Abstract
Contagious caprine pleuropneumonia (CCPP) in goats is defined as a highly contagious and rapidly spreading mycoplasmal disease that is now among the leading causes of major economic losses on many continents (Asia, Africa and the Middle East). In this study, we aimed to evaluate immunohistochemically mast cells (MCs) profile and local interleukin (IL)-17 and IL-1β protein expressions in naturally infected CCPP according to the course of the inflammation (peracute-acute, subacute-chronic). The material of the study consisted of 40 naturally infected CCPP and 6 healthy control goat lung tissues. Appropriate samples were taken from the necropsied goats and subjected to histopathological and immunohistochemical examination. In the histopathological examination of the samples, it was determined that 29 samples had a peracute-acute course and 11 had a subacute-chronic course. In immuno-histochemical examination, MC profile and local IL-17 and IL-1β protein expressions were evaluated in the peracute-acute and subacute-chronic course. Immunohistochemically, significant increases in MC number, local IL-17 and IL-1β scores were detected in the peracute-acute course compared to the control group. There were significant decreases in the relevant scores in the subacute-chronic course compared to the peracute-acute course. Current findings indicated that MC, IL-17, and IL-1β expressions played important roles in the pathogenesis of infection in naturally infected CCPP, especially in the peracute-acute course. Additionally, MC profile was evaluated for the first time in naturally infected CCPP.
Key Words: Caprine, Interleukin-1β, Interleukin-17, Immunohistochemistry, Mast cell
Introduction
Contagious caprine pleuropneumonia (CCPP) in goats is a highly contagious and rapidly spreading mycoplasmal disease that is now among the leading causes of major economic losses on many continents (Asia, Africa, and the Middle East). The CCPP is associated with very high morbidity (100%) and mortality (80.00 - 100%).1,2 The causative agent of CCPP is Mycoplasma capricolum sub-species capripneumoniae (Mccp). In addition to goats, the disease can also be seen in sheep and wild ruminants.3,4 Clinically, severe respiratory distress associated with fever, depression, sero-mucous nasal discharge, cough, pleurodynia and dyspnea is observed. Macroscopic lesions of CCPP are characterized by fibrinous pleuropneumonia, lung hepatization, consolidation and pleural fluid accumulation. Additionally, mastitis, arthritis, peritonitis, meningitis and abortion may occur in pregnant animals.5-7 Three different stages are defined in animals infected with the disease according to clinical and pathological findings (macroscopic), namely peracute, acute and chronic. In peracute and acute stages, it is characterized by sudden death, fever, severe respiratory symptoms, fibrinous pleuropneumonia, lung hepatization and pleural fluid accumulation. In chronic cases, it is characterized by weight loss, mild respiratory symptoms, sequestrum formation and adhesive pleuritis.7-9
Mast cells (MC) are known as part of the body first line of defense providing protection against many microbial pathogens and other environmental insults. MCs are located in host- environment regions that are in contact with the environment such as skin and mucosal tissues.10,11 The MCs are immune cells that have functions in many processes ranging from the initiation of a rapid inflammatory response to the suppression of the immune system.12,13 The most characteristic feature of MCs is secretory granules densely packed with preformed mediators that can be rapidly released upon activation by degranulation. It can also induce de novo synthesis and release of cytokines like tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-17, eicosanoids, chemokines, lipid mediators and growth factors.11,14,15
The IL-17 is a pro-inflammatory cytokine that contributes to the pathogenesis of many inflammatory diseases. T helper 17 cells are known to be the main source of IL-17 production.16,17 The IL-17 is known to be its main function to stimulate chemokines such as granulocyte colony-stimulating factor (G-CSF), chemokine (C-X-C motif) ligand 1 (CXCL1) and CXCL8 (IL-8), which promote neutrophil accumulation and activation at the site of infection.17,18 The IL-1β is a highly functional and potent proinflammatory cytokine that plays an important role in inflammatory and infectious diseases. The IL-1β is an important part of the inflammatory response and is involved in various cellular activities such as cell proliferation, differentiation and apoptosis.19
There are not enough studies in the literature to reveal the pathogenesis of CCPP disease which is an important problem in small ruminant breeding (goats). In this study, MC profile and local IL-17A and IL-1β protein expressions were evaluated immunohistochemically according to the course of inflammation (peracute-acute, subacute-chronic) in naturally infected CCPP.
Materials and Methods
Animals. The material of the study consisted of fibrinous pneumonia/pleuropneumonia-infected lung tissues of 40 goats (6 - 18 months old, hair goats) whose Mccp agent was determined immunohistochemically obtained from enterprises in Sivas and Yozgat provinces. Relevant samples were collected after autopsy from animals that died of pneumonia from enterprises in Sivas and Yozgat provinces. In addition, lung tissue from 6 healthy goats (6 - 18 months, hair goat) was used as a control group. The relevant study was approved by Sivas Cumhuriyet University HADYEK ethics committee (31.07.2023-618).
Histopathological examination. Lung tissues obtained after necropsy were fixed in 10.00% neutral formaldehyde for 24 - 48 hr. Afterwards, paraffin blocks were obtained by going through routine tissue processing procedures. The 4.00 - 5.00 µm sections were taken from paraffin blocks onto grinding slides and stained with Hematoxylin & Eosin. Relevant sections were examined under light microscopy (BX51; Olympus, Tokyo, Japan). As stated in a previous study, lung tissue samples with pneumonia were examined according to the course of inflammation in histopathological examination. They were divided into two groups: Peracute-acute and subacute-chronic.8
Immunohistochemical examination. The 4.00 – 5.00 μm sections were taken from paraffin blocks onto adhesive slides. Paraffin extraction and rehydration were performed on adhesive slides. Immunohistochemical staining was performed with a commercial kit (Lab Vision Corp., Fremont, USA) in accordance with the kit procedure. Polyclonal Mccp hyperimmune sera were obtained from rabbits in a special laboratory and used for immuno-histochemical staining at a dilution of 1/5,000 with an incubation time of 1 hr. As the primary antibody (IL-17, IL-1β, and MC tryptase) used in the study, its properties are given in Table 1. Canine mastocytoma paraffin blocks were used as a positive control for MC tryptase staining. Additionally, the relevant tissues (subacute-chronic cases) were evaluated by immunohistochemical staining for respiratory syncytial virus , parainfluenza virus type 3 (PI-3) and adenovirus. The 3,3′-diaminobenzidine (DAB) was used as chromogen and counterstaining was performed with Mayer's-Hematoxylin. The sections were then examined under a light microscope at 20.00× magnification by a blinded pathologist. Scoring of immunohistochemical staining was 0; none, 1; light, 2; middle, 3; intensive and 4; very intensive.20 Using MC tryptase immunohistochemical staining, MCs were counted in five different areas at 20.00× and a total score was obtained (0; none, 1; 1 to 5 MCs, 2; 6 to 10 MCs, 3; 11 to 16 MCs, 4; 17 to 25 MCs).21
Table 1.
Immunohistochemistry primers used in the current study.
| Primer antibody | Clone number | Dilution rate | Incubation period |
|---|---|---|---|
| Interleukin-17 | sc-374218* | 1/200 | 2 hr |
| Interleukin-1β | 52012* | 1/200 | 2 hr |
| Mast cell tryptase | ab2378† | 1/200 | 1 hr |
* Santacruz Biotechnology (Dallas, USA), and † Abcam (Cambridge, UK).
Statistical analysis. Statistical analysis was done in SPSS Software (version 25.0; IBM Corp., Armonk, USA). Nonparametric Kruskal Wallis was used for immunohistochemical scores and Mann Witney U was used for differences between groups. A p < 0.05 was considered statistically significant.
Results
Histopathology. Control group showed normal histology (Fig. 1). In the histopathological examination of CCPP-infected animals, they were divided into two groups, peracute-acute and subacute-chronic according to the course of inflammation. The 29 lung samples showed a peracute-acute course and 11 lung samples showed a subacute-chronic course. In peracute-acute cases, the alveoli, bronchus and bronchiole lumens were observed to be filled with serofibrinous and/or fibrinous exudate and were determined to be sloughed epithelial cells.
Fig. 1.
Microscopic examination shows the normal histological appearance of healthy control animals (Hematoxylin and Eosin staining; Bar = 200 µm).
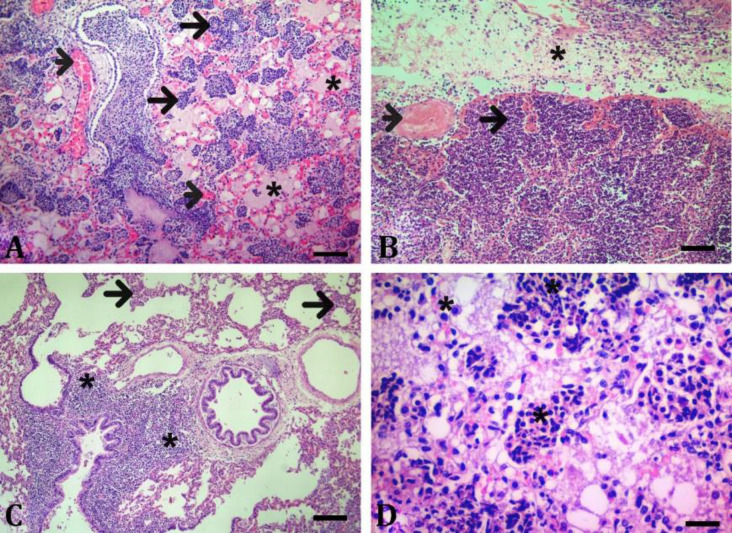
Acute inflammatory (neutrophil granulocyte) cell infiltration and severe hyperemia in the interalveolar septum were observed in these regions (Figs. 2A and 2B). Congested vessels with serofibrinous exudate and acute inflammatory (neutrophil granulocyte) cell infiltration were detected in the interlobular septum. Additionally, occasional mild vasculitis and thrombi were detected. Additionally, it was determined that the pleura was adorned with a serofibrinous exudate (Fig. 2B). In subacute-chronic cases, necrosis of the bronchus and bronchiolar epithelium and peribronchial lymphocyte infiltrates were observed (Fig. 2C).
Fig. 2.
Microscopic examination of animals with contagious caprine pleuropneumonia (CCPP), using Hematoxylin and Eosin staining. A) Inflammatory infiltration (neutrophil granulocyte) in the alveoli (arrows), serofibrinous exudate (asteriks) in the alveoli, hyperemia (arrowheads), peracute-acute course (Bar = 200 µm). B) Inflammatory infiltration (neutrophil granulocyte) in the alveoli (arrow), thrombus mass (arrowhead), inflammatory infiltration (neutrophil granulocyte) and serofibrinous fluid (asteriks) in the interlobular septum, peracute-acute course (Bar = 100 µm). C) Mononuclear cell infiltration in the interaralveolar septum (arrows), peribronchial lymphocyte infiltrates (asteriks), subacute-chronic course (Bar = 200 µm). D) Alveolar macrophage and mononuclear cell infiltration (asteriks), subacute-chronic course (Bar = 50.00 µm).
In these cases severe vasculitis, thrombus formations, mild hyperemia, very rare neutrophil granulocyte infiltrates and fibrinous exudate were detected. In addition, it was determined that there were occasionally multifocal lymphocyte infiltrates as well as mononuclear cell infiltrates in the interalveolar septum and alveolar lumens. In relevant cases, dense macro-phage infiltrates were seen in the affected alveoli and bronchioles (Figs. 2C and 2D).
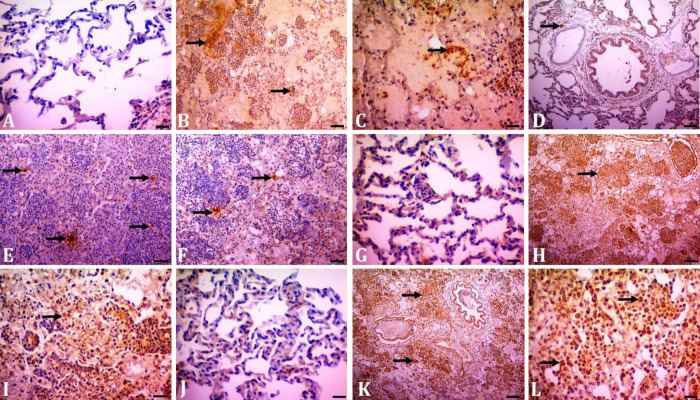
Immunohistochemistry. In the immunohistochemical examination, no immunopositivity was found in the control groups for the agent (Fig. 3A). In immuno-histochemical examination, immunopositive staining was obtained in Mccp staining in the alveolus, bronchus-bronchiolar epithelium and neutrophil granulocytes in the lumens in the per-acute course. Additionally, Mccp positivity was found in neutrophil granulocytes in the interlobular septum (Figs. 3B and 3C). In the subacute-chronic period, Mccp positivity was detected in alveolar macrophages and mononuclear cell infiltrates. In the peracute-acute course, immuno-positivity for Mccp was more severe and widespread compared to the subacute-chronic course.
Fig. 3.
Immunohistochemical staining in contagious caprine pleuropneumonia. A) Healthy control negative staining for Mycoplasma capricolum subspecies capripneumoniae (Mccp) agent (Bar = 50.00 µm). B and C) Positivity for Mccp agent in inflammatory infiltrates and serofibrinous content (arrows), peracute-acute course (Bars = 200 and 50.00 µm, respectively). D) Mast cell tryptase immunopositivity (arrow) in the control group (Bar = 50.00 µm). E) Mast cell tryptase immunopositivity (arrows) in the peracute-acute course (Bar = 200 µm). F) Mast cell tryptase immunopositivity in the subacute-chronic course. Bar = 50.00 µm. G) Mild interleukin (IL)-17 immunopositivity in healthy control (Bar = 50.00 µm). H) Severe IL-17 immunopositivity in inflammatory cell infiltrates (arrow) peracute-acute course (Bar = 200 µm). I) Moderate IL-17 immunopositivity in the subacute-chronic course. Scale bar: 50.00 µm. J) Mild IL-1β immunopositivity in healthy control (Bar = 50.00 µm). K) Severe IL-1β immunopositivity in inflammatory infiltrates (arrows) and bronchiol epithelium in peracute-acute course (Bar = 200 µm). L) Moderate IL-1β immunopositivity in the subacute-chronic course (Bar = 50 µm).
The level of MC tryptase staining was significantly increased in the peracute-acute course compared to the control group (Figs. 3D and 3E). It was found that there was a significant decrease in the relevant scores in the subacute-chronic course compared to the peracute-acute course (Figs. 3E and 3F, p < 0.001). Immunopositive stainings related to IL-17 were found in alveolar, bronchi and bronchiolar epitheliums in inflammatory infiltrates in the alveoli and bronchiole lumen and in alveolar macrophages. In IL-17 immunostaining, the highest scores were observed in the peracute-acute course and the lowest scores were in the control group, respectively (Figs. 3G and 3H). It was determined that IL-17 immunostaining scores were decreased significantly in the subacute-chronic course compared to the peracute-acute course (Figs. 3H and 3I, p < 0.001).
The IL-1β immuno-positive staining, similar to IL-17, was found in alveolar, bronchi and bronchiolar epitheliums, inflammatory infiltrates in the alveoli and bronchiole lumen and alveolar macrophages. The highest IL-1β immunopositive staining was observed in the peracute-acute course (Fig. 3K) and the relevant scores were decreased significantly in the subacute-chronic course (Fig. 3L, p < 0.01). No positivity was detected for respiratory syncytial virus, parainfluenza virus type and adenovirus in the relevant samples (subacute-chronic cases) immunohistochemically. Immunohistochemical scores are given in Figure 4.
Fig. 4.
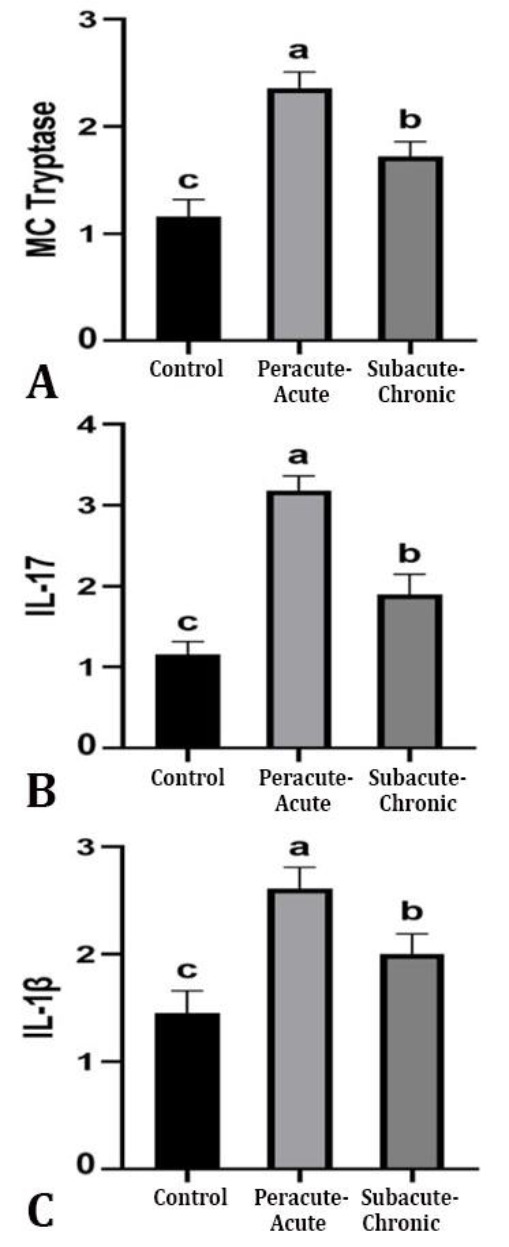
Immunohistochemical evaluation of A) mast cell (MC) tryptase, B) interleukin-17 (IL-17), and C) interleukin-1β (IL-1β) according to the course of inflammation (score) in contagious caprine pleuro-pneumonia. a-c Different superscripts in columns indicate statistical significance (p < 0.05).
Discussion
The pathogenesis mechanism of pathogenic myco-plasmal diseases has not been fully elucidated, although the host immune system is thought to play an important role. It is reported that the factors taken into the body adhere to epithelial cells and this is a prerequisite for colonization and pathogenesis.22 Due to the many disadvantages of CCPP disease, uncovering new findings about its pathophysiology and/or pathogenesis will shed light on a number of agents that can be developed to treat the disease in the future. In this study, we aimed to evaluate immunohistochemically MC profile, local IL-17 and IL-1β protein expressions in naturally infected CCPP goats according to the course of the inflammation (peracute-acute, subacute-chronic). The data of our study were, to our knowledge, the first report evaluating the MC profile of naturally infected CCPP. Indeed, recently, the evaluation of MCs not only in allergic reactions but also in cases of infection has become the focus of attention.10,21
When MCs are activated, they can undergo degranulation, a process in which the contents of secretory granules are released to the outside. In addition to binding to immunoglobulin E molecules, which classically bind to high-affinity immunoglobulin E receptor, or Fc epsilon RI, it can be activated by peptides produced from pathogenic bacteria and various cell wall products. In addition to the secretion of mediators from previously formed stores, activated MCs also induce the de novo synthesis of platelet-activating factor, prostaglandins and leukotrienes, as well as the transcription and release of many cytokines and chemokines.10,23 In this study, the high scores of MCs counts in the peracute-acute course revealed that Mccp caused effective MC induction. In addition, the fact that the causative agent staining in the peracute-acute period was more severe and widespread compared to the subacute-chronic period strengthened this opinion.
Experimental studies suggest that animals suffering from MCs exhibit a pathology that gradually worsens in bacterial infections. On the other hand, MCs have been reported to have a detrimental effect on the agents in bacterial infections and that they are essential for the clearance of pathogens.10,24,25 In an experimental study conducted by Xu et al.25 in mice, it was reported that MCs played an important role against Mycoplasma pneumoniae. In a different experimental study, they stated that mice with MC deficiency were highly vulnerable to Klebsiella pneumonia.26 In the current study, MC count scores in cases in the peracute-acute period were found to be significantly higher compared to the subacute-chronic period. This indicated that MCs proliferated more in the peracute-acute phase of CCPP infection. It is also possible to interpret that increasing the number of MCs might reduce the agent population. Indeed, many previous reports indicated that MCs combated infection by stimulating an inflammatory response that induced the recruitment of phagocytic cells and supported the control of bacterial infection.22,25,26 However, it should not be ignored that the increased number of MCs in the peracute-acute course increases the severity of parenchymal lesions by exacerbating the inflammatory response. Immuno-histochemically, the number of agents in the peracute-acute course was significantly higher than in the subacute-chronic course. As expected, the Mccp agent appeared to contribute to the severity of the lesions.
It is known that lung IL-17 plays important roles in protecting the host against some infections (fungal and bacterial) and in maintaining the barrier function of the epithelial mucosa. IL-17's main function is to stimulate the chemokines granulocyte colony-stimulating factor, CXCL1 and CXCL8 (IL-8). Thus, neutrophil granulocytes can easily infiltrate the infection site.16,27 In a study conducted by Wu et al.,28 they detected intense IL-17 expression in the lungs and a concurrent increase in neutrophil granulocytes in experimental M. pneumoniae infection in mice. They also reported that M. pneumoniae could cause a strong IL-17-associated inflammatory reaction by recruiting neutrophil granulocytes to the lung. In this study, it was determined that there was intense IL-17 expression in the peracute-acute course compared to the subacute-chronic course, depending on the course of the inflammation, and it was parallel to the intense neutrophil granulocyte infiltration in the peracute-acute course. This might be due to the effective stimulation of neutrophil chemoattractants from dense lung epithelial cells by Mccp in CCPP disease especially in the peracute-acute course.
In a recent study emphasized that the role of IL-17 in CCPP disease.29 It has been emphasized that increased IL-17 in CCPP disease causes excessive infiltration of neutrophil granulocytes into the lung and plays an important role in the pathogenesis of the disease.29 In this study, consistent with the relevant study, intense IL-17 expression and neutrophil granulocyte infiltrations were detected in the peracute-acute course depending on the course of inflammation. It shows that IL-17 plays an important role in ensuring neutrophil granulocyte infiltration in the inflammation area, especially in the peracute-acute course. However, it is not possible to say the same for the subacute-chronic course. Inflammation is a highly dynamic process in which neutrophil granulocytes are active in the early stages, whereas, mononuclear cells are involved in the later stages.30 Our findings revealed that IL-17 did not play an effective role in the subacute-chronic course of CCPP disease compared to the peracute-acute course.
Although recent evidence reports that IL-17 is required for a protective immune response, its excessive or unrestricted expression can lead to inflammation-induced tissue pathology.31 Dense neutrophil granulocytes induce a strong inflammatory microenvironment and subsequent tissue immunopathology through the release of proteases, pro-inflammatory cytokines and oxidants.31,32 Recently, in studies conducted in the treatment of inflammatory diseases, the use of antibodies that will neutralize IL-17 has provided some advantages. However, it should not be overlooked that this cytokine also plays important roles in wound healing, tissue regeneration and protection of tissue barriers.16
After MCs activation, de novo synthesis and release of cytokines such as TNF-α, IL-1β and IL-17 are induced. In addition, the role of MCs in regulating the immune response has been proven by secreting TNF and leukotrienes which increase neutrophil granulocyte recruitment to the region at early times.15,33 In this study, the increased expression of IL-1β and IL-17, especially in the peracute-acute course suggested that MCs might play an active role in the relevant expressions in CCPP.
As a result, in this study, MCs and local IL-17 and IL-1β expressions were evaluated according to the course of the inflammation in CCPP. In CCPP, MCs, IL-17 and IL-1β have been found to play important roles in the pathophysiology of the disease in the early stages of the disease (peracute-acute course). This study might shed light on future studies by elucidating the pathogenesis of CCPP from an immunological perspective. Additionally, it could be suggested that MCs, IL-17, and IL-1β might be possible therapeutic candidates in CCPP disease especially in the peracute-acute course.
Acknowledgments
We would like to thank the Department of Pathology, Faculty of Veterinary Medicine, Sivas Cumhuriyet University, Sivas, Türkiye, where the laboratory analysis was performed in the current study.
Conflict of interest
There is no conflict of interest between the authors.
References
- 1.Srivastava AK, Meenowa D, Barden G, et al. Contagious caprine pleuropneumonia in Mauritius. Vet Rec. 2010;167(8):304–305. doi: 10.1136/vr.c3816. [DOI] [PubMed] [Google Scholar]
- 2.Tigga M, Choudhary BK, Ghosh RC, et al. Mycoplasmosis: an emerging threat to developing livestock industry. Int J Adv Res. 2014;2(1):558–564. [Google Scholar]
- 3.Khodakaram-Tafti A, Derakhshandeh A, A Daee A, et al. Identification of Mycoplasma capricolum subspecies capripneumoniae and Mycoplasma arginini by culture, PCR, and histopathology in pneumonic lungs of slaughtered goats in Mashhad, Iran. Iran J Vet Res. 2023;24(2):96–101. doi: 10.22099/IJVR.2023.45321.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radostits OM, Gay C, Hinchcliff KW, et al. A textbook of the diseases of cattle, horses, sheep, pigs and goats. 10th ed. London, UK: Saunders ; 2007. pp. 2045–2050. [Google Scholar]
- 5.Iqbal Yatoo M, Raffiq Parray O, Tauseef Bashir S, et al. Contagious caprine pleuropneumonia - a comprehensive review. Vet Q. 2019;39(1):1–25. doi: 10.1080/01652176.2019.1580826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Çiftçi M, Ortatatlı M, Erer H, Hatipoğlu F, Özdemir Ö. Veterinary systemic pathology 1 [Turkish] 4th ed. Konya, Türkiye: Nobel Bookstore; 2020. 132 pp. [Google Scholar]
- 7.Thiaucourt F, Bölske G, Leneguersh B, et al. Diagnosis and control of contagious caprine pleuropneumonia. Rev Sci Tech. 1996;15(4):1415–1429. doi: 10.20506/rst.15.4.989. [DOI] [PubMed] [Google Scholar]
- 8.Abd-Elrahman AH, Khafaga AF, Abas OM. The first identification of contagious caprine pleuropneumonia (CCPP) in sheep and goats in Egypt: molecular and pathological characterization. Trop Anim Health Prod. 2020;52(3):1179–1186. doi: 10.1007/s11250-019-02116-5. [DOI] [PubMed] [Google Scholar]
- 9.Mondal D, Pramanik A, Basak DK. Clinico-haematology and pathology of caprine mycoplasmal pneumonia in rain fed tropics of West Bengal. Small Rumin Res. 2004;51(3):285–295. [Google Scholar]
- 10.Johnzon CF, Rönnberg E, Pejler G. The role of mast cells in bacterial infection. Am J Pathol. 2016;186(1):4–14. doi: 10.1016/j.ajpath.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Wesolowski J, Paumet F. The impact of bacterial infection on mast cell degranulation. Immunol Res. 2011;51(2-3):215–226. doi: 10.1007/s12026-011-8250-x. [DOI] [PubMed] [Google Scholar]
- 12.Chacón-Salinas R, Chen L, Chávez-Blanco AD, et al. An essential role for platelet-activating factor in activating mast cell migration following ultraviolet irradiation. J Leukoc Biol. 2014;95(1):139–148. doi: 10.1189/jlb.0811409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St John AL, Abraham SN. Innate immunity and its regulation by mast cells. J Immunol. 2013;190(9):4458–4463. doi: 10.4049/jimmunol.1203420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce JA. Eicosanoid mediators of mast cells: receptors, regulation of synthesis, and pathobiologic implications. Chem Immunol Allergy. 2005;87:59–79. doi: 10.1159/000087571. [DOI] [PubMed] [Google Scholar]
- 15.Mukai K, Tsai M, Saito H, et al. Mast cells as sources of cytokines, chemokines, and growth factors. Immunolo Rev. 2018;282(1):121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50(4):892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puerta-Arias JD, Mejía SP, González Á. The role of the interleukin-17 axis and neutrophils in the pathogenesis of endemic and systemic mycoses. Front Cell Infect Microbiol. 2020;10:595301. doi: 10.3389/fcimb.2020.595301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121(24):4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Qian H, Xiao M, et al. Role of signal transduction pathways in IL‐1β‐induced apoptosis: pathological and therapeutic aspects. Immun Inflamm Dis. 2023;11(1):e762. doi: 10.1002/iid3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akcakavak G, Kazak F, Deveci MZY. Eucalyptol protects against cisplatin-induced liver injury in rats. Biol Bull. 2023;50(5):987–994. [Google Scholar]
- 21.Sener S, Ipek V. Investigation of brain mast cells in ovine encephalitic listeriosis. Biotech Histochem. 2022;97(4):247–253. doi: 10.1080/10520295.2021.1941256. [DOI] [PubMed] [Google Scholar]
- 22.Smith BP, VanMetre D, Pusterla N. Large animal internal medicine-E-Book. 6th ed. St Louis, USA: Elsevier; 2019. p. 1949. [Google Scholar]
- 23.Rivera J, Fierro NA, Olivera A, et al. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gekara NO, Weiss S. Mast cells initiate early anti‐Listeria host defences. Cell Microbiol. 2008;10(1):225–236. doi: 10.1111/j.1462-5822.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Zhang D, Lyubynska N, et al. Mast cells protect mice from Mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173(2):219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutherland RE, Olsen JS, McKinstry A, et al. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181(8):5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo Y, Li C, Zhou Z, et al. Biological functions of IL‐17‐producing cells in mycoplasma respiratory infection. Immunology. 2021;164(2):223–230. doi: 10.1111/imm.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Martin RJ, Rino JG, et al. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9(1):78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma WT, Gu K, Yang R, et al. Interleukin-17 mediates lung injury by promoting neutrophil accumulation during the development of contagious caprine pleuropneumonia. Vet Microbiol. 2020;243:108651. doi: 10.1016/j.vetmic.2020.108651. [DOI] [PubMed] [Google Scholar]
- 30.Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19(2):177–191. doi: 10.1038/s41423-021-00832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed M, Morris SH, Owczarczyk AB, et al. Deficiency of autophagy protein Map1-LC3b mediates IL-17-dependent lung pathology during respiratory viral infection via ER stress-associated IL-1. Mucosal Immunol. 2015;8(5):1118–1130. doi: 10.1038/mi.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 33.Abraham SN. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]