pre-emptive anti-emetic and bowel regimen, and early post-operative patient recovery. Although there is extensive evidence demonstrating the effectiveness of these pathways in other specialties, their application in vascular surgery is low. Advanced age and comorbidities of vascular surgery patients, the invasive nature of many operations, and difficult pre-operative optimisation can lead to high rates of post-operative complications, resulting in prolonged hospitalisation, rehabilitation needs, and hospital re-admissions.
The ERAS Society and Society for Vascular Surgery published a consensus statement, in 2022, on the use of enhanced recovery for patients undergoing open aortic vascular surgery.2 Docherty et al.3 reported a reduction in hospitalisation and complication rates with ERAS application in open aortic surgery in a meta-analysis comprising 10 studies. This meta-analysis included one randomis ed controlled trial (RCT) which was reported twice,4,5 presenting 99 patients treated about fifteen years ago, although the inclusion period was not specified in the study published in 2009.4 The RCT4 reported lower post-operative complications (16% vs. 36%; p = 0.039) and a shorter post-operative hospital stay in the fast track group (10 days vs. 11 days; p = 0.016). Furthermore, the prospective study by Giacomelli et al.5 used control patients treated by EVAR and a historical control group of standard open abdominal aortic aneurysm (AAA) repair who had undergone surgery during the six months before the study period. All the remaining studies were single centre and retrospective.
This study aimed to compare early contemporary outcomes of ERAS with a traditional post-operative protocol after open AAA surgery in two tertiary referral centres, with propensity score adjustment for risk factors.
Patients and methods
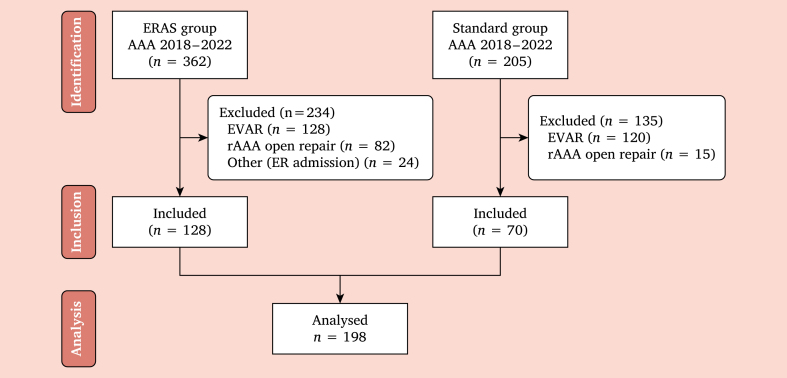
An observational retrospective cohort study was conducted in two tertiary vascular surgery centres, with vast experience in open aortic surgery, including patients treated between January 2018 – January 2022. They are both high volume centres for open and endovascular repair but apply different pre, peri, and post-operative protocols, as ERAS has routinely been implemented in only one unit, while the second centre applied a traditional post-operative protocol. All consecutive patients treated for aortic or aorto-iliac aneurysms in the elective setting were included. Urgent setting and thoraco-abdominal aortic aneurysm repairs were excluded. A STROBE-like flow chart describes the inclusion process (Fig. 1).
Figure 1.
STROBE-like flow chart describing the patient inclusion process in the two groups. ERAS = enhanced recovery after surgery; AAA = abdominal aortic aneurysm; rAAA = ruptured AAA; ER = emergency room; TAP = transversus abdominis plane.
The primary outcome was the post-operative length of stay (pLOS), defined as the time between surgery and discharge. Secondary outcomes were in hospital death, major complications, re-interventions, and re-hospitalisations within 30 days. Acute kidney injury (AKI) was defined as a twofold increase or more in serum creatinine level over the pre-operative value according to RIFLE criteria.6 Chronic kidney disease (CKD), coronary artery disease (CAD), and chronic obstructive pulmonary disease (COPD) were defined according to current guidelines.7, 8, 9 Major complications were defined as the need for significant surgical or medical interventions, prolonged or permanent disability or death, according to Chaikof et al.,10 and were considered as a composite outcome.
Fast track protocol
Pre-admission phase
All patients were evaluated pre-operatively by a team of surgeons and anaesthetists. The caregivers were also involved in this process. The patient was informed about pre-operative suspension of anticoagulant and antiplatelet therapy. In accordance with the Kidney Disease: Improving Global Outcomes recommendations,7 angiotensin converting enzyme inhibitors and angiotensin receptor antagonists were withheld 24 hours before surgery and re-started once normovolaemia had been achieved post-operatively. Bowel preparation consisted of a waste free diet the week before and two tablets of charcoal three times a day during the three days before surgery. Post-operative nausea and vomiting risk was evaluated with the Apfel score.11 The malnutrition screening tool was also calculated;12 therapeutic measures were applied if required. Need for post-operative intensive care unit (ICU) recovery was planned. Patients were informed of the program and learned how to use the pain valuation scale (numerical rating scale).
Pre-operative phase
All patients were allowed to eat up to six hours before the operation and drink clear fluids up to two hours before the operation.13 The analgesia strategy was planned (thoracic epidural catheter placement when feasible) as needed for post-operative ICU admission. Maltodextrins were administered three hours before surgery (except for patients with type I diabetes mellitus).14,15 Targeted surgical shaving and pre-operative shower were performed.
Intra-operative phase
The surgical approach consisted of a limited surgical incision, no evisceration, and no surgical drain (when feasible). General anaesthesia was performed with short acting drugs and curarisation was monitored with train of four. Fluid management was based on goal directed therapy. All patients were awakened in the operating room. Opioid sparing for post-operative analgesia was performed using epidural infusion.
Post-operative phase
In the afternoon of day 0, if the patient's general condition was stable, he or she drank 2–6 hours after surgery and ate a light diet from day 1. Once the patients were able to drink freely with no nausea, the intravenous fluids were discontinued. Patients mobilised from day 0, sitting for two hours in bed; they sat for four hours of bed from day 1. An oxygen mask (2 L/min O2) was provided during the first two post-operative nights. The urinary catheter was removed on day 1. Clinical parameters were recorded every four hours on the day of surgery and every six hours the day after. Laboratory tests were performed daily. Discharge home was determined according to standard criteria (tolerance to solid food, gas canalisation, absence of infection, and ambulation without assistance). Details of post-operative course and instructions for home discharge were given verbally and in writing; a telephone contact was also given if required.
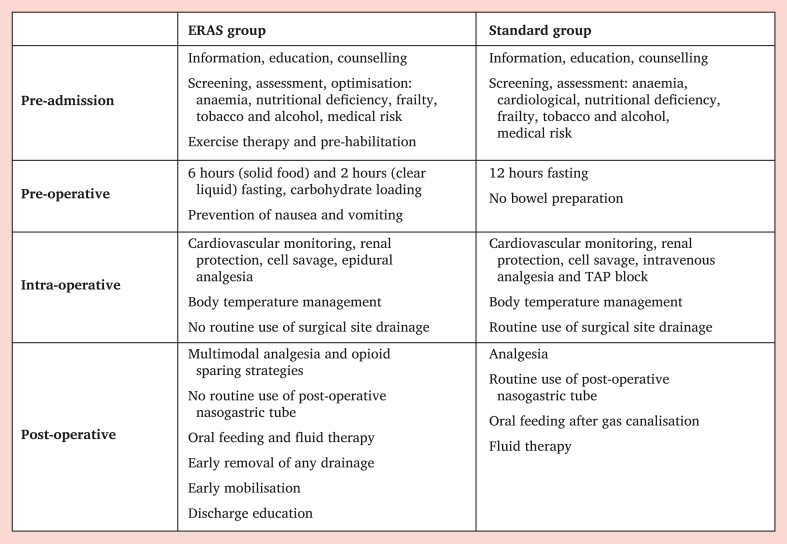
The peri-operative and post-operative care was based on current anaesthetic16,17 and vascular guidelines18 and on best medical practice care in the SG. Intensive care unit admission was planned pre-operatively for older patients, patients with multiple comorbidities, and those who had intra-operative complications (e.g., haemodynamic instability, need for multiple transfusions, prolonged operative time). Only patients with severe comorbidities or intra-operative complications were referred to ICU in the EG. The principal differences between the standard and ERAS protocol are reported in Fig. 2.
Figure 2.
Peri-operative protocol details in the ERAS and Standard groups. ERAS = enhanced recovery after surgery; TAP = Transversus abdominis plane block.
Data collection and statistical analysis
Data were collected prospectively in a dedicated database in both centres and a retrospective analysis was conducted. A descriptive statistical analysis was performed. Normality was assessed using the Shapiro–Wilk test or Kolmogorov–Smirnov test. Continuous variables were compared by Mann Whitney U test or unpaired t test and presented as mean ± standard deviation (SD) or median with interquartile range (IQR). Categorical variables were compared using Chi square test or Fisher's exact test.
A propensity score approach was employed to overcome the bias related to the observational nature of the study. The probability of ERAS was generated by a non-parsimonious logistic regression model including 10 variables listed in Table 1. Propensity score was employed to weight logistic and generalised linear regression models as a balancing method. Propensity score weighting had the advantage of using all the subjects in the two treatments groups for the outcome analysis compared with propensity score matching, with no loss of sample size. The inverse probability of treatment weight (IPTW) uses propensity scores to balance patient characteristics in the exposed and unexposed groups by weighting each individual in the analysis by the inverse probability of receiving their actual exposure. The balance between the weighted groups was evaluated with standardised mean differences. An absolute standardised difference < 0.1 was considered an acceptable balance between covariables.
Table 1.
Baseline characteristics and risk factors.
| Characteristics | EG (n = 128) | SG (n = 70) | p value |
|---|---|---|---|
| Age – y | 70.8 ± 6.7 | 71.1 ± 6.7 | 0.39 |
| Male | 120 (93.7) | 64 (91.4) | 0.57 |
| AAA diameter – mm | 57 ± 9.2 | 62 ± 15.4 | 0.005 |
| Smokers | 51 (39.8) | 26 (37.1) | 0.76 |
| Ex-smokers | 56 (43.8) | 29 (41.4) | 0.78 |
| Diabetes | 12 (9.4) | 7 (10) | 1.0 |
| Hypertension | 102 (79.7) | 59 (84.3) | 0.45 |
| Dyslipidaemia | 65 (50.8) | 30 (42.9) | 0.30 |
| CAD | 30 (23.4) | 12 (17.1) | 0.37 |
| CKD | 9 (7) | 10 (14.3) | 0.13 |
| COPD | 24 (18.8) | 11 (15.7) | 0.69 |
Data are presented as mean ± standard deviation (SD) or n (%). EG = ERAS group; ERAS = enhanced recovery after surgery; SG = standard group; AAA = abdominal aortic aneurysm; CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease.
Data are presented as n (%). EG = ERAS group; ERAS = enhanced recovery after surgery; SG = standard group.
Multivariable logistic regression modelling was applied to dichotomous outcomes, while the relationship between LOS and risk factors was evaluated with a multivariable generalised linear model. A forward stepwise variables selection for models was performed (probability of stay = 0.10, probability of entry = 0.05).
A p value < 0.050 was considered statistically significant. Propensity score was used to weight semi-parametric regression models (Cox and Fine models) as a balancing method. Propensity score weighting is one of the techniques used in controlling for selection biases in non-experimental studies, as well as propensity score matching and stratification on propensity score. Propensity scores can be used as weights to account for selection assignment differences between treatment and comparison groups. A post hoc power calculation of sample size was performed for the primary outcome, which showed a study power of 99.9% (1-β) with an alpha level of 0.050. Analyses were performed with R language (R 4.0.3; R Development Core Team 2020, http://www.R-project.org/) and SPSS Statistics v19 (IBM, Armonk, NY, USA).
Results
The study cohort included 198 patients: 128 in the ERAS group (EG, 120 males and eight females) and 70 in the standard group (SG, 64 males and six females). In the same period, EVAR (endovascular aneurysm repair) for elective aortic aneurysm was performed in 128 patients in the EG centre and in 120 patients in the SG centre.
Table 1 shows demographic characteristics of the two groups. No statistically significant differences were observed in pre-operative cardiovascular risk factors. The mean age was 70.8 ± 6.7 years in EG and 71.1 ± 6.7 in SG (p = 0.34). The mean aneurysm diameter was 56.9 ± 9.2 mm in EG and 62 ± 15.4 mm in SG (p = 0.005). An iliac aneurysm was associated with the aortic aneurysm in 12.5% of cases in EG and 17.1% in SG (p = 0.40). The prevalence of inflammatory aneurysm was 3.1% in EG and 5.7% in SG (p = 0.46).
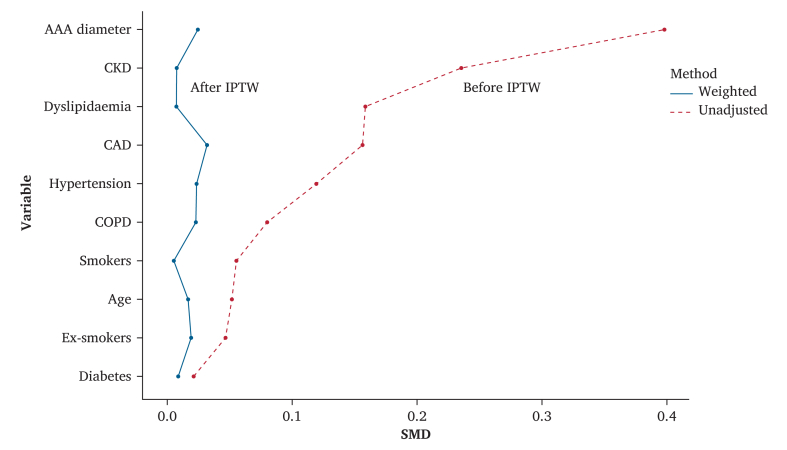
Intra-operative procedure details are reported in Table 2. The baseline characteristics of the study groups before and after IPTW are shown in Fig. 3. The distribution of baseline characteristics was well balanced between groups after IPTW, as demonstrated by the mean standardised differences.
Table 2.
Intra-operative procedure details.
| Details | EG (n = 128) | SG (n = 70) | p value |
|---|---|---|---|
| Surgical approach | |||
| Transperitoneal | 76 (59.4) | 63 (90) | <0.001 |
| Extraperitoneal | 41 (32) | 2 (2.9) | <0.001 |
| Subcostal | 11 (8.6) | 5 (7.1) | 0.79 |
| Procedure | |||
| Aorto–aortic | 84 (65.6) | 46 (65.7) | 0.88 |
| Aortobi–iliac | 31 (24.2) | 22 (31.4) | 0.40 |
| Aortobifemoral | 13 (10.2) | 2 (2.9) | 0.02 |
| Clamp site | |||
| Suprarenal | 17 (13.3) | 8 (11.4) | 0.83 |
| Infrarenal | 111 (86.7) | 62 (88.6) | 1.0 |
Figure 3.
Groups balancing for risk factors with IPTW based on propensity score. Y axis represents risk factors as dependent variables and X axis represents standardised mean difference (SMD) needed to assess covariable balance after matching. Continuous line represents matched groups weighted after propensity score. The dashed line represents unadjusted groups. An absolute standardised difference < 0.1 was considered an acceptable balance between covariables. IPTW = inverse probability of treatment weight; AAA = abdominal aortic aneurism; CKD = chronic kidney disease; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease.
The median pLOS was five days (IQR 3, 6) in the EG group and 8 days (IQR 6, 11) in the SG group (p < 0.001). Sixty patients (46.9%) in the EG group were discharged within the fourth post-operative day; hospitalisation lasted seven or more days in 21.9% of cases. All patients were discharged to home. Table 3 shows the final reduced multivariable generalised linear regression model before and after IPTW. Enhanced recovery after surgery benefitted pLOS by reducing it by 40%. Moreover, COPD was significantly associated with an increase in pLOS by 45%.
Table 3.
Multivariable model results for pLOS (days) with propensity score weighting.
| Factors | pLOS before IPTW | p value | pLOS after IPTW | p value |
|---|---|---|---|---|
| ERAS | −3.4 (−4.9–2.0) | <0.001 | −3.5 (−4.9–2.2) | <0.001 |
| COPD | 3.3 (1.6–5.1) | <0.001 | 3.9 (2.1–5.7) | <0.001 |
Data are presented as beta coefficient (95% CI). CI = confidence interval; pLOS = pre-operative length of stay; IPTW = inverse probability of treatment weight; ERAS = enhanced recovery after surgery; COPD = chronic obstructive pulmonary disease.
First, all single complications, death, re-operations, and re-hospitalisations in both groups were compared. Post-operative ICU admission rates were 48.4% in EG and 75.7% in SG (p < 0.001). No differences in terms of death, complications, re-operations, and rehospitalisations were observed, as shown in Table 4. Six patients died: four in EG and two in SG. Causes of death in EG were: one intestinal ischaemia, one intestinal perforation, one multiorgan failure due to acute kidney injury, and one ruptured spleen with early aorto-enteric fistula. One patient died of intra-operative haemorrhage due to a ruptured vena cava and one from disseminated intravascular coagulation in SG.
Table 4.
Thirty day post-operative complications, re-interventions, and re-hospitalisations.
| Complications | EG (n = 128) | SG (n = 70) | p value |
|---|---|---|---|
| Pancreatitis | 0 (0) | 2 (2.9) | 0.12 |
| Acute limb ischaemia | 7 (5.5) | 3 (4.3) | 1.0 |
| Acute myocardial infarction | 0 (0) | 0 (0) | – |
| Acute kidney injury | 15 (11.7) | 9 (12.9) | 0.82 |
| Stroke | 0 (0) | 0 (0) | – |
| Pneumonia and or ARDS | 6 (4.7) | 7 (10) | 0.23 |
| Gastrointestinal complications | 7 (5.5) | 8 (11.4) | 0.16 |
| Death | 4 (3.1) | 2 (2.9) | 1.0 |
| Re-interventions | 8 (6.3) | 8 (11.4) | 0.28 |
| Re-hospitalisations | 0 (0) | 1 (1.4) | 0.35 |
Data are presented as n (%). EG = ERAS group; ERAS = enhanced recovery after surgery; SG = standard group; ARDS = acute respiratory distress syndrome.
In the multivariable logistic model, after IPTW, the ERAS protocol was associated with a lower risk of major complications in the 30 day post-operative period (OR 0.41, 95% CI 0.26–0.66; p < 0.001) and a lower risk of ICU admission (OR 0.32, 95% CI 0.20–0.50; p < 0.001). Risk factors influencing post-operative outcome were COPD, CAD, and CKD (all p < 0.050). Furthermore, COPD and CKD became risk factors affecting the ICU admission rate (both with p < 0.050). The main results for complications and ICU admission in the multivariable model are shown in Table 5.
Table 5.
Multivariable model results for complications and ICU admission with propensity score weighting.
| Factors | Major complications after IPTW | p value |
|---|---|---|
| COPD | 2.8 (1.6–5.0) | <0.001 |
| CAD | 2.3 (1.2–4.2) | <0.001 |
| CKD | 2.8 (1.35.8) | <0.001 |
| ICU admission after IPTW | ||
| COPD | 6.1 (2.813.1) | <0.001 |
| CKD | 3.9 (1.69.8) | <0.001 |
Data are presented as odds ratio (95% CI). CI = confidence interval; ICU = intensive care unit; IPTW = inverse probability of treatment weight; COPD = chronic obstructive pulmonary disease; CAD = coronary artery disease; CKD = chronic kidney disease.
Discussion
This study compared patients undergoing open aortic surgery with two different peri-operative management protocols and found no significant difference in 30 day mortality rate. Complication rates are in line with current literature19 and, after propensity score weighting, a significantly lower peri-operative complication rate and shorter hospitalisation time were observed with the application of the ERAS protocol; COPD, CAD, and CKD seemed to have a negative influence on these outcomes.
Evidence supporting enhanced recovery after surgery protocols in surgery, including vascular surgery, has grown in recent years.4 These multimodal pathways have several goals, including: optimisation of pain control, avoiding the use of opioids, and early mobilisation and nutrition; all through pre-operative education and standardised intrahospital pathways. Fast track protocols in vascular surgery have not been widely adopted, despite the encouraging results highlighted in several papers.3 In 2022, the SVS formulated a consensus statement in accordance with the ERAS Society regarding the application of this protocol in abdominal aortic surgery.2 The fast track protocol adopted and described previous aims to reduce surgical stress, optimising pain therapy with the use of an epidural catheter, and to achieve an early recovery, reducing the hospitalisation time.
Pasin et al. registered almost double the number of pulmonary complications without fast track protocol application,20 while Malik et al. hypothesised that early mobilisation and pain control favoured the respiratory mechanics and may have contributed to a reduction in respiratory complications.21 In the current cohort, SG presented a higher, but not statistically significant, incidence of pulmonary complications (4.7% EG vs. 10% SG; p = 0.23); this may have been due to the small number of registered events, due both to small sample size and inclusion criteria. The current study considered complications to be pneumonia, need for tracheal reintubation, invasive or non-invasive ventilation, while Pasin et al. also included atelectasis, pleural effusion, prolonged mechanical ventilation, pneumothorax, pulmonary embolism, and haemothorax.20 Conversely, a possible confounding factor was the higher number of extraperitoneal accesses in EG (32% vs. 2.9%; p < 0.001), which may have contributed to the reduced respiratory complications in this group.14 In addition, COPD led to a 45% increase in pLOS in both groups, confirming the correlation between COPD and longer hospitalisation, again underlining the importance of post-operative physiotherapy and early mobilisation.22 The different surgical access described in the two groups may also represent a bias.
All patients included in this study were discharged home. The pLOS was significantly shorter in the EG (5 days, IQR 3, 6 vs. 8 days, IQR 6, 11; p < 0.001), with a mean reduction of three days of hospitalisation. This finding corroborates the recent literature regarding fast track protocols in vascular surgery.15,23 A 40% reduction in pLOS was found in the multivariable model with application of the ERAS protocol, with at home discharge within the fourth post-operative day in 46.9% of the included patients. The meta-analysis on fast track protocols after open aortic surgery published in 2022 by Docherty et al. confirmed these results in terms of pLOS and complication reduction with ERAS application.3 Even though the current study was multicentre, all papers included in this meta-analysis we re single centre studies. A strength of this analysis is that a direct comparison was performed between two groups of patients with similar risk factors, operated on in the same period, but managed with two different protocols.
Post-operative ICU admission was significantly more common in the SG than the EG (75.7% and 48.4%, respectively; p < 0.001). The ERAS protocol became an independent protective factor, with a 3.1 fold reduction in ICU admissions. Therefore, a post-operative non-ICU setting seems possible and safe with this type of procedure. Furthermore, in this analysis, COPD and CKD became risk factors affecting the ICU admission rate. A cost analysis was not carried out, but a reduction in ICU stay and hospitalisation days are essentials for reducing the costs related to this type of surgery.
Limitations
Limitations of this study were its retrospective nature and the two group coming from two different centres with different operators. The study design could have hidden some effects on outcomes due to other management factors in each single centre and not related to the ERAS protocol (risk of type II statistical error). As the ERAS protocol plans fewer ICU admissions during pre-operative evaluation compared with the standard protocol, this could have influenced the results on different ICU admission rates. There were also differences in the number of patients treated in the two groups (128 EG vs. 70 SG), patient characteristics such as AAA diameter, and surgical approach. Furthermore, there may have been biases related to data collection carried out in different centres and the diagnostic criteria of some risk factors and or complications.
Conclusions
The ERAS protocol is safe and feasible, without exclusion criteria for age or comorbidities, when applied to abdominal aortic surgery. According to the data it may be associated with lower complication rates and similar mortality rates compared with a standard procedure protocol, reducing the need for ICU admissions and post-operative length of stay. Chronic obstructive pulmonary disease was found to be a major risk factor for increased length of post-operative stay after AAA open repair.
The ERAS protocol is also promising in terms of resource utilisation. A cost effectiveness comparison between aortic open surgery applying ERAS and standard protocols with EVAR treatment should be undertaken.
Funding
None.
Conflict of interest
None.
References
- 1.Ljungqvist O., Scott M., Fearon K.C. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 2.McGinigle K.L., Spangler E.L., Pichel A.C., Ayyash K., Arya S., Settembrini A.M., et al. Perioperative care in open aortic vascular surgery: a consensus statement by the Enhanced Recovery After Surgery (ERAS) Society and Society for Vascular Surgery. J Vasc Surg. 2022;75:1796–1820. doi: 10.1016/j.jvs.2022.01.131. [DOI] [PubMed] [Google Scholar]
- 3.Docherty J., Morgan-Bates K., Stather P. A systematic review and meta-analysis of enhanced recovery for open abdominal aortic aneurysm surgery. Vasc Endovascular Surg. 2022;56:655–664. doi: 10.1177/15385744221098810. [DOI] [PubMed] [Google Scholar]
- 4.Muehling B., Schelzig H., Steffen P., Meierhenrich R., Sunder-Plassmann L., Orend K.H. A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World J Surg. 2009;33:577–585. doi: 10.1007/s00268-008-9892-2. [DOI] [PubMed] [Google Scholar]
- 5.Giacomelli E., Dorigo W., Campolmi M., Casini A., Fargion A., Bush R.L., et al. A pilot study of the enhanced recovery after surgery protocol in aortic surgery. J Vasc Surg. 2021;74:90–96.e2. doi: 10.1016/j.jvs.2020.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Lopes J.A., Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. 2013;6:8–14. doi: 10.1093/ckj/sfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KDIGO Evaluation and Management of Chronic Kidney Disease Guideline Summary. Guideline Central. Publication Date: January 1, 2013. Last Updated: March 14, 2022. Available from: https://www.guidelinecentral.com/guideline/25092.
- 8.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 9.Agustí A., Celli B.R., Criner G.J., Halpin D., Anzueto A., Barnes P., et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD Executive Summary. Arch Bronconeumol. 2023;59:232–248. doi: 10.1016/j.arbres.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Chaikof E.L., Blankensteijn J.D., Harris P.L., White G.H., Zarins C.K., Bernhard V.M., et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 11.Apfel C.C., Heidrich F.M., Jukar-Rao S., Jalota L., Hornuss C., Whelan R.P., et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:742–753. doi: 10.1093/bja/aes276. [DOI] [PubMed] [Google Scholar]
- 12.Lomivorotov V.V., Efremov S.M., Boboshko V.A., Nikolaev D.A., Vedernikov P.E., Deryagin M.N., et al. Prognostic value of nutritional screening tools for patients scheduled for cardiac surgery. Interact Cardiovasc Thorac Surg. 2013;16:612–618. doi: 10.1093/icvts/ivs549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith I., Kranke P., Murat I., Smith A., O'Sullivan G., Søreide E., et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556–569. doi: 10.1097/EJA.0b013e3283495ba1. [DOI] [PubMed] [Google Scholar]
- 14.Feo C.V., Portinari M., Tsolaki E., Romagnoni G., Verri M., Camerani S., et al. The effect of an enhanced recovery program in elective retroperitoneal abdominal aortic aneurysm repair. J Vasc Surg. 2016;63:888–894. doi: 10.1016/j.jvs.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 15.McGinigle K.L., Eldrup-Jorgensen J., McCall R., Freeman N.L., Pascarella L., Farber M.A., et al. A systematic review of enhanced recovery after surgery for vascular operations. J Vasc Surg. 2019;70:629–640.e1. doi: 10.1016/j.jvs.2019.01.050. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen S.D., Knuuti J., Saraste A., Anker S., Bøtker H.E., Hert S.D., et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA) Eur Heart J. 2014;35:2383–2431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 17.Lindholm E.E., Aune E., Norén C.B., Seljeflot I., Hayes T., Otterstad J.E., et al. The Anesthesia in Abdominal Aortic Surgery (ABSENT) s tudy: a prospective, randomized, controlled trial comparing troponin T release with fentanyl–sevoflurane and propofol–remifentanil anesthesia in major vascular surgery. Anesthesiology. 2013;119:802–812. doi: 10.1097/ALN.0b013e31829bd883. [DOI] [PubMed] [Google Scholar]
- 18.Wanhainen A., Herzeele I.V., Goncalves F.B., Montoya S.B., Berard X., Boyle J.R., et al. Editor's choice – European society for vascular surgery (ESVS) 2024 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2024;67:192–331. doi: 10.1016/j.ejvs.2023.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Trenner M., Kuehnl A., Salvermoser M., Reutersberg B., Geisbuesch S., Schmid V., et al. Editor's Choice – high annual hospital volume is associated with decreased in hospital mortality and complication rates following treatment of abdominal aortic aneurysms: secondary data analysis of the nationwide German DRG statistics from 2005 to 2013. Eur J Vasc Endovasc Surg. 2018;55:185–194. doi: 10.1016/j.ejvs.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Pasin L., Nardelli P., Landoni G., Beretta L., Piras D., Baccellieri D., et al. Enhanced recovery after surgery program in elective infrarenal abdominal aortic aneurysm repair. J Cardiovasc Surg. 2019;60:369–374. doi: 10.23736/S0021-9509.16.09194-1. [DOI] [PubMed] [Google Scholar]
- 21.Malik K., Poletto G., Musto L., Giustiniano E., Cecconi M., Civilini E. Implementation of a perioperative protocol to enhance open aortic repair. J Vasc Surg. 2021;74:434–441.e2. doi: 10.1016/j.jvs.2020.12.102. [DOI] [PubMed] [Google Scholar]
- 22.Smetana G.W. Preoperative pulmonary evaluation. N Engl J Med. 1999;340:937–944. doi: 10.1056/NEJM199903253401207. [DOI] [PubMed] [Google Scholar]
- 23.Chisci E., Simongini S., Lazzarotto T., Ercolini L., Frosini P., Nerini A., et al. Lessons learned with enhanced recovery for open abdominal aortic aneurysm surgery: a long term regional network experience. Eur J Vasc Endovasc Surg. 2024;S1078–5884(24) doi: 10.1016/j.ejvs.2024.07.033. 00645-2. [DOI] [PubMed] [Google Scholar]