To the Editor,
The integration of mass spectrometry – with its fundamental advantages over previously implemented analytical technologies – into the streamlined workflows of routine high-throughput clinical laboratories is a highly attractive goal in laboratory medicine [1], [2], [3]. A fully automated mass spectrometry system for the clinical routine laboratory is being developed by a major manufacturer of laboratory diagnostics and is now close to being launched on the market. In our laboratory, an advanced prototype of this system was tested for the first time under intended-user conditions in a clinical laboratory.
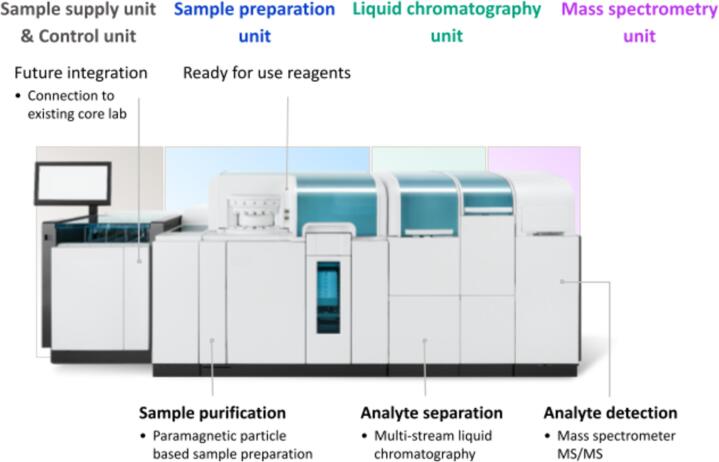
The system under evaluation, Cobas® Mass Spec solution2 (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) (Fig. 1), was designed to be a fully automated and integrated liquid chromatography-tandem mass spectrometry (LC-MS/MS) solution with a broad in vitro diagnostics test menu. At the time of the study execution, both the reagents and the analyzer were still under development. The study was carried out in the second half of 2022 at the Institute of Laboratory Medicine, LMU University Hospital, LMU Munich, Germany, according to a pre-specified study program and after approval from the Ethics Committee. The study focused on assessing the system's functionality, including the interaction between software, hardware, reagents, auxiliaries, and biological samples; evaluating practicability; assessing robustness; and examining sample throughput under intended user random access conditions in a stand-alone configuration. It was not within the scope of the prototype study to assess analytical performance data; this will be the subject of subsequent studies.
Fig. 1.
Prototype of an automated LC-MS/MS clinical analyzer system under study.
Sample preparation was based on paramagnetic particles with specified surfaces [4], including immunocapture functionalities. For sample concentration in some assays, an integrated evaporation station was used. Stable-isotope labeled compounds served as internal standards for quantification. Internal standard solutions, paramagnetic particles, and buffer solutions were delivered in a three-chamber container configuration identical to an already routinely used system of the same manufacturer. The instrument incorporated two distinct chromatographic configurations: one for conventional HPLC and a second based on Rapid LC for very fast matrix fractionation and separation, both featuring several HPLC streams configured in parallel. A triple-quadrupole mass spectrometer was employed for analyte detection and quantification.
For calibration of the assays, a pre-defined master calibration curve was adjusted using a two-point calibrator set. Each assay was calibrated at the beginning of the study, and subsequent calibrations were performed based on preliminary stability data for each assay and the accuracy of quality control materials. Evaluation of chromatograms and quantification were conducted fully automatically by the system, without peak review or interpretation of metadata by the operator. Data management included downloading requests from a host and uploading results.
The following analytes were addressed in diverse experiments during the study: Tacrolimus, Cyclosporine, Sirolimus, Everolimus, Mycophenolic Acid and its glucuronide, Voriconazole, Lamotrigine, Meropenem, Estradiol, Testosterone, and 25-OH- Vitamin D2/3. For evaluation, anonymized leftover samples from the institute’s routine service were used. All analyses were controlled via host-instrument interaction, with request downloads and result uploads to a laboratory information system-like tool. Routine simulations were performed in batch mode and random-access working mode, assessing throughput and turnaround time.
It was found that a three-day training session for laboratory technicians was sufficient to operate the system successfully, without requiring any previous skills in mass spectrometry or chromatography. Practicability at the level of state-of-the-art high-throughput analytical systems for clinical laboratories was observed, featuring automated timed start-up procedures, automatic shutdown, and extended walk-away times.
The time to first result was 37 min. We observed the quantification of mycophenolic acid (MPA) in a batch of 200 plasma samples within 2 h and 37 min. A throughput of up to 100 samples per hour was also noted in random access mode with nine different analytes. We calculated that our conventional mass spectrometry routine methods with manual handling would have required about 10-fold more time for processing, 5-fold more time for measurement, and 10-fold more time until the results were available.
Systematic evaluation of calibration stability of the assays and other aspects of robustness are addressed in ongoing studies of the final launch-version of the system.
Based on the results of this scientific interim method development report and our observations and handling experience, we believe that further development and maturation of the system under investigation will most likely lead to a fully automated LC-MS/MS analyzer system suitable for use in high-throughput routine clinical laboratories.
Ethics statement
As only fully anonymized patient samples were used that were not obtained specifically for use in this study through an interaction or intervention with living individuals, neither informed consent nor IRB review were required; however, approval from Ethics Committee of the Ludwig-Maximilians-University Munichwas obtained.
Funding support
The study was sponsored by Roche Diagnostics Germany GmbH, Mannheim, Germany.
CRediT authorship contribution statement
Michael Vogeser: Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Katharina Habler: Writing – review & editing, Validation, Project administration, Investigation, Data curation. Antje Reuter: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Christian Schneider-Thauern: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CST and AR are employees of Roche Diagnostics Germany. CST and AR hold non-voting equity securities in Roche. MV and KH receive research grants and honoraria for scientific presentations from Roche Diagnostics.
Acknowledgements
The authors thank the technicians and support staff at their respective institutions for their contributions to this study.
Footnotes
Cobas Mass Spec solution (including Cobas i601 analytical unit and Ionify reagent line) is in development, not approved by regulatory bodies, and not commercially available. COBAS and IONIFY are trademarks of Roche.
References
- 1.Adaway J.E., Keevil B.G., Owen L.J. Liquid chromatography tandem mass spectrometry in the clinical laboratory. Ann. Clin. Biochem. 2015;52(Pt 1):18–38. doi: 10.1177/0004563214557678. Epub 2014 Oct 13 PMID: 25313226. [DOI] [PubMed] [Google Scholar]
- 2.Vogeser M., Zhang Y.M. Understanding the strategic landscape surrounding the implementation of mass spectrometry in the clinical laboratory: a SWOT analysis. Clin. Mass Spectrometry. 2018;9:1–6. doi: 10.1016/j.clinms.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junger S., Hoene M., Shipkova M., Danzl G., Schöberl C., Peter A., Lehmann R., Wieland E., Braitmaier H. Automated LC-MS/MS: ready for the clinical routine laboratory? J Mass Spectrom Adv Clin Lab. 2023;2(30):1–9. doi: 10.1016/j.jmsacl.2023.07.001. PMID: 37583571; PMCID: PMC10423925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogeser M., Geiger A., Herrmann R., Kobold U. Sample preparation for liquid chromatography-tandem mass spectrometry using functionalized ferromagnetic micro-particles. Clin. Biochem. 2008;41(16–17):1417–1419. doi: 10.1016/j.clinbiochem.2008.08.001. Epub 2008 Aug 13 PMID: 18755176. [DOI] [PubMed] [Google Scholar]