Abstract
Klebsiella pneumoniae is a pathogenic bacterium responsible for otorhinolaryngology-head and neck infections. Hypervirulent K. pneumoniae (hvKp), an alarming subtype of K. pneumoniae, causes life-threatening hematogenous infection. However, there are few reports on the character of hvKp strain in the field of otorhinolaryngology-head and neck surgery. We report the case of a 60-year-old Japanese man with a peritonsillar abscess caused by hvKp. K. pneumoniae isolated from pus was positive in a string test. Genetic analysis revealed that the strain had K2, rmpA and aerobactin genes. There was no evidence of hematogenous infections such as bacteremia and liver abscess, and there was improvement by surgical drainage and intravenous antimicrobial treatment. To the best of our knowledge, this is the first reported case of peritonsillar abscess caused by hvKp that did not have hematogenous infections. The string test is a simple and inexpensive method for screening hvKp. This case highlights the need for strategies to inhibit the spread of these highly virulent strains by early drainage and appropriate antimicrobial treatment.
Keywords: Hypervirulent Klebsiella pneumoniae, Peritonsillar abscess
Highlights
-
•
Peritonsillar abscess is a common, potentially life-threatening disease.
-
•
Hypervirulent K. pneumoniae can cause upper respiratory tract infection.
-
•
String test is useful for screening, but detection of virulent factors is required.
-
•
Ruling out systemic infection is highly recommended.
-
•
Early drainage and appropriate antimicrobial treatment are highly recommended.
Introduction
Peritonsillar abscess (PTA) is a common deep-neck infection. Despite being rare, PTA is potentially life-threatening, so early diagnosis is crucial. Appropriate antimicrobial treatment and surgery to remove the abscess are essential. The most frequent pathogens identified from pus in patients with PTA are Streptococcus viridans and anaerobes such as Prevotella and Fusobacterium spp [1]. The prevalence of Klebsiella pneumoniae has not been widely reported, but there are increasing reports showing detection of K. pneumoniae in patients with abscesses in the field of otorhinolaryngology-head and neck surgery [1].
K. pneumoniae is a Gram-negative, non-motile, encapsulated, anaerobic bacteria. Classical K. pneumoniae (cKp) is known to be an opportunistic pathogen capable of causing life-threatening infections including pneumonia, urinary tract infection, abdominal cavity infection and intravascular device infection in immunocompromised patients or those frequently exposed to healthcare facilities [2], [3]. Hypervirulent K. pneumoniae (hvKp) is a distinct and alarming subtype. Since its initial documentation in the 1980s, it has been predominantly reported in East and South-East Asia [2], [4]. The pathogen has since demonstrated a remarkable ability to disseminate and establish itself globally.
We present a case of PTA caused by a hvKp strain. This is the first known report of hvKp developing locoregional invasive infection in otorhinolaryngology-head and neck surgery without any metastatic infections.
Case presentation
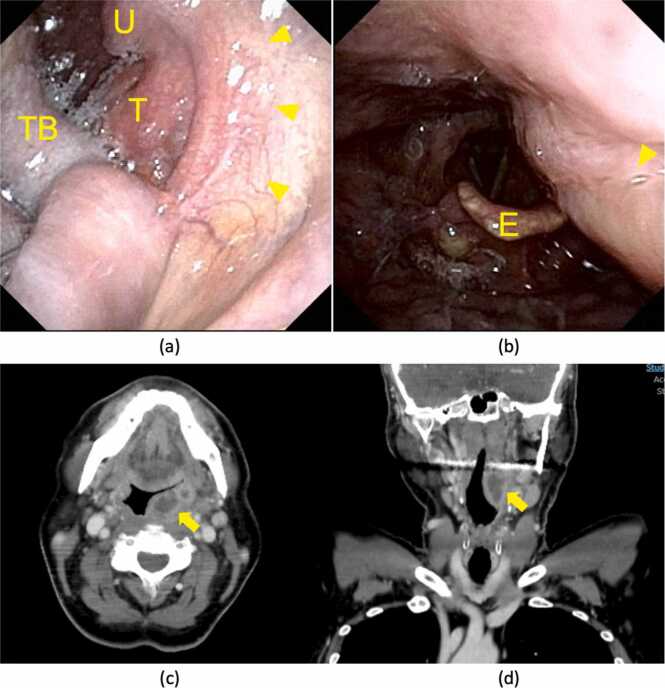
A sixty-year-old Japanese man without notable medical history presented with a sore throat for two days. He had marked swelling and redness of the left peritonsillar tissue and trismus without enlarged or tender cervical lymph nodes (Fig. 1a). Laryngoscopy also revealed swelling of the left lateral wall of the mesopharynx, but there were no findings in the hypopharynx or larynx (Fig. 1b).
Fig. 1.
Clinical findings. Pharyngeal findings by fiberscope via the oral cavity (a) and via the nasal cavity (b). The left tonsil and the lateral pharyngeal wall were markedly swollen (arrowhead). Contrast-enhanced CT of pharynx and neck. (c) axial view (d) coronal view. 1.0 × 2.5 × 2.5 cm PTA is indicated by the arrow. E; epiglottis, T; left tonsil, TB; tongue base, U; uvula.
Contrast-enhanced computed tomography (CT) showed 1.0 × 2.5 × 2.5 cm of left-side PTA (Fig. 1c, 1d). We performed transoral drainage of the abscess under local anesthesia. The patient was then treated with intravenous ampicillin/sulbactam (9 g/day) for six days and intravenous hydrocortisone (300 mg/day) for the first three days. Blood examination showed an undiagnosed glucose intolerance (HbA1c of 6.7 %).
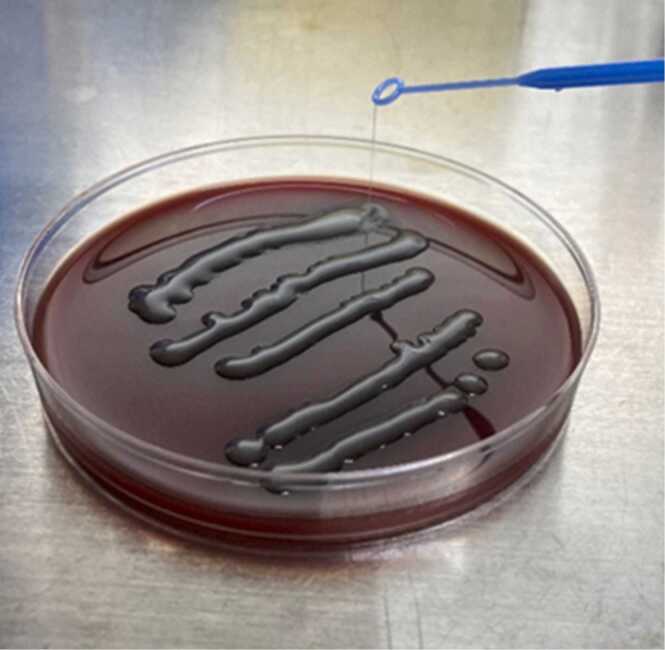
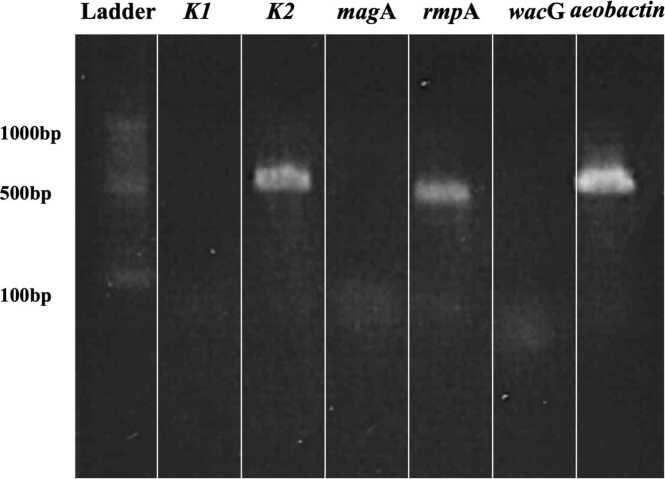
A strain of K. pneumoniae was identified by using matrix assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF/MS) in a bacterial culture of pus from the PTA. The strain showed to be hypermucoviscous by a string test (Fig. 2). Serotyping and identification of virulence factors by polymerase chain reaction (PCR) identified K2, rmpA and aerobactin (Fig. 3). Primer sequences were based on previous literature and displayed in Table 1 [5], [6], [7], [8]. Drug susceptibility test (DST) by broth microdilution method did not show any drug resistance (Table 2). Multilocus sequence typing (MLST) was performed using seven housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB) according to the method of previous reports [9]. The current strain was identified as ST3690 by reference for an MLST database for K. pneumoniae (https://pubmlst.org/mlst). Blood culture and liver ultrasonography demonstrated that he had neither bacteremia nor liver abscess. A year after treatment, the patient had not had recurrence of the PTA. The patient provided written informed consent for publication of this case report and accompanying images.
Fig. 2.
String test. String test was positive. The string test in an isolated colony of K. pneumoniae from the CSF on an agar plate, which showed hypermucoviscosity with a >5-mm-long viscous filament.
Fig. 3.
Genetic analysis. k2 (serotype), rmpA and aerobactin gene (virulent factor) were detected in PCR study of the extracted DNA.
Table 1.
Primer sequences.
| Gene | Primer name | Primer sequence (5′ to 3′) | Reference |
|---|---|---|---|
| K1 | K1 forward | GTAGGTATTGCAAGCCATGC | [5] |
| K1 reverse | GCCCAGGTTAATGAATCCGT | ||
| K2 | K2 forward | CAACCATGGTGGTCGATTAG | [6] |
| K2 reverse | TGGTAGCCATATCCCTTTGG | ||
| magA | magA forward | GGTGCTCTTTACATCATTGC | [6] |
| magA reverse | GCAATGGCCATTTGCGTTAG | ||
| rmpA | rmpA forward | CATAAGAGTATTGGTTGACAG | [6] |
| rmpA reverse | CTTGCATGAGCCATCTTTCA | ||
| wcaG | wcaG forward | GGTTGGKTCAGCAATCGTA | [7] |
| wcaG reverse | ACTATTCCGCCAACTTTTGC | ||
| aerobactin | aerobactin forward | GCATAGGCGGATACGAACAT | [8] |
| aerobactin reverse | CACAGGGCAATTGCTTACCT |
Primers for serotyping and detecting virulence factors were listed.
Table 2.
Drug susceptibility test.
| Drug | Minimum inhibitory concentration (µg/mL) | *Susceptibility |
|---|---|---|
| Meropenem | ≤0.06 | susceptible |
| Ceftriaxone | ≤1 | susceptible |
| Levofloxacin | ≤0.12 | susceptible |
| Ampicillin/Sulbactam | 4 | susceptible |
| Tazobactam/Piperacillin | ≤2 | susceptible |
| Cefepime | ≤0.5 | susceptible |
| Amikacin | ≤4 | susceptible |
Susceptibility for each drug was determined by Clinical Laboratory Standards Institute (CLSI).
Discussion
Detection of K. pneumoniae as a causative pathogen in infectious diseases in otorhinolaryngology head and neck surgery is considered to be relatively rare. The nasopharyngeal colonization rate has been reported to be 15 % [2]. This case suggests that hvKp is a pathogen of concern for PTA.
K. pneumoniae has been associated with aspiration pneumonia or liver abscess in immunocompromised patients and in patients with diabetes [10], [11]. Elderly patients with PTA are also reported to be at high risk of K. pneumoniae infection [1]. Unlike cKp, hvKp are capable of causing life-threatening metastatic infections spreading via the bloodstream including liver abscesses, meningitis, brain abscesses and necrotizing fasciitis. Even in healthy individuals in the community, the mortality rate is estimated to be 3–42 % [11]. This new type of K. pneumoniae was firstly reported in 1986 from Taiwan as a liver abscess associated with metastatic septic endophthalmitis [11]. Recent studies have shown the prevalence of hvKp amongst cases of K. pneumoniae to be as high as 12–45 % mainly in East Asia [3].
In previous reports, there are three cases of infection caused by hvKp in the field of otorhinolaryngology-head and neck surgery. The cases of deep neck abscess and Lemierre’s syndrome were reported and all cases were described to be positive for blood culture [12], [13], [14] (Table 3). However, these cases presented no pharyngo-laryngeal findings and were considered to be spread by hematogenous metastasis from the infected site. Locoregional virulence of hvKp at the colonized site in the field of otorhinolaryngology-head and neck surgery has not been previously reported. Our case showed locoregional abscess without evidence of hematogenous infections including bacteremia and liver abscess. The characteristics of the strain isolated in the present case may be related to the course of infection.
Table 3.
Reported cases of hvKp infection in head and neck region.
| Reference | Country | Age | Sex | Findings in head and neck | Complications | Blood culture | Underlying disease (s) | Capsule strain | Sequence type | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Lee SE. 2021 [12] | United States | 63 | Female | Lemierre’s syndrome | Suppurative lymphadenitis | Positive | Diabetes mellitus | K2 | 65 | Survived |
| McHardy JA. 2021 [13] | United Kingdom | 53 | Male | Deep neck abscess | None | Positive | Diabetes mellitus Pulmonary tuberculosis |
K20 | 420 | Survived |
| Lan P. 2021 [14] | China | 74 | Male | Deep neck abscess | None | Positive | Diabetes mellitus Liver cirrhosis |
K5 | 1049 | Survived |
| Present case | Japan | 60 | Male | Peritonsillar abscess | None | Negative | Diabetes mellitus | K2 | 3690 | Survived |
Characteristics of reported four cases with hvKp infection in head and neck region including current case were listed.
HvKp is highly mucoviscous and is determined by string test, which evaluates the viscosity of K. pneumoniae. The hyper-mucoviscosity aids in evading the host’s immune defenses and facilitating colonization. A string test is a simple and inexpensive screening test for detecting hvKp. However, some studies described that 17–23 % of putative cKp isolates had a positive string test result [15]. It is important to recognize that hyper-mucoviscosity alone does not necessarily lead to invasive disease in immunocompetent individuals [15]. The identification of genetic biomarker is required.
Serotyping and identification of virulence factors were performed using the strains isolated in this study. K2, rmpA and Aerobactin were identified as virulence factors. HvKp strain frequently carries genes encoding for a highly efficient capsular polysaccharide (K antigen) that provides resistance against phagocytosis. K1 and K2 strains have been widely reported in cases of hvKp. K1 strains are reportedly more prone to hematogenous infection than K2 strains [3], [16]. Therefore, the infection was thought to be limited in this case to PTA, a localized infection, reflecting a characteristic of the K2 strain. The current strain, ST3690, was detected in a patient with sepsis that developed from severe pneumonia in a previous report [17]. Although ST3690 is less prevalent to major sequence types such as ST23 and ST65 among hvKp strains, it is reported to be relatively prevalent in East Asia and be associated with highly invasive infections [18].
In addition, hvKp possesses several iron acquisition systems and factors contributing to the bacteria's resistance to phagocytosis and complement-mediated killing. Aerobactin has been reported as a factor of iron acquisition system [19]. The gene rmpA is recognized as a player in the enhancement of virulence by regulating the production of capsular polysaccharide and it is also associated with the hyper-mucoviscous phenotype, which is detectable by using string test [3].
Aerobactin has high sensitivity in hyperviscous Klebsiella and rmpA has high specificity in highly pathogenic Klebsiella [20]. These virulence attributes underscore the formidable pathogenic potential of hvKp and its ability to cause life-threatening invasive infections [2].
HvKp is usually intrinsically resistant to ampicillin, but it is surprisingly sensitive to the most commonly used antimicrobials, generally with favorable outcomes [2]. Antimicrobial susceptibility patterns of hvKp have remained consistent since the first report. However, there has been an alarming increase in the prevalence of antibiotic-resistant hvKp strains in recent years [2]. Furthermore, virulent genes associated with hvKp have also been identified in cKp, leading to outbreaks of hypervirulent strains. To address this new threat, urgent and comprehensive strategies are needed to inhibit the spread of these resistant and highly virulent strains.
In conclusion, we reported the first known case in which hvKp positive for K2, rmpA, and Aerobactin was detected in a patient with peritonsillar abscess. We believe that the clinical course may be predicted by searching for serotype and pathogen type if the pathogen is string test positive.
CRediT authorship contribution statement
Kazuya Mizobata: Writing - original draft, Data curation. Daichi Murakami: Writing - original draft, Conceptualization. Ryo Ueda: Data curation. Yuki Suzuki: Formal analysis. Yusuke Koizumi: Writing - review & editing, Validation. Hisakazu Yano: Formal analysis, Validation. Masamitsu Kono: Writing - review & editing, Validation. Muneki Hotomi: Writing - review & editing, Supervision.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the Wakayama Medical University Hospital Ethics Committee, Wakayama, Japan, no. 3837.
Consent
Informed consent was obtained from the patient involved in the study.
Funding
We did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We acknowledge proofreading and editing by Benjamin Phillis at the Clinical Study Support Center at Wakayama Medical University.
References
- 1.Tsai Y.W., Liu Y.H., Su H.H. Bacteriology of peritonsillar abscess: the changing trend and predisposing factors. Braz J Otorhinolaryngol. 2018;84:532–539. doi: 10.1016/j.bjorl.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo T.A., Marr C.M. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. 2019;32:e00001–e00019. doi: 10.1128/CMR.00001-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choby J.E., Howard-Anderson J., Weiss D.S. Hypervirulent Klebsiella pneumoniae - clinical and molecular perspectives. J Intern Med. 2020;287:283–300. doi: 10.1111/joim.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee C.R., Lee J.H., Park K.S., Jeon J.H., Kim Y.B., Cha C.J., Jeong B.C., Lee S.H. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front Cell Infect Microbiol. 2017;7:483. doi: 10.3389/fcimb.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang C.T., Lai S.Y., Yi W.C., Hsueh P.R., Liu K.L., Chang S.C. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45(3):284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 6.Compain F., Babosan A., Brisse S., Genel N., Audo J., Ailloud F., Kassis-Chikhani N., Arlet G., Decré D. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol. 2014;52(12):4377–4380. doi: 10.1128/jcm.02316-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turton J.F., Perry C., Elgohari S., Hampton C.V. PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J Med Microbiol. 2010;59(Pt 5):541–547. doi: 10.1099/jmm.0.015198-0. [DOI] [PubMed] [Google Scholar]
- 8.Yu W.L., Ko W.C., Cheng K.C., Lee C.C., Lai C.C., Chuang Y.C. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn Microbiol Infect Dis. 2008;62(1):1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Kakuta N., Nakano R., Nakano A., Suzuki Y., Masui T., Horiuchi S., Kakuta R., Tsubaki K., Ogawa M., Yano H. Molecular characteristics of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in Japan: predominance of CTX-M-15 and emergence of hypervirulent clones. Int J Infect Dis. 2020;98:281–286. doi: 10.1016/j.ijid.2020.06.083. [DOI] [PubMed] [Google Scholar]
- 10.Siu L.K., Yeh K.M., Lin J.C., Fung C.P., Chang F.Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Liu Y.C., Lin D.L. CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146(10):1913–1916. doi: 10.1001/archinte.1986.00360220057011. [DOI] [PubMed] [Google Scholar]
- 12.Lee S.E., Mushtaq A., Gitman M., Paniz-Mondolfi A., Chung M., Obla A., Sordillo E.M., Nowak M.D., van Bakel H., Ramírez J.D., Muñoz M., Lee M. Lemierre's syndrome associated with hypervirulent Klebsiella pneumoniae: a case report and genomic characterization of the isolate. IDCases. 2021;25 doi: 10.1016/j.idcr.2021.e01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McHardy J.A., Selvaganeshapillai V., Khanna P., Whittington A.M., Turton J., Gopal Rao G. A case of neck abscess caused by rare hypervirulent Klebsiella pneumoniae, capsular type K20 and sequence type 420. Ann Clin Microbiol Antimicrob. 2021;20(1):46. doi: 10.1186/s12941-021-00453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan P., Zhao D., Gu J., Shi Q., Yan R., Jiang Y., Zhou J., Yu Y. Genome-based analysis of a sequence type 1049 hypervirulent Klebsiella pneumoniae causing bacteremic neck abscess. Front Microbiol. 2021;11 doi: 10.3389/fmicb.2020.617651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catalán-Nájera J.C., Garza-Ramos U., Barrios-Camacho H. Hypervirulence and hypermucoviscosity: two different but complementary Klebsiella spp. phenotypes? Virulence. 2017;8:1111–1123. doi: 10.1080/21505594.2017.1317412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X., Sun Q., Li J., Jiang Y., Li Y., Lin J., Chen K., Chan E.W., Zhang R., Chen S. Molecular epidemiology of carbapenem-resistant hypervirulent Klebsiella pneumoniae in China. Emerg Microbes Infect. 2022;11:841–849. doi: 10.1080/22221751.2022.2049458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parrott A.M., Shi J., Aaron J., Green D.A., Whittier S., Wu F. Detection of multiple hypervirulent Klebsiella pneumoniae strains in a New York City hospital through screening of virulence genes. Clin Microbiol Infect. 2021;27(4):583–589. doi: 10.1016/j.cmi.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Sohrabi M., Alizade Naini M., Rasekhi A., Oloomi M., Moradhaseli F., Ayoub A., Bazargani A., Hashemizadeh Z., Shahcheraghi F., Badmasti F. Emergence of K1 ST23 and K2 ST65 hypervirulent Klebsiella pneumoniae as true pathogens with specific virulence genes in cryptogenic pyogenic liver abscesses Shiraz Iran. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.964290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J., Zhang H., Liao X. Hypervirulent Klebsiella pneumoniae. Infect Drug Resist. 2023;16:5243–5249. doi: 10.2147/IDR.S418523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G., Sun S., Zhao Z.Y., Sun Y. The pathogenicity of rmpA or aerobactin-positive Klebsiella pneumoniae in infected mice. J Int Med Res. 2019;47:4344–4352. doi: 10.1177/0300060519863544. [DOI] [PMC free article] [PubMed] [Google Scholar]