Abstract
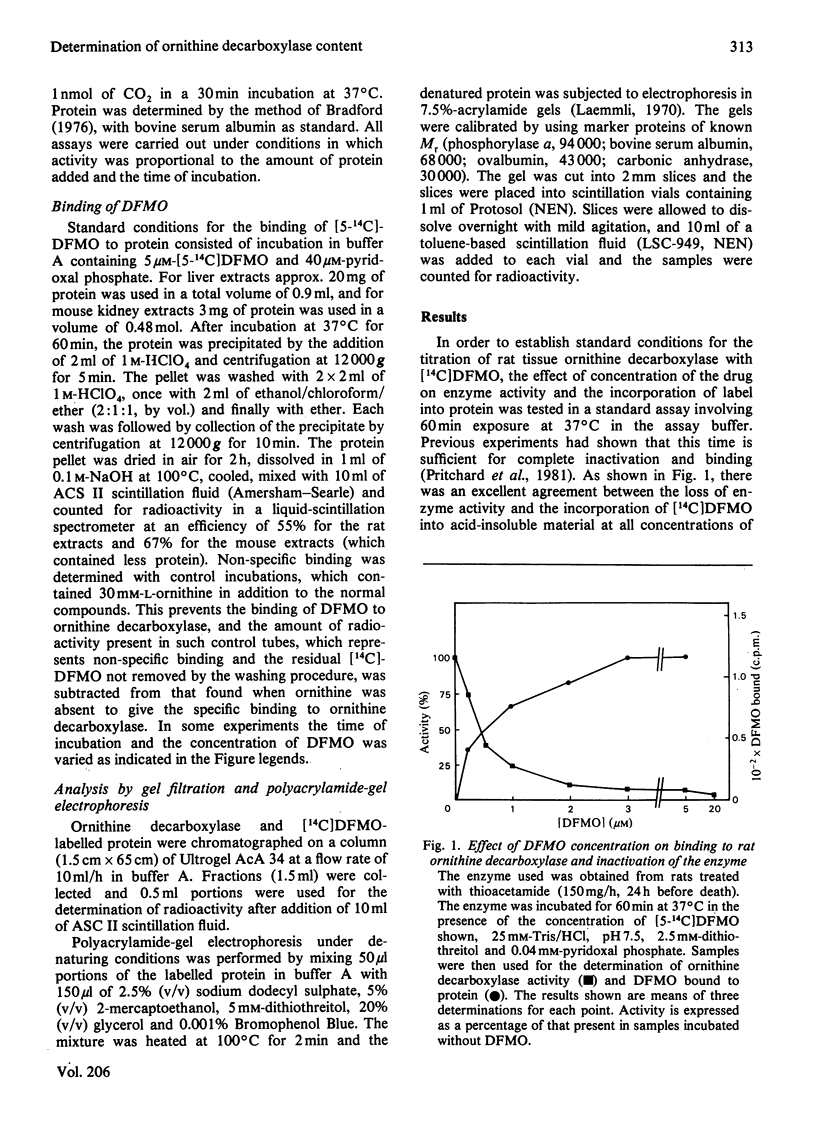
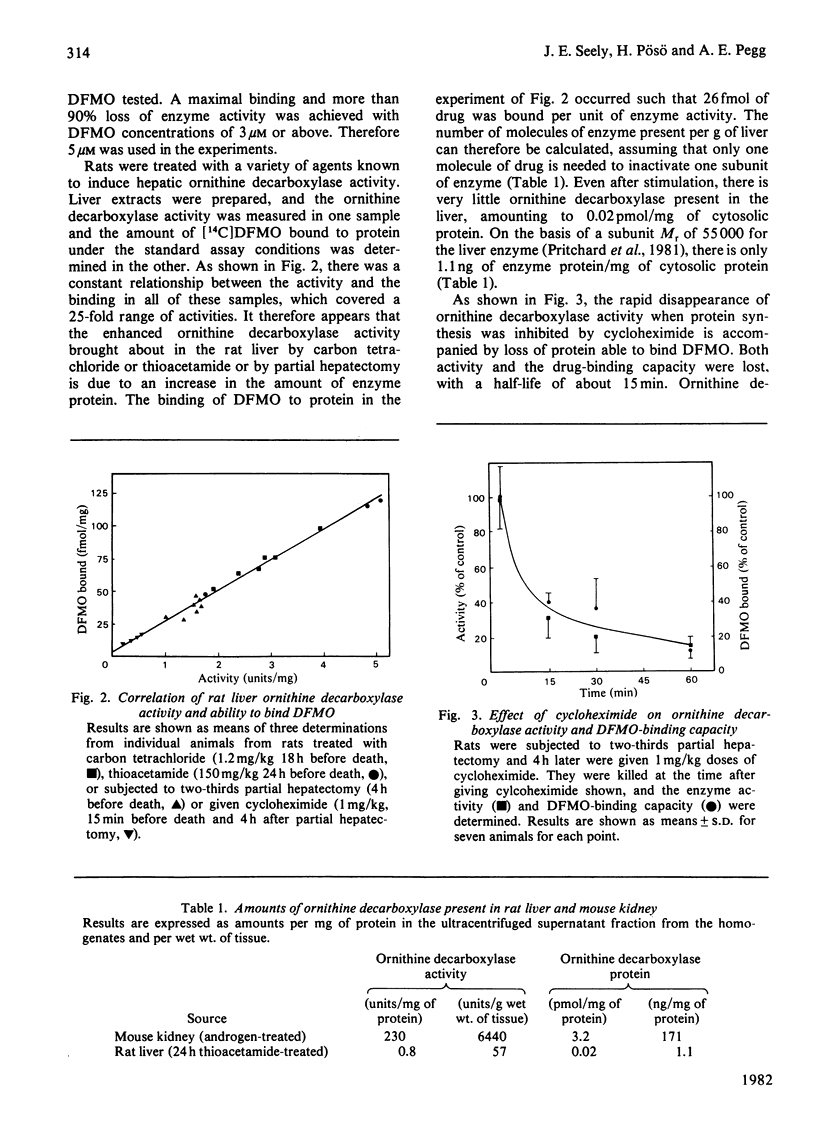
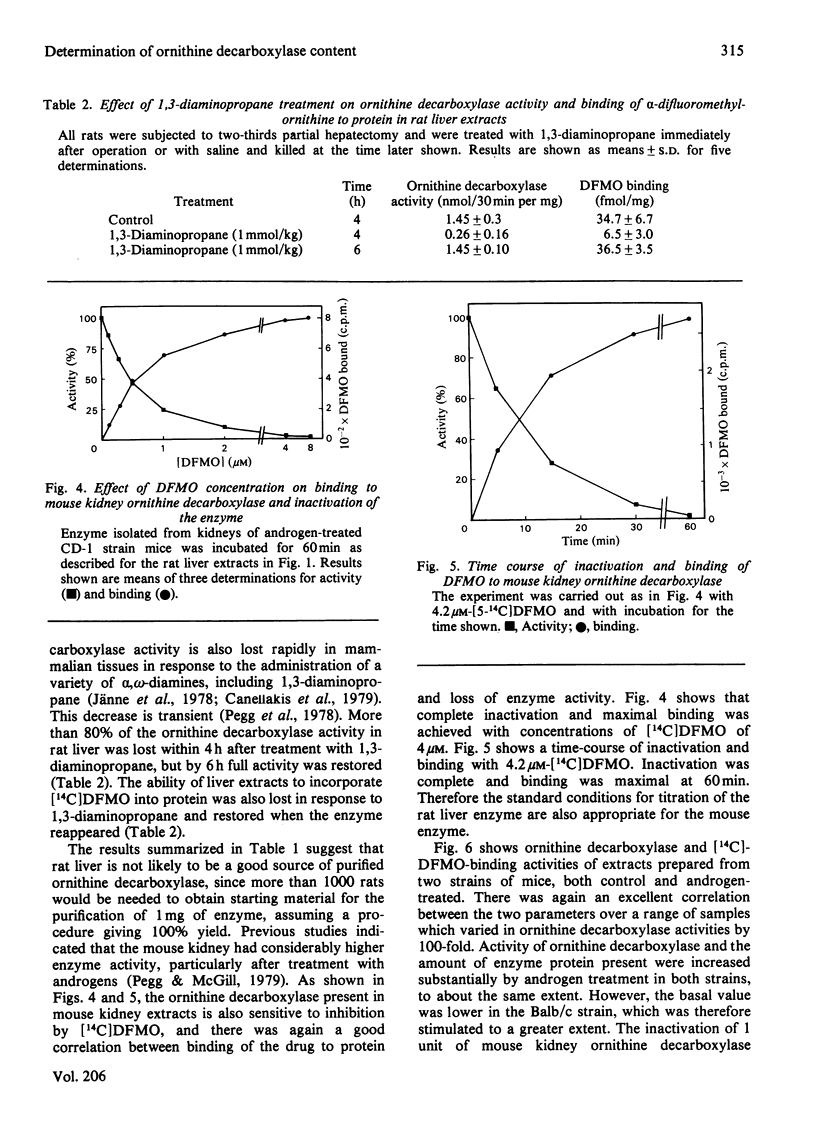
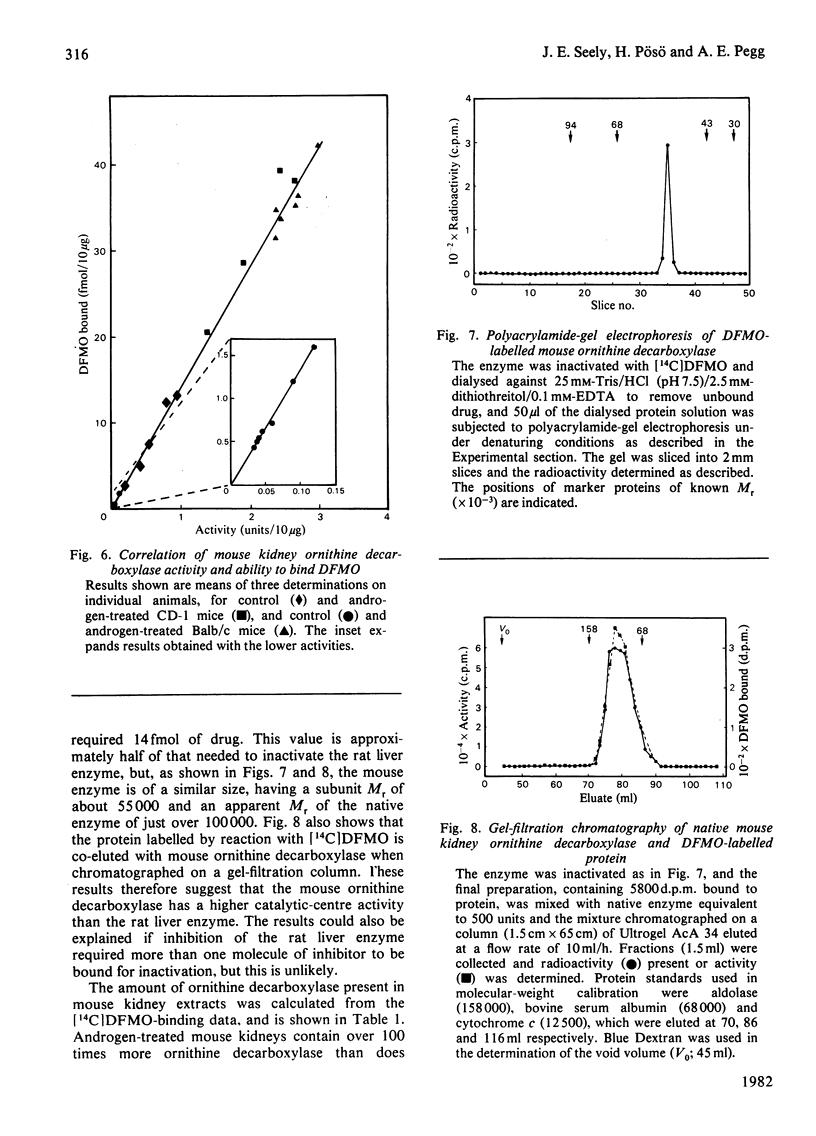
The binding of α-difluoromethylornithine, an irreversible inhibitor, to ornithine decarboxylase was used to investigate the amount of enzyme present in rat liver under various conditions and in mouse kidney after treatment with androgens. Maximal binding of the drug occurred on incubation of the tissue extract for 60min with 3μm-difluoromethyl[5-14C]ornithine in the presence of pyridoxal phosphate. Under these conditions, only one protein became labelled, and this corresponded to ornithine decarboxylase, having Mr about 100000 and subunit Mr about 55000. Treatment of rats with thioacetamide or carbon tetrachloride or by partial hepatectomy produced substantial increases in ornithine decarboxylase activity and parallel increases in the amount of enzyme protein as determined by the extent of binding of difluoromethyl[5-14C]ornithine. Similarly, treatment with cycloheximide or 1,3-diaminopropane greatly decreased both the enzyme activity and the amount of difluoromethyl-[5-14C]ornithine bound to protein. In all cases, the ratio of drug bound to activity was 26fmol/unit, where 1 unit corresponds to 1nmol of substrate decarboxylated in 30min. These results indicate that even after maximal induction of the enzyme in rat liver there is only about 1ng of enzyme present per mg of protein. When mice were treated with androgens there was a substantial increase in renal ornithine decarboxylase activity, the magnitude of which depended on the strain. There was an excellent correspondence between the amount of activity present and the capacity to bind labelled α-difluoromethylornithine in the mouse kidney extracts, but in this case the ratio of drug bound to activity was 14fmol/unit, suggesting that the mouse enzyme has a higher catalytic-centre activity. After androgen induction, the mouse kidney extracts contain about 170ng of enzyme/mg of protein. These results indicate that titration with α-difluoromethylornithine provides a valuable method by which to quantify the amount of active ornithine decarboxylase present in mammalian tissues, and that the androgen-treated mouse kidney is a much better source for purification of the enzyme than is rat liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Canellakis Z. N., Theoharides T. C. Stimulation of ornithine decarboxylase synthesis and its control by polyamines in regenerating rat liver and cultured rat hepatoma cells. J Biol Chem. 1976 Jul 25;251(14):4436–4441. [PubMed] [Google Scholar]
- Haddox M. K., Russell D. H. Ornithine decarboxylase from calf liver. Purification and properties. Biochemistry. 1981 Nov 10;20(23):6721–6729. doi: 10.1021/bi00526a030. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Canellakis E. S. Cellular control of ornithine decarboxylase activity by its antizyme. J Cell Physiol. 1981 May;107(2):209–217. doi: 10.1002/jcp.1041070206. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kallio A., Löfman M., Pösö H., Jänne J. Diamine-induced inhibition of liver ornithine decarboxylase. Biochem J. 1979 Jan 1;177(1):63–69. doi: 10.1042/bj1770063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., Löfman M., Pösö H., Jänne J. Inhibition of ornithine decarboxylase by diamines in regenerating rat liver. Evidence for direct action on the accumulation of the enzyme protein. FEBS Lett. 1977 Jul 1;79(1):195–199. [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Noguchi T., Hayashi S. Rat liver ornithine decarboxylase purification and some immunochemical studies. Med Biol. 1981 Dec;59(5-6):296–299. [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Effects of detergent on ornithine decarboxylase from rat liver. Stabilization and renaturation. Eur J Biochem. 1981 Sep;119(1):177–181. doi: 10.1111/j.1432-1033.1981.tb05591.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Obenrader M. F., Prouty W. F. Production of monospecific antibodies to rat liver ornithine decarboxylase and their use in turnover studies. J Biol Chem. 1977 May 10;252(9):2866–2872. [PubMed] [Google Scholar]
- Pegg A. E., Conover C., Wrona A. Effects of aliphatic diamines on rat liver ornithine decarboxylase activity. Biochem J. 1978 Mar 15;170(3):651–660. doi: 10.1042/bj1700651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Matsui I., Seely J. E., Pritchard M. L., Pösö H. Formation of putrescine in rat liver. Med Biol. 1981 Dec;59(5-6):327–333. [PubMed] [Google Scholar]
- Pegg A. E., McGill S. Decarboxylation of ornithine and lysine in rat tissues. Biochim Biophys Acta. 1979 Jun 6;568(2):416–427. doi: 10.1016/0005-2744(79)90310-3. [DOI] [PubMed] [Google Scholar]
- Persson L. Decarboxylation of ornithine and lysine by ornithine decarboxylase from kidneys of testosterone treated mice. Acta Chem Scand B. 1981;35(6):451–459. doi: 10.3891/acta.chem.scand.35b-0451. [DOI] [PubMed] [Google Scholar]
- Pritchard M. L., Seely J. E., Pösö H., Jefferson L. S., Pegg A. E. Binding of radioactive alpha-difluoromethylornithine to rat liver ornithine decarboxylase. Biochem Biophys Res Commun. 1981 Jun;100(4):1597–1603. doi: 10.1016/0006-291x(81)90701-4. [DOI] [PubMed] [Google Scholar]
- Pösö H., Pegg A. E. Effect of alpha-difluoromethylornithine on polyamine and DNA synthesis in regenerating rat liver: reversal of inhibition of DNA synthesis by putrescine. Biochim Biophys Acta. 1982 Feb 26;696(2):179–186. doi: 10.1016/0167-4781(82)90026-4. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Ornithine decarboxylase as a biological and pharmacological tool. Pharmacology. 1980;20(3):117–129. doi: 10.1159/000137355. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Theoharides T. C., Canellakis Z. N. Antiserum monospecific to hepatic ornithine decarboxylase. J Biol Chem. 1976 Mar 25;251(6):1781–1784. [PubMed] [Google Scholar]
- Weiss J. M., Lembach K. J., Boucek R. J., Jr A comparison of ornithine decarboxylases from normal and SV40-transformed 3T3 mouse fibroblasts. Biochem J. 1981 Jan 15;194(1):229–239. doi: 10.1042/bj1940229. [DOI] [PMC free article] [PubMed] [Google Scholar]


