Abstract
Multiple sclerosis (MS) is a chronic immune-mediated and heterogeneous disease characterized by demyelination, axonal damage, and physical and cognitive impairment. Recent studies have highlighted alterations in the microbiota of people with MS (pwMS). However, the intricate nature of the disease and the wide range of treatments available make it challenging to identify specific microbial populations or functions associated with MS symptoms and disease progression. This study aimed to characterize the microbiota of pwMS treated with the oral drug teriflunomide (TF) and compare it with that of pwMS treated with beta interferons (IFNβ), pwMS treated with no previous disease modifying therapies (naïve), and healthy controls. Our findings demonstrate significant alterations in both the composition and function of the gut microbiota in pwMS that are further influenced by disease-modifying therapies. Specifically, oral treatment with TF had a notable impact on the gut microbiota of pwMS. Importantly, the dysregulated microbial environment within the gut was associated with symptoms commonly experienced by pwMS, including fatigue, anxiety, and depression.
Keywords: Microbiome, Disease-modifying therapies, Microbial metabolism, Fatigue, Anxiety
Introduction
Multiple Sclerosis (MS) is an immune-mediated disease characterized by demyelination and axonal damage. The incidence and prevalence of MS are increasing in both developed and developing countries, with the female-to-male ratio steadily rising and nearing 3:1 [1]. Fortunately, the course of the disease has dramatically changed in recent decades with the commercialization of several disease-modifying therapies (DMTs), yet it remains the leading cause of no traumatic disability in young adults [2]. The etiological factors of the disease are currently unknown, but consistent evidence points to the interaction of genetic, epigenetic and environmental factors. Low levels of vitamin D, reduced sunlight exposure, previous virus infections or smoking are the most explored environmental factors [[3], [4], [5], [6]]. In recent decades, the gut microbiota has been recognized as a contributing factor in neuroinflammatory diseases, including MS [7,8]. The microbiota encompasses the microorganisms that live in or on our bodies. The richest, densest and most studied one is the gut microbiota. Numerous functions have been attributed to the microbiota, most notably its role in the development and modulation of the immune system and its interaction with the central nervous system (CNS) via the microbiota-gut-brain axis [[9], [10], [11]]. These systems are the main ones involved in the progression of MS.
The first commercialized DMTs were interferon beta (IFNβ) based therapies. These compounds are a type 1 family of cytokines whose mechanism of action is complex, and not completely understood. The IFNβs used include injectable drugs that are administered at different frequencies. Broadly speaking, IFNβ treatment provokes a shift in the balance from a proinflammatory Th1/Th17 response to a Th2 anti-inflammatory response, as well as a reduction in the number of inflammatory cells able to cross the blood-brain barrier (BBB). A large body of data supports the long-term efficacy and safety of IFNβ for lowering the relapse rate (by approximately one third), slowing disability worsening, and decreasing the number of CNS lesions [[12], [13], [14]]. Teriflunomide (TF) is included along with Fingolimod (FIN) and Dimethyl Fumarate (DMF), which are among the traditional oral treatments for MS. TF was approved in Europe in 2013 at a daily dose of 14 mg for the treatment of relapsing-remitting MS adults and in 2021 in pediatric patients with a recommended dose dependent on body weight [15]. TF is a selective and reversible inhibitor of dihydroorotate dehydrogenase, a mitochondrial enzyme necessary for the de novo pyrimidine synthesis, leading to a reduction in the proliferation of activated T and B cells without causing cell death. The beneficial effects of TF include not only a significant improvement in the reduction of inflammation and prevention of relapses but also in the improvement of disability worsening rates and MRI outcomes [15,16]. Moreover, unlike other immune-targeted MS treatments, TF provides therapeutic benefit without causing immune suppression [16]. In addition to reducing inflammation at the peripheral level, both IFNβ and TF, can to cross the BBB, decrease microgliosis and reduce the M1 proinflammatory microglial phenotype. IFNβs also have some effect in decreasing astrogliosis, but are less effective in reducing the damage to neurons and oligodendrocytes caused by the disease. TF has some effect on neuronal survival and neurogenesis, although its effect on oligodendrocytes is very modest [17].
Considering the effects that treatments have on the immune system and CNS, it is not surprising that they also have some modulatory effect on the microbiota. In this respect, oral treatments may be of particular interest because they involve direct contact with the microbiota in the gastrointestinal tract. In recent years, several articles describing not only the alteration of the microbiota in people with MS (pwMS) but also the changes caused by DMTs have been published. For example, a recent publication of the International Multiple Sclerosis Microbiome Study (iMSMS) consortium revealed that DMF reduces the populations of Bacteroides stercoris and several species of Clostridium and Eubacterium genera. FIN reduces Bacteroides finegoldii CAG:203, Roseburia faecis, and Blautia spp; while IFNβ treatment is associated with lower levels of Short Chain Fatty Acid (SCFA)-producing species, such as Ruminococcus sp., Clostridium sp., Faecalibacterium prausnitzii, Roseburia inulinivorans and Roseburia intestinalis; and increased Parabacteroides distasonis populations. In addition, some species show treatment-dependent modulation, e.g., Bacteroides uniformis increases with IFNβs but decreases with natalizumab (NTZ) and glatiramer acetate (GA) therapies. NTZ and anti CD20 antibodies significantly alter the microbiota, increasing the presence of Phascolarctobacterium sp. CAG:207 and reducing the abundancies of Bifidobacterium longum and various Prevotella species. A reduction in the abundance of Bacteroides finegoldii CAG:203 and Blautia sp. CAG:37 was observed in the microbiota of patients treated with anti CD20 [7]. Other studies have also noted an increase in Prevotella abundance with IFNβ treatment [18], a reduction in the Lachnospiraceae and Veillonellaceae families with DMF and GA, a general reduction in the Firmicutes and Fusobacteria phyla, and an increase in Bacteroidetes abundance with DMF [19]. Expansion of the bacterial species F. prausnitzii with anti CD20, Roseburia intestinalis with DMF and Ruminococcaceae PAC001607 with NTZ and FIN has also been previously reported [20,21]. Limited information is available on how TF treatment influences the composition of the microbiota. Vacaras et al. explored its role in their study, though it was assessed alongside IFNβs and the optional use of homeopathic treatments [22].
In this work, we explored the effect of oral DMT TF on the composition and function of the microbiota of pwMS compared to that of healthy controls (HCs), naïve pwMS (with no previous DMT) or IFNβ-treated pwMS.
Material and Methods
Subjects
We carried out a prospective observational case-control study. The sample included 60 individuals diagnosed with MS, from the Hospital Universitario Donostia (Donostia-San Sebastian, Spain) and Hospital Universitario Araba-Txagorritxu (Vitoria-Gasteiz, Spain), between June 2019 and November 2022, and 20 HCs of the same age range. The local ethics committee approved the research protocol with reference TCT-UEM-2019-01 on March 26, 2019. After signing the informed consent, participants were provided with a kit for the hygienic collection of the fecal samples at their own residence. The kit contains a stool collector that adheres to the toilet, two tubes for collecting stool samples, a safety bag, a hydrated cold accumulator and an isothermal bag for the transport of the frozen samples. Once the participant collected the sample in the tube, it was immediately frozen at −20 °C and subsequently transported to the hospital protected with cold accumulators from the kit. When the samples arrived at our center, they were reviewed to assess their suitability and stored at −80 °C until analysis. Several questionnaires were also distributed to the participants, including a food frequency test to evaluate lifestyle habits and questionnaires to assess the degree of fatigue, anxiety and depression of patients, as well as a self-assessment of the impact of the disease on their daily lives.
The MS group included adult persons with confirmed relapsing remitting MS (RRMS) according to the revised McDonald diagnosis criteria [23] who did not suffer gastrointestinal or chronic infectious diseases, who had no undergone steroids in the last month or chemotherapy or antibiotics in the last three months and who were not pregnant or who were more than 6 months postpartum. pwMS must have been receiving the same DMT for at least the last 3 months. PwMS were either naïve, IFNβ-treated or TF-treated. Patients treated with IFNβ have been treated for an average of 11 years and 3 months (2 years and 3 months- 17 years and 8 months), while a treatment duration of 3 years and 1 months (8 months- 6 years and 8 months) characterizes TF-treated patients. In regard to HCs, in addition to not being relative to the pwMS, they must not suffer any autoimmune, gastrointestinal or chronic infectious disease.
The relevant clinical and demographic data, together with the main results of the questionnaires, can be found in Table 1.
Table 1.
Demographic and clinical data of the studied population and resume of the questionnaires in the MS group.
| Variable | HC (20) | Naïve MS (20) | IFNβ MS (18) | TF MS (22) | P-values | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age | 48.50 | 49.95 | 50.94 | 41.77 | 0.049 | ||||
| Sex | M 17 (85 %) | F 3 (15 %) | M 7 (35 %) | F 13 (65 %) | M 6 (33 %) | F 12 (67 %) | M 4 (18 %) | F 18 (82 %) | 0.000b |
| Dietary habits | |||||||||
| Protein (%kcal) | 26.86 | 26.90 | 27.18 | 24.96 | 0.775 | ||||
| Total fats (%Kcal) | 34.61 | 35.22 | 38.90 | 35.52 | 0.570 | ||||
| SF (%Kcal) | 8.98 | 8.84 | 10.31 | 9.21 | 0.138a | ||||
| MUFA (%Kcal) | 16.70 | 16.40 | 15.72 | 14.85 | 0.623 | ||||
| PUFA (%Kcal) | 3.72 | 4.12 | 4.71 | 4.94 | 0.002 | ||||
| Carbohydrates (%Kcal) | 45.23 | 47.82 | 44.44 | 47.09 | 0.568 | ||||
| Fiber (g) | 29.15 | 41.46 | 35.37 | 33.61 | 0.546 | ||||
| Cholesterol (g) | 192.29 | 282.80 | 352.43 | 349.14 | 0.001 | ||||
| Clinical conditions | |||||||||
| Evolution | – | 12.00 | 15.00 | 3.50 | 0.000 | ||||
| EDSS | – | 1.00 | 1.00 | 1.50 | 0.390 | ||||
| MSSS | – | 0.71 | 0.82 | 2.62 | 0.001 | ||||
| Fatigue | – | N 0 (0 %) | Y 4 (100 %) | N 1 (20 %) | Y 4 (80 %) | N 2 (14 %) | Y 12 (86 %) | 1.000b | |
| Anxiety | – | N 2 (50 %) | Y 2 (50 %) | N 1 (20 %) | Y 4 (80 %) | N 4 (29 %) | Y 10 (71 %) | 0.673b | |
| Depression | – | N 1 (25 %) | Y 3 (75 %) | N 4 (80 %) | Y 1 (20 %) | N 10 (71 %) | Y 4 (29 %) | 0.241b | |
| MS impact physical | – | 48.00 | 36.20 | 35.50 | 0.476 | ||||
| MS impact psycological | – | 55.00 | 54.20 | 51.30 | 0.927a | ||||
SF: Saturated fat; MUFA: Monounsaturated Fatty Acids; PUFA: Polyunsaturated Fatty Acids.
EDSS: Expanded disability Score Status; MSSS: MS Severity Score.
M: Male; F: Female; N: No; Y: Yes.
Anova test. Other p-values comes from Kruskal Wallis test.
Fisher exact test.
DNA extraction and 16S rRNA gene sequencing
Fecal samples were thawed on ice and diluted in 1X Dulbecco's Phosphate Buffered Saline (DPBS) (Gibco, USA). Subsequently, the DNA extraction was assessed using mechanical and enzymatic lysis of the cells, and the QIAamp DNA Stool Mini Kit (Qiagen, Germany). Mechanical disruption was performed by three rounds of bead-beating with 0.1 mm diameter zirconia/silica beads (Sigma, St.Louis, USA) using a Bead Ruptor 12 (OMNI International, Georgia, USA). Enzymatic lysis was performed with the enzyme lysozyme (Sigma, St.Louis, USA) at a concentration of 10 mg/ml. The obtained DNA was subjected to quality and quantity controls. A negative control was extracted in parallel with fecal samples to evaluate the influence of reactants and possible contamination background on the samples. This control was performed in an empty sterile tube (similar to those containing feces) without the addition of any biological sample.
Microbiome analysis was performed on Ion Torrent PGM equipment (Life Technologies, MA, USA) using a 318 chip. The hypervariable regions V2, V4 and V8, and V3, V6-7 and V9 of the 16S rRNA gene were amplified in separate tubes and subjected to quality control. Between 300 and 400 ng of DNA from each sample was used for amplification and sequencing.
Questionnaires
Several questionnaires to evaluate a variety of symptoms related to the disease were used in the present study. Fatigue was determined using the FSMC, a 20-item scale developed as a measure of cognitive and motor fatigue for pwMS that was validated in 2009 [24]. The Hospital Anxiety and Depression Scale (HADS) used in this study was developed in 1983 [25] with the purpose of providing clinicians with an acceptable, reliable, valid and easy to use practical tool for identifying and quantifying depression and anxiety. Subsequently, it was validated and reviewed in the general population and hospital patients [[24], [25], [26], [27], [28], [29]]. The impact of the disease on the day-to-day basis of the patients was evaluated with the MS Impact Scale (MSIS-29, v2). This questionnaire is a disease specific outcome measure for clinical trials of MS that combines patient perspectives with rigorous psychometric methods. It consists of a 29-item self-report measure comprising 20 items associated with a physical scale and 9 items associated with a psychological scale validated for use in the pwMS to across disability levels and practice settings of MS [30,31].
Microbiome data processing
The QIIME2 microbiome informatics platform was used to obtain 16S rRNA gene abundance tables. The pipeline used included data import, quality filtering and denoising, ASV identification (using DADA2 denoise-pyro) and taxonomic classification using the GreenGenes database (with the VSEARCH feature-classifier). Due to the lack of available pipelines for the analysis of Ion Torrent (IT) sequences (partly because of the increased use of Illumina vs IT), the best way to perform this analysis has been discussed several times by the community in the forum that QIIME2 offers. The above described steps, which were carried out in our workflow, were selected based on discussions from the forum [[32], [33], [34], [35]]. The pipeline designed and used for the microbiota analysis has been deposited on GitHub, https://github.com/MGorostidi/mbiome [36].
16S rRNA gene PICRUSt metagenome analysis
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) was used to generate predictive pathways based on the Greengenes classified OTU table and KEGG database according to the PICRUSt2 tutorial (https://github.com/picrust/picrust2/wiki/PICRUSt2-Tutorial-(v2.5.2)).
Statistics
The overall composition of the collected stool samples was analyzed in Excel and R. The Shannon diversity index (SDI) was calculated by using the R vegan package. Sample clustering and statistical analyses were carried out in R. Multifactor ANOVA and the Kruskal-Wallis test were used to compare the microbial taxon abundance and alpha diversity between the groups for parametric and nonparametric data respectively. Fisher's exact test was used to compare proportions. Principal coordinate analysis (PCoA) was carried out to explore the relationships between the family-/genus-level datasets. Concurrently, the Galaxy Huttenhower platform, an online resource, was used to determine the LDA effect size (LEfse algorithm) and obtain the cladograms in which represent the microbial taxa that are significantly different in the samples. Extended error bar plot for two-group analysis of predicted KEGG functional data was made using Statistical Analysis of Taxonomic and Functional Profiles (STAMP) with Welch's two-sided t-test with 95 % confidence intervals [37].
Results
A total of 80 participants with a median age of 47.6 years were recruited for the study. Participants were distributed in almost homogeneous groups of 20 persons (18 in the IFNβ group and 22 in the TF group). In general, females dominated the MS group, especially the TF group (82 %), and males exceeded the HC group (85 %). No gender differences in beta diversity were observed in either the HC or MS groups; consequently, the analyses were not stratified by gender. In terms of macronutrient intake, minimal differences in the diets of MS patients and HCs were found, with a greater intake of cholesterol (p-value = 0.000) and polyunsaturated fatty acids (PUFAs) (p = 0.001) in patients. In general, the diets of both groups were characterized by an excess of protein and fat and a low intake of carbohydrates (Table 1). On the other hand, there were greater differences in micronutrient intake between MS and HC. These differences are generally characterized by the greater presence of micronutrients in the diet of patients, particularly the minerals Na and K and group B vitamins, vitamin C, and the precursor of vitamin A, carotenes (data not shown).
PwMS in the TF group had greater EDSS scores but fewer years of disease progression, resulting in a worse disease prognosis (higher MSSS). In general, the patients in the naïve MS group had high scores on the fatigue and depression questionnaires, even if no significant differences could be found between the groups (Table 1).
The mean amount of DNA per gram of feces was 30.79 μg in HC and 120.39 μg in pwMS; while no DNA was detected in the negative control. The quantity of DNA was adjusted before amplification and again to carry out sequencing. The total number of reads in the samples ranged from 23179 to 384405 (mean of 153013.1 reads), and a total of 38–116 families/genera (mean of 69 families/genera) were detected for each of them.
There were 3286 reads in the negative control, and 31 families/genera were detected. However, the diversity parameters and the bacterial profile clearly differed from those detected in the samples; therefore, the interference of the negative control on the samples was considered negligible.
Gut microbiota composition and function in MS. Influence of DMTs
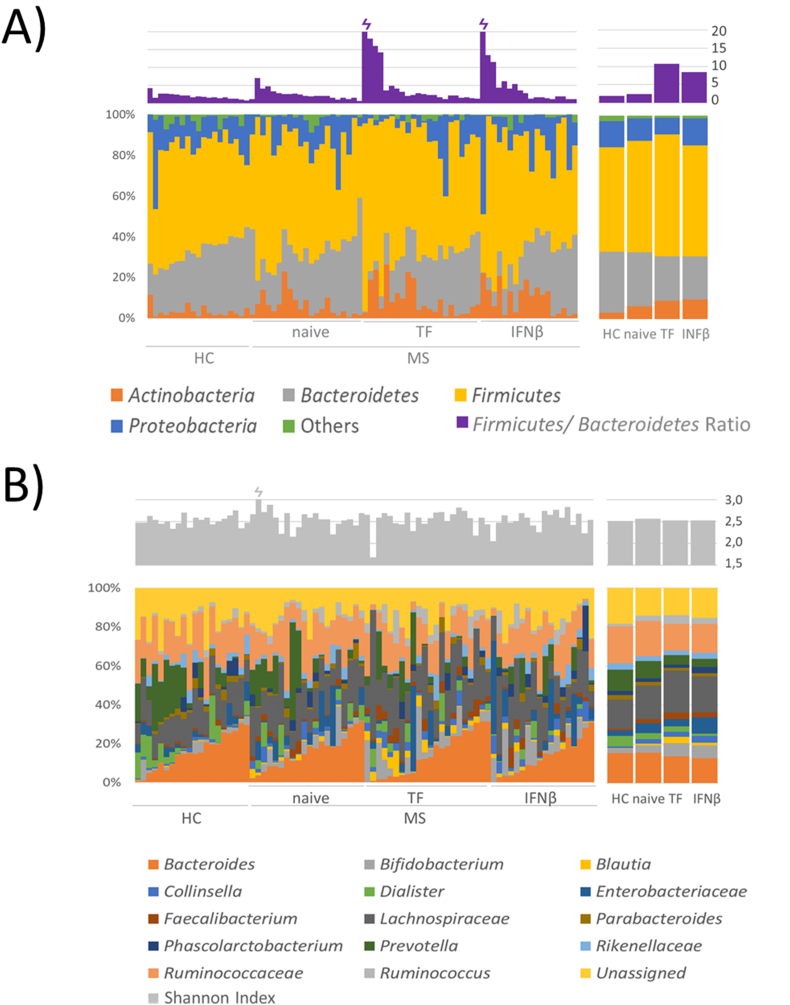
The microbiota composition was analyzed at the phylum and genus level. An increase in Actinobacteria and Firmicutes and a decrease in Bacteroidetes and Proteobacteria were observed in the pwMS group compared to the HC group. These differences (not statistically significant) were especially pronounced in TF-treated patients (Fig. 1A). However, the Firmicutes/Bacteroidetes ratio significantly increased in pwMS (p = 0.021) and patients treated with TF (p = 0.022) compared with HCs (Fig. 1A). α-Diversity (Shannon index, Fig. 1B) did not differ according to disease status or DMT followed by pwMS. The analysis of the top 10 dominant families/genera in the samples (note, where resolution at the genus level was not possible taxa are described at family level) revealed an increase in Bifidobacterium, Blautia, Collinsella and Enterobacteriaceae in pwMS and a raise in Dialister and Prevotella in the HC microbiota. In general, these differences were especially clear in treated patients (Fig. 1B).
Fig. 1.
Taxonomic analysis of microbiota between samples and groups. A) Taxonomy bar plot of the main phyla (lower graph) and the Firmicutes/Bacteroidetes ratio (upper graph). B) Taxonomy bar plot of the top 10 most abundant taxa at the genus level for all groups (n = 80). Note, where resolution at the genus level was not possible, taxa are described at the family level, the lowest feature level obtained (lower graph). Less abundant taxa are not displayed. The α-diversity measured by the Shannon index is represented in the upper graph.
A total of 120 genera and 84 families were identified in the fecal samples of the participants. Higher bacterial richness, considered as the number of taxa at the lowest feature level obtained, was observed in the HC group. The lowest richness was found in naïve patients and it was partly recovered in pwMS following DMTs.
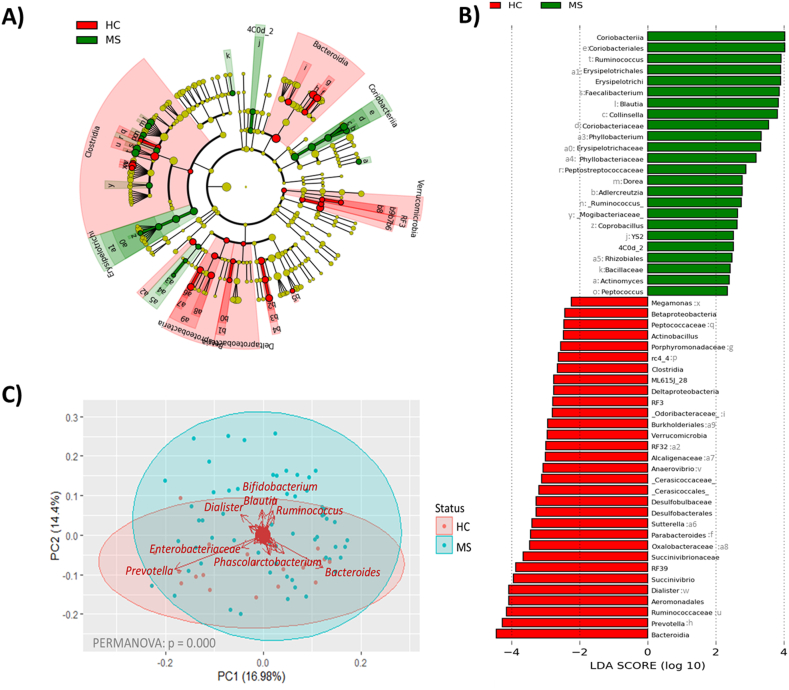
β-Diversity analysis revealed significant differences at the phylum level in the minority phylum Verrucomicrobia (p = 0.001). At the class level Bacteroidia (p = 0.009) (Bacteroidetes phylum) dominated the HC microbiota, while Coriobacteria (p = 0.000) (Actinobacteria phylum) and Erysipelotrichia (p = 0.020) (Firmicutes phylum) dominated the microbiota in the MS samples. Alterations at the genus level in the MS microbiota affected the bacterial groups Blautia (p = 0.000), Ruminococcus (p = 0.044), Sutterella (p = 0.005), Faecalibacterium (p = 0.002), Prevotella (p = 0.027), Parabacteroides (p = 0.015) and Collinsella (p = 0.005), as previously described in the literature. In addition, the abundances of the genera Phyllobacterium (p = 0.032), Anaerovibrio (p = 0.000), Dialister (p = 0.008) and Succinivibrio (p = 0.016) differed between MS patients and HCs in terms of their microbiota characteristics specific to the studied cohort (Fig. 2A and B). The microorganisms with more influence on the composition of the HC's microbiota was the genus Prevotella, while the genera Dialister, Blauttia, Bifidobacterium and Ruminococcus were in the microbiota of pwMS (Fig. 2C).
Fig. 2.
Cladogram plot (A) and linear discriminant analysis (LDA) analysis (B) showing differentially abundant bacterial groups as biomarkers determined using Kruskal-Wallis test (P < 0.05) with an LDA score >2.0. The cladograms plot shows the different taxonomic levels by rings; the root of the cladogram denotes the domain bacteria, phyla are represented in the inner ring and genera are represented in the outer one. The plots represent the microbial differences in the HC (red), and pwMS (green) groups. The LDA plot represents the microbial differences between HCs and pwMS. (C) Scatter plot representing samples grouped by HC and MS. Clustered samples were represented in different colors and the bacterial groups that had a higher influence placing the samples were represented with arrows. The bidimensional PCoA plot explains 31.4 % of the sample's variability.
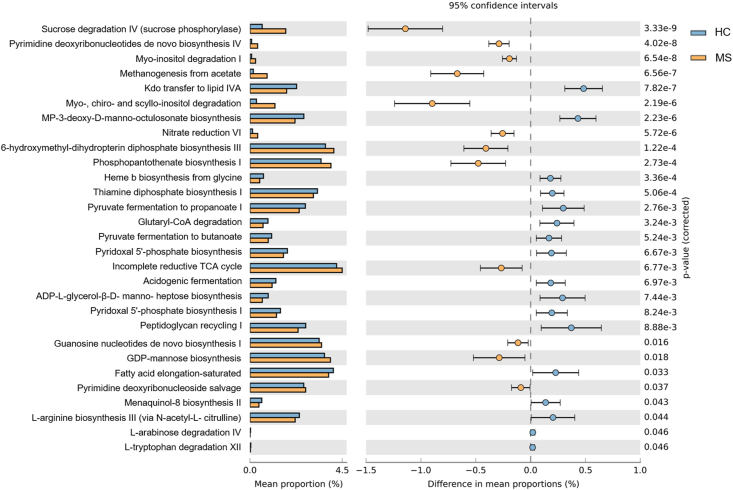
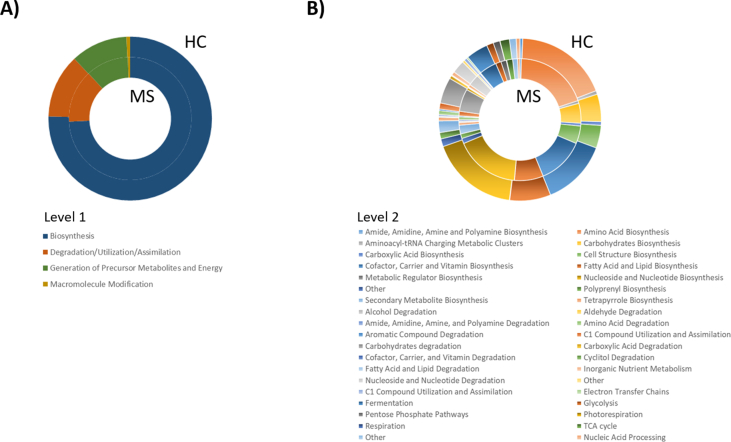
To assess the metabolic potentials of the HC and MS microbiomes, operational taxonomic units (OTUs) were entered into PICRUSt2, and the inferred gene families were annotated against KEGG Orthologies (KOs) and then collapsed into Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. In general, 283 pathways were depleted and 101 pathways were enriched in MS. Enriched pathways in MS included those related to degradation and/or assimilation processes of different compounds (54 pathways), energy acquisition (7 routes) and compound biosynthesis (40 pathways). The abundances of genes related to the bacterial biosynthesis of amino acids (p = 0.001) and cell structures (p = 0.028) increased in the MS microbiota, while the abundances of genes related to the biosynthesis of amides, amidines, amines and polyamines (p = 0.001), carbohydrates (p = 0.004), cofactors, carriers and vitamins (p = 0.000), fatty acids and lipids (p = 0.013), metabolic regulators (p = 0.023), nuclesides and nucleotides (p = 0.013); and polyprenyls (p = 0.023) decreased. Genes encoding pathways involved in bacterial degradation and/or assimilation were generally more intense in the MS microbiota, especially those related to alcohol (p = 0.001), amide, amidine, amine and polyamine (p = 0.000), carbohydrates (p = 0.000) and cyclitol (p = 0.000). Pathways involved in cofactor, carrier, and vitamin degradation and fatty acid and lipid degradation were increased in the microbiota of HCs. Regarding the pathways involved in the generation of energy, the abundances of genes involved in bacterial electron transfer chains (p = 0.006), the TCA cycle (p = 0.005) and C1 compound utilization and assimilation (p = 0.005) were increased in the MS microbiota while decreases in the abundances of genes involved in fermentation (p = 0.002), glycolysis (p = 0.023), respiration (p = 0.000) and pentose phosphate (p = 0.002) pathways were detected (Supplementary Fig. 1).
A total of 395 KEGG level 3 pathways were identified in the samples. Seven pathways were exclusively assigned to the MS microbiome, among which the 1,5-anhydrofructose degradation (PWY-6992), vitamin B6 degradation I (PWY-5499) and β-alanine biosynthesis II (PWY-3941) pathways could be identified (present in at least 20 % of MS microbiome samples). Regarding differences in predicted bacterial functional pathways, the dominance of cofactors, carriers and vitamin biosynthesis (PWY-5920, PWY0-845, PWY-6263, THISYN-PWY and PYRIDOXSYN-PWY) was highlighted in the HC microbiota, while pathways related to nucleoside and nucleotide biosynthesis (PWY-7198, PWY-7228 and PWY-7200) and to cyclitol degradation (P562-PWY and PWY-7237) were highlighted in the MS microbiota. The metabolic pathway involved in the generation of precursor metabolites and energy by fermentation (P108-PWY, CENTFERM-PWY and PWY-6590) dominated the HC pathways, while those involved in the generation of precursor metabolites and energy by respiration (METH-ACETATE-PWY) were predominant in the MS microbiota. The functional prediction results also revealed the dominance of pathways related to pyrimidine biosynthesis (PWY-7198 and PWY-7200) in the MS microbiota (Fig. 3).
Fig. 3.
Functional prediction analysis of the two groups using PICRUSt2. Extended error bar plot for two-group analysis of predicted KEGG functional data based on disease status (HC and MS) using Welch's t-test for two groups (only predicted functions with p < 0.05 are shown). Bar plots on the left side display the mean proportion of each KEGG pathway while the dot plots on the right show the differences in mean proportions using p-values. A total of 29 KEGG pathways were significantly different between HCs (blue) and MS patients (orange).
Dietary habits interfere with the microbiota composition of pwMS in a specific way
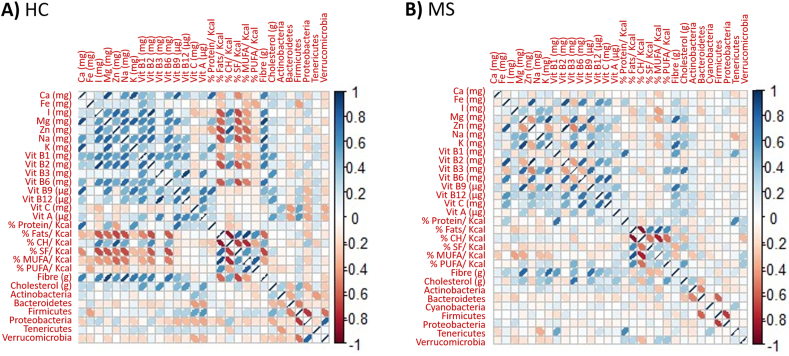
Some nutrients correlated with the bacterial population in the gut (Fig. 4), highlighting the positive correlation of vitamins A and C and cholesterol with the Firmicutes phylum in HCs (p-values of 0.017, 0.007 and 0.034 respectively). More relevant are the changes observed in pwMS, which consisted of a positive correlation of cholesterol and a negative correlation of monounsaturated fatty acids (MUFAs) with Actinobacteria (p-values of 0.040 and 0.008 respectively), a negative correlation of cholesterol and iodine (I) with Bacteroidetes (p-values of 0.036 and 0.046 respectively), a negative influence of sodium (Na), and a positive correlation of vitamin B1 and proteins in Tenericutes (p-values of 0.006, 0.000 and 0.000 respectively) and a positive correlation of the main macronutrient proteins, total fats, PUFAs and MUFAs and a negative correlation of carbohydrates with the Verrucomicrobia phylum (p-values of 0.041, 0.031, 0.005, 0.011 and 0.014 respectively) in pwMS (Supplementary Table 1).
Fig. 4.
Pearson's correlation matrix of dietary components and main phyla (present in at least 10 % of the group samples) observed in the HC (A) and MS (B) groups. Blue indicates positive correlations, while red indicates negative correlations; the color intensity and width of the ellipses are proportional to the correlation.
TF as modulator of the gut microbiota composition and function
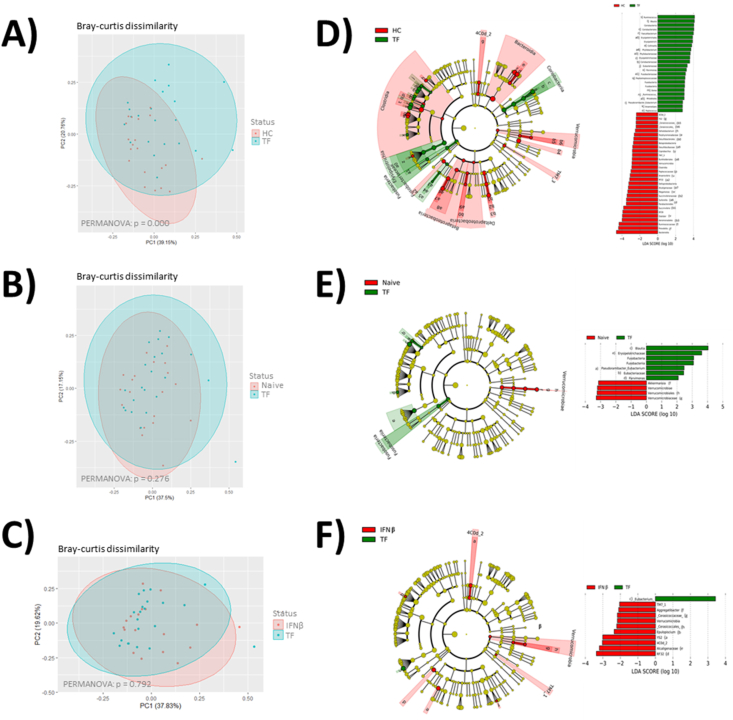
Analysis of the microbiome of pwMS treated with TF revealed that the treatment affected both the microbiota composition and its function. Major differences in microbiome composition were observed between the microbiota of patients treated with TF and HCs (Bray-Curtis dissimilarity PERMANOVA p = 0.000). Many of them coincided with those previously described between HCs and MS patients. Some specific differences could be found including those affecting the Firmicutes groups Parvimonas, Anaerostipes and Pseudoramibacter_Eubacterium; and the phylum Fusobacteria, which characterize the microbiome of patients treated with TF (Fig. 5A and D).
Fig. 5.
Scatter plot of Bray-Curtis dissimilarity, cladogram plot and linear discriminant analysis (LDA) analysis showing differentially abundant bacterial groups as biomarkers determined using Kruskal-Wallis test (P < 0.05) with an LDA score >2.0. Plots represent the microbial differences in pwMS treated with TF and HC (A and D), naïve pwMS (B and E) and pwMS treated with IFNβ (red) (C and F). The LDA plot represents the microbial differences in the study groups.
The microbiome modulation by TF has been explored by studying the differences between patient groups. Compared with those of naïve patients, the microbiomes of TF treated patients were characterized by a decrease in the Verrucomicrobia phylum and Akkermansia genus and the abundance of Aeromodales and the family Succinivibrionaceae, which belonged to the Proteobacteria phylum, but no differences in Bray-Curtis dissimilarity were found (PERMANOVA p = 0.276) (Fig. 5B and E). Compared to the microbiota of IFNβ-treated patients, that of TF-treated patients was characterized by the underrepresentation of the Cyanobacteria groups 4C0d-2 and YS2, the Verrucomicrobia group Cerasicoccaceae, the Proteobacteria groups RF32 and Alcaligenaceae, the class TM7-1 and the genus Epulopiscium, nevertheless no differences in Bray-Curtis dissimilarity were found (PERMANOVA p = 0.792) (Fig. 5C and F).
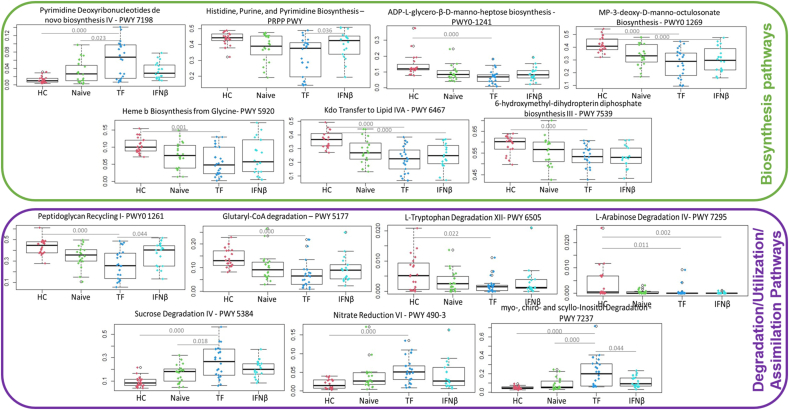
Concerning microbial functionality, the analysis of predicted KEGG functions revealed that TF treatment was able to modulate several pathways. Specifically, it was capable of increasing the levels of pyrimidine deoxyribonucleotides de novo biosynthesis IV (PWY-7198) compared with those in HC and naïve patients and decreasing the levels of the carbohydrates ADP-L-glycero-β-D-manno-heptose (PWY0-1241) and MP-3-deoxy-D-manno-octulosonate (PWY-1269), and the biosynthesis of heme b (PWY-5920), lipid IVA (PWY-6467) and 6-hydroxymethyl-dihydropterin diphosphate (PWY-7539) compared with those in HCs (Fig. 5). TF treatment also affected several degradation pathways including the reduction of peptidoglycan recycling (PWY0-1261), the degradation of glutaryl-CoA (PWY-5177), L-tryptophan (PWY-6505) and L-arabinose (PWY 7295); and the increase in sucrose degradation (PWY 5384), nitrate reduction (PWY 490-3) and myo-, chiro- and scyllo-inositol (PWY 7237) degradation with respect to those of HCs (Fig. 6).
Fig. 6.
Box plots showing the predicted bacterial functions that significantly differed between TF-treated patients and HCs or naïve or IFNβ pwMS.
Effects of MS symptoms on the gut microbiota composition and function
To evaluate the effect of common MS symptoms, microbiome composition and function were analyzed in pwMS in the presence or absence of these symptoms. The results revealed that anxiety, depression and, above all, fatigue affect the microbiome. Even the self-evaluation of the impact of MS on everyday life could be related to changes in microbiome composition and function.
Fatigue leads to an increase in several members of the Firmicutes phylum, such as the genera Oscillospira, rc4-4, Enterococcus, and Coprococcus, and unclassified genera of the families Erysipelotrichaceae, Peptococcaceae, Peptostreptococcaceae and Clostridiaceae. In contrast, unclassified genera of the family Ruminococcaceae decreased in the microbiota of patients suffering fatigue. Members of other phyla were also implicated in fatigue, such as the genera Phyllobacterium (Proteobacteria phylum), Atopobium and Actinomyces (Actinobacteria phylum), which increased when symptoms of fatigue appeared. These changes in the microbiota composition result in the major degradation of glycol (GLYCOL-GLYOXDEG-PWY), gallate and methylgallate (GALLATE-DEGRADATION-I-PWY, GALLATE-DEGRADATION-II-PWY and METHYLGALLATE-DEGRADATION-PWY), myo inositol (P562-PWY) and heparin (PWY-7644); the increased fermentation of hexitol (P461-PWY), and the major biosynthesis of the nucleotides guanosine (PWY-7222) and adenosine (PWY-7220), the amino acid L-tryptophan (TRPSYN-PWY), the carbohydrate rhamnose (DTDPRHAMSYN-PWY) and the cofactor 1,4-dihydroxy-6-naphthoate (PWY-7371) (Fig. 7A).
Fig. 7.
Taxonomic differences and functional prediction analysis from all MS participants grouped based on the results obtained from questionnaires assessing MS-related symptoms using PICRUSt2.A) Extended error bar plot for two-group analysis of genera composition (up), functional prediction (down) and fatigue symptoms using Welch's t-test for two groups (only taxonomical groups with p < 0.05 are shown). Bar plots on the left side display the mean proportion of each genus, while the dot plots on the right show the differences in mean proportions using p-values. A total of 12 taxonomical groups and 1 predicted pathway were significantly different between patients with (orange) or without (blue) fatigue symptoms according to the FSMC. B) Extended error bar plot for two-group analysis of genera composition (up), functional prediction (down) and anxiety probability using Welch's t-test for two groups. A total of 6 taxonomical groups and 1 predicted pathway were significantly different between patients with (orange) or without (blue) probable anxiety according to HADS. C) Extended error bar plot for two-group analysis of genera composition (up), functional prediction (down) and depression probability using Welch's t-test for two groups. Two taxonomical groups and two predicted pathways were significantly different between patients with (orange) or without (blue) probable depression according to HADS. D) Extended error bar plot for two-group analysis of genera composition (up) functional prediction (down) and MS psycological impact using Welch's t-test for two groups. Four taxonomical groups and eight predicted pathways were significantly different between MSIS-29 patients with scores greater than 50 (orange) or less than 50 (blue). E) Extended error bar plot for two-group analysis of genera composition (up) functional prediction (down) and MS physical impact (<37.5, 37.5–50 and > 50) using Welch's t-test for two groups. Five taxonomical groups and 12 predicted metabolic routes were significantly different between MSIS-29 patients with scores of 37.5–50 (orange) or <37.5 (blue); and 3 predicted pathways were significantly different between MSIS-29 patients with scores >50 (green) or < 37.5 (blue).
The probability of anxiety is related to the dominance of the genera Atopobium, Parabacteroides, Enterococcus and Granulicatella, and the enriched biosynthesis of sucrose (SUCSYN-PWY), legionaminate (PWY-6749) and 1,4-dihydroxy-6-naphthoate (PWY-7371) in pwMS. However, the biosynthesis of heme b (PWY0-1415), adenosylcobalamin (P381-PWY), thiamine diphosphate (PWY-6891), Factor 420 (PWY-5198) and CDP-diacylglycerol (PWY-5667) decreased. The fermentation of hexitol (P461-PWY) increased, and the degradation of sulfolactate (PWY-6641) decreased in patients with symptoms of anxiety (Fig. 7B). Symptoms of depression reduced the abundance of Oxalobacter and other unclassified genera of the family Oxalobacteriaceae. In addittion, depression is related to the reduced biosynthesis of the carbohydrates pseudaminate (PWY-6143) and acetylthomosamine (PWY-7315), the biosynthesis of S-adenosyl-L-methionine (PWY-6151), the degradation of aromatic biogenic amine (PWY-7431) and taurine (PWY-1541); and pathways related to the generation of precursor metabolites and energy, such as the TCA cycle (PWY-5913) and the ethylmalonyl-CoA pathway (PWY-5741) (Fig. 7C).
Regarding the self-evaluation of the impact of MS, the questionnaire allows the distinction of the physical and psychological impacts of the disease. Our results showed that the minor presence of the families Christensenellaceae and Oxalobacteraceae and the increase in the genera Dialister and Collinsella are related to the perception of a greater psychological impact of the disease. These compositional changes are associated with a decreased degradation of the carbohydrates D-glucarate and D-galactarate (GLUCARGALACTSUPER-PWY, GALACTARDEG-PWY and GLUCARDEG-PWY), the degradation of catechol (PWY-5415) and 2-methylcitrate (PWY0-42); and increased biosynthesis of peptidoglycan (PWY-6470), ubiquinol-10 (PWY-5857) and demethylmenaquinol-6 (PWY-7373) (Fig. 7D). The perception of increased physical impairment associated with MS is related with the major presence of the families Christensenellaceae, Clostridiaceae, Paraprevotelladaceae and Rikenellaceae and the genus Oxalobacter in the microbiota. The increased degradation of carbohydrates (GLUCARGALACTSUPER-PWY, GALACTARDEG-PWY, GLUCARDEG-PWY and KETOGLUCONMET-PWY) and other compounds, such as allantoin (PWY-5705) and formaldehyde (RUMP-PWY and PWY-1861), the major biosynthesis of the cofactor cob(II)yrinate a,c-diamide (PWY-7376), the amino acids L-isoleucine (PWY-5101) and L-methionine (HSERMETANA-PWY) and the reduced biosynthesis of adenosine (PWY-7229) were observed in patients who reported greater physical impairment. The urea cycle (PWY-4984) increased in patients who reported the major physical impact of the MS (Fig. 7E).
Discussion
The aim of this study was to determine the effect of oral DMT TF on the composition and function of the microbiota of pwMS compared to that of HCs and naïve or IFNβ-treated pwMS. First, we compared the fecal microbiome structure and function of pwMS and HCs. We also investigated the effect of diet on the composition of the microbiome in each group of participants. Then, we directed the study to DMTs, specifically the effect of oral treatment with TF versus IFNβ and naïve patients. Finally, we evaluated the effect of alterations in the microbiota on common disease symptoms such as fatigue, anxiety and depression, and self-assessed of the impact of MS on the studied participants.
Our data revealed differences in the microbiome composition and function of pwMS in comparison to HCs. β-Diversity analysis revealed differences previously described in the literature in MS as those affecting the bacterial groups Blautia, Ruminococcus, Sutterella, Faecalibacterium, Prevotella, Parabacteroides and Collinsella [8,38]; but also differences in the bacterial genera Dorea, Peptococcus, Anaerovibrio, Dialister, Megamonas, Coprobacillus and Phyllobacterium specific to the studied cohort. Regarding microbiota functionality, the general tendencies to decrease biosynthetic pathways and to increase degradation routes were observed in the microbiota of the gut of pwMS, suggesting a decrease in the microbial metabolism of patients. Daiki Takewaki et al. performed functional analysis with metagenomic data from pwMS and HCs and identified 97 KOs that were significantly enriched and 117 that were significantly depleted in MS. The authors noted the enrichment of pathways involved in energy acquisition, related to SCFA production and vitamin B12 biosynthesis [39]. We found 7 pathways significantly enriched in MS, 2 of which are involved in energy acquisition (methanogenesis from acetate and nitrate reduction), 1 in the biosynthesis of pyrimidine deoxyribonucleotides and 3 in the degradation of sugars (sucrose degradation) and inositol (myo-, chiro- and scyllo-inositol degradation and myo-inositol degradation). The increase in pyrimidine synthesis observed in the microbiota of patients may be due to patient selection, as a significant proportion of patients are treated with TF, an inhibitor of the novo synthesis of this compound. Inositol is an interesting compound that is part of the vitamin B12 complex and is a biologically active metabolite with important functions in physiological processes including reproductive, hormonal, and metabolic modulation [40,41]. The most common and bioavailable form of inositol, myo-inositol, mediates cell osmoregulation, and its derivatives act as second messengers in signal transduction pathways, and participate in proteins phosphorylation, chromatin remodeling and gene expression. Myo-inositol homeostasis depends on endogenous synthesis and catabolism, transmembrane transport, intestinal adsorption and renal excretion [41]. Myo-inositol deficiency has been related to intestinal lipodystrophy in animals [40] and high levels of triacylglycerol, cholesterol, and nonesterified lipids in the mammalian liver [40]. In recent years, the study of cholesterol metabolism in the context of MS has gained importance among the scientific community since evidence suggests that cholesterol levels might have a causal relationship with disease and disability progression [42]. On the other hand, it has been described that myo-inositol deficiency leads to immune depression in animal models [43]. In this context, disease-specific alterations in the intestinal microbiota may compromise the body's myo-inositol levels, contributing to the development and progression of MS.
The dietary habits of the studied MS patients were subtly different from those of HCs. The differences were characterized by the greater intake of fats, especially PUFAs and cholesterol (p = 0.002 and 0.001 respectively). The only group of fats with lower consumption in MS patients was MUFAs. In addittion, CH and fiber intake are generally greater in pwMS, also showing a major correlation between dietary habits and microbiota, highlighting the influence of the intake of cholesterol and the reduction in Bacteroidetes; and the consumption of proteins and fats in the increase in Akkermansia.
These results suggest that dietary protocols directed to reduce the intake of protein and total fats (with special care for cholesterol consumption), such as well-balanced plant-based diets, could help to compensate for the bacterial dysbiosis in MS. In this context, a connection has been established between meat consumption and an increased presence of blood metabolites associated with meat, a reduction in bacteria that digest polysaccharides, and a rise in circulating proinflammatory markers. Additionally, there is an enrichment of bile acid metabolites in individuals with MS [38].
Besides the differing correlations between major dietary components and the microbiota in HC and pwMS, suggest a distinct microbial metabolization or interaction with micronutrients between HC and pwMS. Whether these differences are attributed to variations in microbial composition at lower taxonomical levels (species or strains) or due to the disease itself requires further investigation.
Currently, a wide variety of drugs are available to treat relapsing remitting MS, the most common form of the disease. Most DMTs are aimed at regulating the immune system. Given the close relationship between the immune system and the microbial community in the human body, the modulatory effect of DMTs on the microbiota is not surprising. In this regard, oral treatments may be of particular interest because they involve direct contact with the microbiota in the gastrointestinal tract. To our knowledge, little is known about the effect of TF treatment on the microbiota. Nevertheless, some years ago, the effect of TF in gut-associated lymphoid tissue (GALT) in a murine model of MS was widely explored. The authors identified a mechanism by which the expansion of specific Treg cells produced in the GALT could contribute to the efficacy of TF in MS and speculated that TF may have an effect on the microbiota composition [44]. Our results showed that TF promoted microbial dysbiosis in the studied cohort of patients, with both compositional and functional microbial changes generally more pronounced in patients treated with TF. Remarkably, TF treatment reduced the abundance of Akkermansia, a bacterial genus broadly associated with MS and characteristic of our naïve pwMS population. In addittion, compared with those of both naïve and IFNβ-treated patients, the concentrations of the Proteobacteria phylum in the TF-treated group decreased. The expansion of this bacterial phylum has been previously described by our group as characteristic of people with MS from the same geographical area [45].
TF also play a modulatory role in microbial functionality. The results revealed that the increase in de novo biosynthesis of pyrimidine was especially pronounced in patients treated with TF, as if the microbiota were somehow trying to compensate for the inhibition caused by the treatment. To evaluate how this compensation contributes to immune system modulation or TF treatment efficacy, further studies are needed. The reduced synthesis of lipopolysaccharide (LPS) precursors (MP-3-deoxy-D-manno-octulosonate biosynthesis and Kdo transfer to lipid A pathways) by the microbiota of patients treated with TF should also be highlighted. LPSs are important components of the outer membrane of gram-negative bacteria and are bacterial toxins. This may compromise the proliferation of gram-negative bacteria and explain the reduced presence of Proteobacteria in the microbiota of TF-treated patients. Finally, TF seemed to affect the bacterial degradation of tryptophan (L-tryptophan degradation and glutaryl-CoA degradation). This amino acid promotes the release of serotonin, a neurotransmitter broadly associated with fatigue [46,47]. In support of this theory, tryptophan synthesis was greater in patients who reported fatigue in this study.
Fatigue is a common symptom in pwMS (reported in 75 % of patients) and is considered to be the single most debilitating symptom of MS [48]. Recent studies have evaluated the role of the microbiota in physical and mental fatigue symptomatology. A reduction in bacterial diversity and beneficial bacteria, and the accumulation of pathogens are common characteristics previously reported in individuals with fatigue. In addittion, the enrichement of the Proteobacteria and Bacteroidetes phyla, Erysipelotrichia class and Anaerostipes, Kebsiella, Bacteroides, Collinsella and Enterococcus genera; and the deprivation of the Ruminococcaceae and Lachnospiraceae families and the Dorea, Holdemania, Faecalibacterium, Lactobacillus and Eubacterium genera were previously related to extreme tiredness and reduced motivation [47,49,50]. Our results support some of these findings, such as the enrichment of Erysipelotrichiaceae and Enterococcus and the reduced presence of Ruminococcaceae in pwMS who report fatigue. Additionally, we found an association between the enriched microbiota in unclassified genera of the families Peptostreptococcaceae, Peptococcaceae, rc4-4 and Clostridiaceae; and the genera Oscillospira, Atopobium, Actinomyces, Phylobacterium and Coprococcus and symptoms of fatigue. Some of these microorganisms, such as the dominance of Actinomyces, Phyllobacterium, Erysipelotrichiaceae and Peptpstreptococcaceae members, along with the reduction of Ruminococcaceae members, have been associated with MS in our study, suggesting a potential role in fatigue symptomatology. Nevertheless, it should be noted that the lack of a standardized method for fatigue measurement and other factors associated with MS could introduce bias into the results.
Among the mental disorders of particular concern for individuals with MS, the most prevalent are anxiety and depression. Research on the microbiome–gut–brain axis revealed the critical role of the gut microbiota in maintaining mental health. In fact, a variety of studies have associated microbial dysbiosis with different mental disorders. Current research describes the enrichment of Parabacteroides (member of the phylum Bacteroidetes) and Atopobium (member of the phylum Actinobacteria) and the members of the Lactobacillales classes Enterococcus and Granulicatella in those pwMS that have reported anxiety. Previous studies revealed a reduction in the Firmicutes/Bacteroidetes ratio; the Lactobacillales class; and the abundances of the genera Faecalibacterium, Prevotella, Lachnospira and Butyricimonas in individuals with anxiety [51]. Therefore, our results mostly agree with those previously reported. The genus Atopobium, belongs to the bacterial class Coriobacteriales, and microorganisms of this class are related to human disease, as they have been associated with tumor tissue in colorectal cancer [52], higher levels of intestinal permeability and inflammation biomarkers [53] and other mental disorders, such as depression [54]. Considering microbiota functionality, to our knowledge, no differences in anxiety have been previously found [55]. Among the metabolic pathways significantly affected by anxiety, an altered vitamin production profile was detected in our study (reduced adenosylcobalamin biosynthesis II, the biologically active form of vitamin B12; production of thiamine (thiazole component of thiamine diphosphate biosynthesis II); and increased 1,4-dihydroxy-6-naphthoate biosynthesis II, an intermediate in vitamin K synthesis). Lower levels of thiamine have been associated with anxiety; in fact, thiamine supplementation significantly improved anxiety scores [56,57]. The reduction of β-Proteobacteria, as Oxalobacteraceae family and Oxalobacter genus detected in our MS population, has been previously described in depression [51,58]. In terms of microbial functionality alterations in depression, reduced taurine degradation, an amino acid that contributes to energy production and confers some neuroprotection, was observed in patients who reported depression.
Finally, regarding the impact of the disease on the everyday life of patients, prominent bacterial groups (Dialister, Collinsella, Prevotella, Rickenellaceae, and Oxalobacteriaceae) and metabolic pathways (sugars degradation and energy production pathways) previously associated with the disease stand out, suggesting that microbial dysbiosis is more common in patients with more severe symptoms or everyday challenges. Additionally, other metabolic pathways that increase with the reported impact of MS include the major synthesis of the antioxidant ubiquinol-10 and the oxidation and assimilation of formaldehyde. These results suggest that oxidative stress and formaldehyde levels could be increase in the gut of patients with severe MS.
The modulation of the microbiota by TF treatment in patients is not sufficiently pronounced to allow assessment of its potential impact on MS-related symptoms. While subtle changes in microbial composition may occur, they do not appear to be significant enough to establish a clear connection between TF treatment and the modulation of symptoms such as fatigue, anxiety, or depression in MS patients. Further research with more pronounced microbial shifts or larger patient cohorts may be required to better understand the role of TF treatment in influencing MS-related symptomatology through microbiota alterations.
The results of the current study contribute to the identification of key microbial genera and microbial metabolic pathways, providing insights into which ones influence MS microbiota modulation. Nevertheless, further research is necessary to determine the causal associations between specific genera in the gut microbiota and MS development and progression, the suitability of treatments and the prevention of MS associated symptoms.
Ethics approval and consent to participate
The study was approved by the local ethics committee on March 26, 2019 under the research protocol TCT-UEM-2019-01. All participants signed an informed consent form before entering the study.
Consent for publication
Not applicable.
Availability of data and materials
The datasets presented in this study were submitted to the SRA NCBI repository under the BioProject ID PRJNA1123472 and the BioSample accessions SAMN41812384- SAMN41812463.
Funding
This work was supported by the Department of Health of the Basque Government under Grant 2018111038. L.M was supported by the Sara Borrell contract (the National Institute of Health Carlos III). A.O.C was supported by the Department of Education of the Basque Government and M. G. A by the University of the Basque Country.
Authors' Contributions
DO, TCT and LM conceived the study concept and design. TCT, MA, AAA, EF, LR and IM recruited the participants and collected the samples. HCV, AOC and LM processed the biological samples. MGA, AOC and LM supported the microbial data and performed the statistical analysis. LM wrote the first draft of the manuscript, and all the authors contributed to its revision. All the authors have read and approved the final manuscript.
Declaration of competing interest
The authors declare that they do not have conflicts of interest. The founding sponsors had no role in the design of the study or the decision to publish the results.
Acknowledgments
We would like to thank all the donors who participated in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurot.2024.e00457.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Figure S1.
References
- 1.Koch-henriksen N., Thygesen L.C., Stenager E., Laursen B. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology. 2018;90:e1954–e1963. doi: 10.1212/WNL.0000000000005612. [DOI] [PubMed] [Google Scholar]
- 2.Miller A.L., Bessho S., Grando K., Tükel Ç. Microbiome or infections: amyloid-containing biofilms as a trigger for complex human diseases. Front Immunol. 2021;12(February):1–16. doi: 10.3389/fimmu.2021.638867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sintzel M.B., Rametta M., Reder A.T. Vitamin D and multiple sclerosis: a comprehensive review. Neurol Ther [Internet] 2018;7(1):59–85. doi: 10.1007/s40120-017-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremlett H., Zhu F., Ascherio A., Munger K.L. Sun exposure over the life course and associations with multiple sclerosis. Neurology. 2018;90(14):E1191–E1199. doi: 10.1212/WNL.0000000000005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishanth K., Tariq E., Nzvere F.P., Miqdad M., Cancarevic I. Role of smoking in the pathogenesis of multiple sclerosis: a review article. Cureus. 2020;12(8) doi: 10.7759/cureus.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sedighi S., Gholizadeh O., Yasamineh S., Akbarzadeh S., Amini P., Favakehi P., et al. Comprehensive investigations relationship between viral infections and multiple sclerosis pathogenesis. Curr Microbiol [Internet] 2023;80(1):1–11. doi: 10.1007/s00284-022-03112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X., Baumann R., Gao X., Mendoza M., Singh S., Katz Sand I., et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. 2022;185(19):3467–3486.e16. doi: 10.1016/j.cell.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochoa-Repáraz J., Kirby T.O., Kasper L.H. The gut microbiome and multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(6):1–16. doi: 10.1101/cshperspect.a029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morais L.H., Schreiber H.L., Mazmanian S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol [Internet] 2021;19(4):241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 10.Rutsch A., Kantsjö J.B., Ronchi F. The gut-brain Axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. 2020;11(December):1–24. doi: 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res [Internet] 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipi M., Jack S. Interferons in the treatment of multiple sclerosis: a clinical efficacy, safety, and tolerability update. Int J MS Care. 2020;22(4):165–172. doi: 10.7224/1537-2073.2018-063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rommer P.S., Milo R., Han M.H., Satyanarayan S., Sellner J., Hauer L., et al. Immunological aspects of approved MS therapeutics. Front Immunol. 2019;10(JULY):1–24. doi: 10.3389/fimmu.2019.01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermersch P., Oh J., Cascione M., Oreja-Guevara C., Gobbi C., Travis L.H., et al. Teriflunomide vs injectable disease modifying therapies for relapsing forms of MS. Mult Scler Relat Disord [Internet] 2020;43(October 2019) doi: 10.1016/j.msard.2020.102158. [DOI] [PubMed] [Google Scholar]
- 15.Miller A.E. An updated review of teriflunomide's use in multiple sclerosis. Neurodegener Dis Manag. 2021;11(5):387–409. doi: 10.2217/nmt-2021-0014. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Or A., Pachner A., Menguy-Vacheron F., Kaplan J., Wiendl H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs. 2014;74(6):659–674. doi: 10.1007/s40265-014-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Kleijn K.M.A., Martens G.J.M. Molecular effects of FDA-approved multiple sclerosis drugs on glial cells and neurons of the central nervous system. Int J Mol Sci. 2020;21(12):1–49. doi: 10.3390/ijms21124229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo-Álvarez F., Pérez-Matute P., Oteo J.A., Marzo-Sola M.E. The influence of interferon β-1b on gut microbiota composition in patients with multiple sclerosis. Neurol (English Ed [Internet] 2021;36(7):495–503. doi: 10.1016/j.nrleng.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Katz Sand I., Zhu Y., Ntranos A., Clemente J.C., Cekanaviciute E., Brandstadter R., et al. Disease-modifying therapies alter gut microbial composition in MS. Neurol Neuroimmunol NeuroInflammation. 2019;6(1):1–13. doi: 10.1212/NXI.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox L.M., Maghzi A.H., Liu S., Tankou S.K., Dhang F.H., Willocq V., et al. Gut microbiome in progressive multiple sclerosis. Ann Neurol. 2021;89(6):1195–1211. doi: 10.1002/ana.26084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilotto S., Zoledziewska M., Fenu G., Cocco E., Lorefice L. Disease-modifying therapy for multiple sclerosis: implications for gut microbiota. Mult Scler Relat Disord [Internet] 2023;73(March) doi: 10.1016/j.msard.2023.104671. [DOI] [PubMed] [Google Scholar]
- 22.Vacaras V., Muresanu D.F., Buzoianu A.D., Nistor C., Vesa S.C., Paraschiv A.C., et al. The role of multiple sclerosis therapies on the dynamic of human gut microbiota. J Neuroimmunol [Internet] 2023;378(February) doi: 10.1016/j.jneuroim.2023.578087. [DOI] [PubMed] [Google Scholar]
- 23.Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 24.Penner I.K., Raselli C., Stöcklin M., Opwis K., Kappos L., Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler. 2009;15(12):1509–1517. doi: 10.1177/1352458509348519. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann C. International experiences with the hospital anxiety and depression scale - a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41. doi: 10.1016/s0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 27.Lisspers J., Nygren A., Söderman E. Hospital anxiety and depression scale (HAD): some psychometric data for a Swedish sample. Acta Psychiatr Scand. 1997;96(4):281–286. doi: 10.1111/j.1600-0447.1997.tb10164.x. [DOI] [PubMed] [Google Scholar]
- 28.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the hospital anxiety and depression scale. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 29.Johnston M., Pollard B., Hennessey P. Construct validation of the hospital anxiety and depression scale with clinical populations. J Psychosom Res. 2000;48(6):579–584. doi: 10.1016/s0022-3999(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 30.Hobart J., Lamping D., Fitzpatrick R., Riazi A., Thompson A. The multiple sclerosis impact scale (MSIS-29) a new patient-based outcome measure. Brain. 2001;124(5):962–973. doi: 10.1093/brain/124.5.962. [DOI] [PubMed] [Google Scholar]
- 31.Widener G.L., Allen D.D. Measurement characteristics and clinical utility of the 29-item multiple sclerosis impact scale. Arch Phys Med Rehabil [Internet] 2014;95(3):593–594. doi: 10.1016/j.apmr.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 32.QIIME2 forum. Available from: https://forum.qiime2.org/t/dada2-denoise-pyro-for-ion-torrent/17605.
- 33.QIIME2 forum. Available from: https://forum.qiime2.org/t/rarefying-when-having-multiple-runs/22087/18.
- 34.QIIME2 forum [Internet]. Available from: https://forum.qiime2.org/t/cutadapt-in-ion-torrent-sequences/22165/10.
- 35.QIIME2 forum. Available from: https://forum.qiime2.org/t/classifier-for-ion-torrent-data/3675/9.
- 36.Gorostidi-Aicua M. Mbiome. GitHub repository. https://github.com/MGorostidi/mbiome
- 37.Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30(21):3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantoni C., Lin Q., Dorsett Y., Ghezzi L., Liu Z., Pan Y., et al. Alterations of host-gut microbiome interactions in multiple sclerosis. eBioMedicine [Internet] 2022;76 doi: 10.1016/j.ebiom.2021.103798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takewaki D., Suda W., Sato W., Takayasu L., Kumar N., Kimura K., et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc Natl Acad Sci U S A. 2020;117(36):22402–22412. doi: 10.1073/pnas.2011703117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chhetri D.R. Myo-inositol and its derivatives: their emerging role in the treatment of human diseases. Front Pharmacol. 2019;10(October):1–8. doi: 10.3389/fphar.2019.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lepore E., Lauretta R., Bianchini M., Mormando M., Di Lorenzo C., Unfer V. Inositols depletion and resistance: principal mechanisms and therapeutic strategies. Int J Mol Sci. 2021;22(13) doi: 10.3390/ijms22136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandra A., Xu Y.M. Neuroimmunology. 2016;7:145–157. [Google Scholar]
- 43.Jiang W.D., Hu K., Liu Y., Jiang J., Wu P., Zhao J., et al. Dietary myo-inositol modulates immunity through antioxidant activity and the Nrf2 and E2F4/cyclin signalling factors in the head kidney and spleen following infection of juvenile fish with Aeromonas hydrophila. Fish Shellfish Immunol [Internet] 2016;49:374–386. doi: 10.1016/j.fsi.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Ochoa-Repáraz J., Colpitts S.L., Kircher C., Kasper E.J., Telesford K.M., Begum-Haque S., et al. Induction of gut regulatory CD39+ T cells by teriflunomide protects against EAE. Neurol Neuroimmunol NeuroInflammation. 2016;3(6):1–9. doi: 10.1212/NXI.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moles L., Delgado S., Gorostidi-Aicua M., Sepúlveda L., Alberro A., Iparraguirre L., et al. Microbial dysbiosis and lack of SCFA production in a Spanish cohort of patients with multiple sclerosis. Front Immunol. 2022;13(October):1–10. doi: 10.3389/fimmu.2022.960761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita M. Potential role of neuroactive tryptophan metabolites in central fatigue: establishment of the fatigue circuit. Int J Tryptophan Res. 2020;13 doi: 10.1177/1178646920936279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boolani A., Gallivan K.M., Ondrak K.S., Christopher C.J., Castro H.F., Campagna S.R., et al. Trait energy and fatigue may Be connected to gut bacteria among young physically active adults: an exploratory study. Nutrients. 2022;14(3):1–16. doi: 10.3390/nu14030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braley T.J., Chervin R.D. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep. 2010;33(8):1061–1067. doi: 10.1093/sleep/33.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farhadfar N., Gharaibeh R.Z., Dahl W.J., Mead L., Alabasi K.M., Newsome R., et al. Gut microbiota dysbiosis associated with persistent fatigue in hematopoietic cell transplantation survivors: N. Farhadfar et al. Transplant Cell Ther [Internet] 2021;27(6):498.e1–498.e8. doi: 10.1016/j.jtct.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Guo C., Che X., Briese T., Ranjan A., Allicock O., Yates R.A., et al. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell Host Microbe. 2023;31(2):288–304.e8. doi: 10.1016/j.chom.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong R., Li J., Cheng J., Zhou D., Wu S., Huang S., et al. The role of gut microbiota in anxiety , depression , and other. Nutrient. 2023;15(3258) doi: 10.3390/nu15143258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arthur J.C., Jobin C. The complex interplay between inflammation, the microbiota and colorectal cancer. Gut Microb. 2013;4(3):253–258. doi: 10.4161/gmic.24220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaczmarczyk M., Löber U., Adamek K., Węgrzyn D., Skonieczna-Żydecka K., Malinowski D., et al. The gut microbiota is associated with the small intestinal paracellular permeability and the development of the immune system in healthy children during the first two years of life. J Transl Med [Internet] 2021;19(1):1–26. doi: 10.1186/s12967-021-02839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar A., Pramanik J., Goyal N., Chauhan D., Sivamaruthi B.S., Prajapati B.G., et al. Gut microbiota in anxiety and depression: unveiling the relationships and management options. Pharmaceuticals. 2023;16(4) doi: 10.3390/ph16040565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butler M.I., Bastiaanssen T.F.S., Long-Smith C., Morkl S., Berding K., Ritz N.L., et al. The gut microbiome in social anxiety disorder: evidence of altered composition and function. Transl Psychiatry. 2023;13(1):1–12. doi: 10.1038/s41398-023-02325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.vinh quốc Lương K., Nguyễn L.T.H. The impact of thiamine treatment on generalized anxiety disorder. Int J Clin Med. 2011;2(4):439–443. [Google Scholar]
- 57.Li H., Xu H., Wen W., Wu L., Xu M., Luo J. Thiamine deficiency causes long-lasting neurobehavioral deficits in mice. Brain Sci. 2020;10(8):1–14. doi: 10.3390/brainsci10080565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bibbò S., Fusco S., Ianiro G., Settanni C.R., Ferrarese D., Grassi C., et al. Gut microbiota in anxiety and depression: pathogenesis and therapeutics. Front Gastroenterol. 2022;1(October):1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study were submitted to the SRA NCBI repository under the BioProject ID PRJNA1123472 and the BioSample accessions SAMN41812384- SAMN41812463.