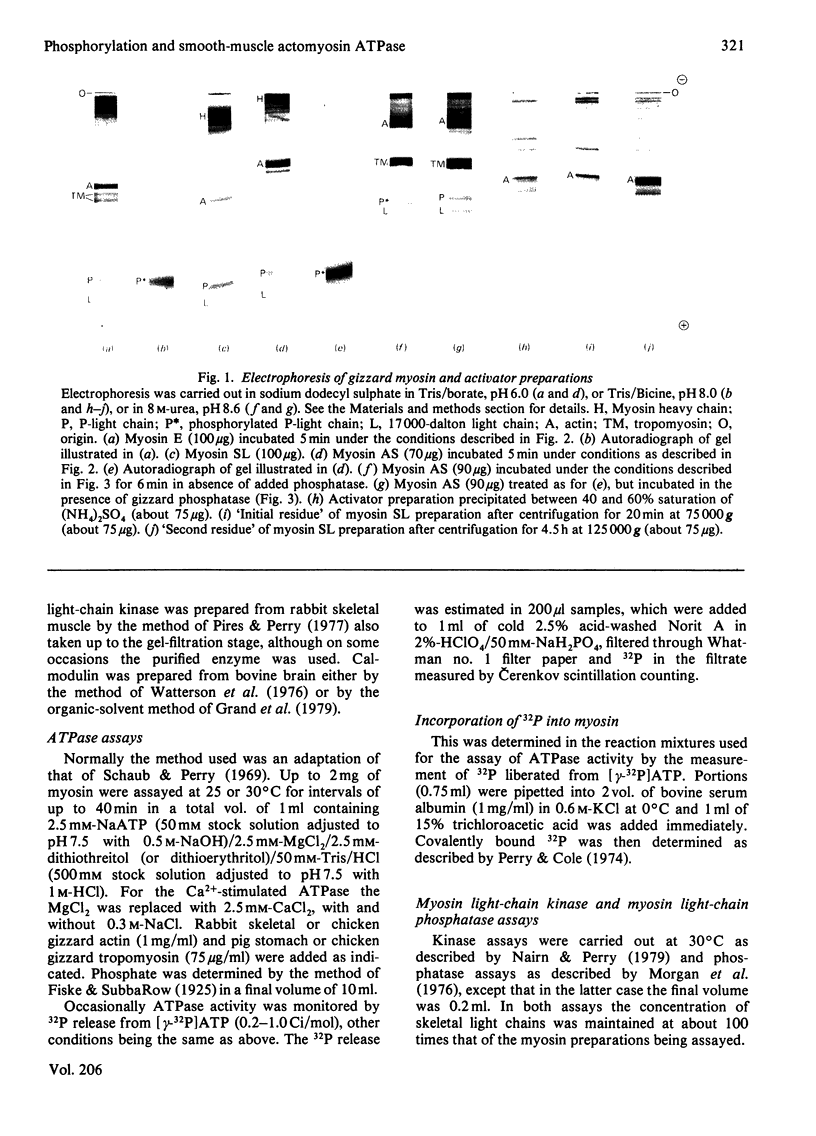
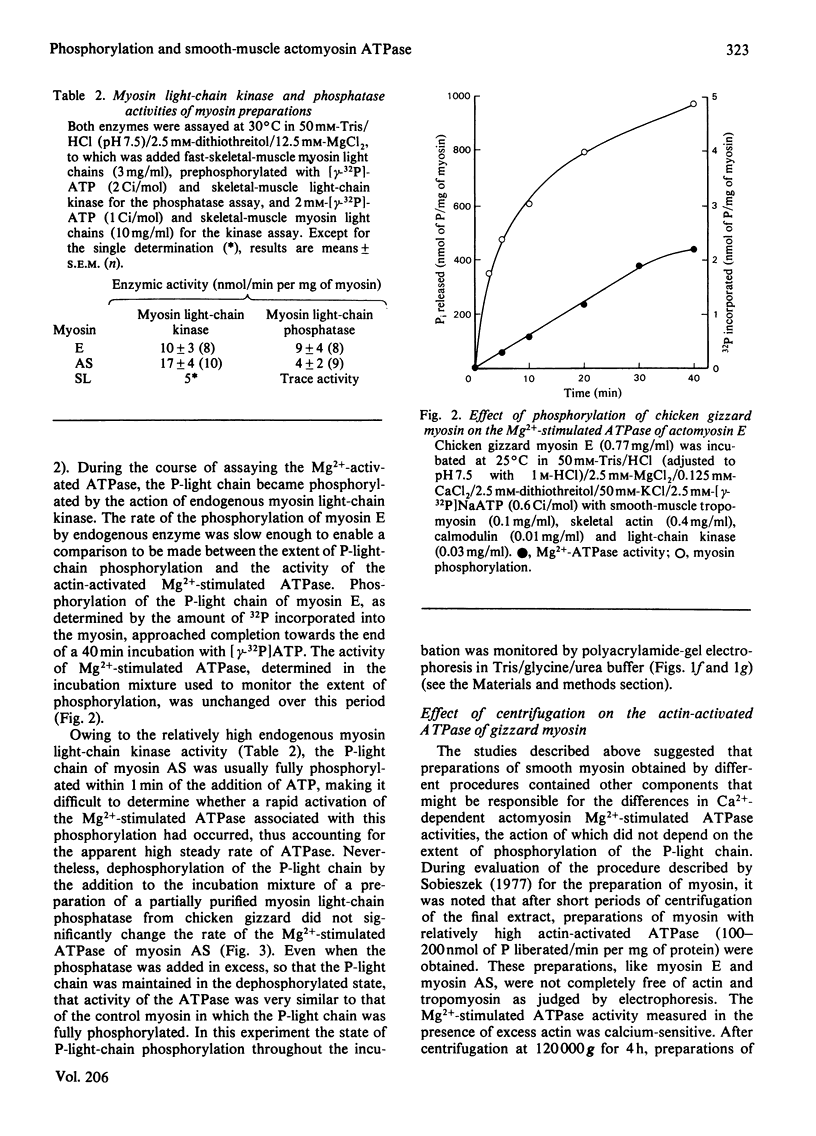
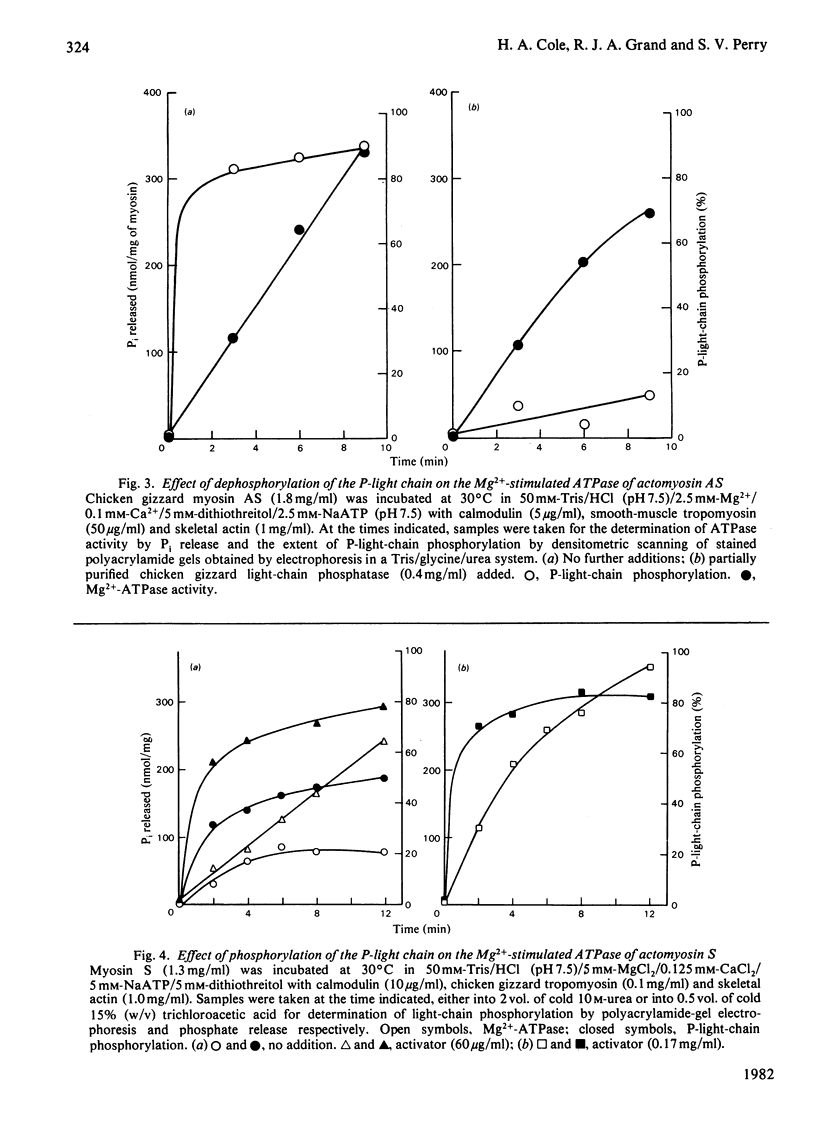
Abstract
1. The enzymic properties of myosin isolated from chicken gizzard by three different methods have been compared. 2. Although the specific Ca2+-stimulated ATPases of all preparations were similar and high, there were significant differences in the specific activities of the Mg2+-stimulated actomyosin ATPases. 3. There was no direct correlation between the Mg2+-stimulated actomyosin ATPase activity and the extent of P-light-chain phosphorylation in any of the three myosin preparations. 4. A fraction that activates the Mg2+-stimulated actomyosin ATPase of gizzard muscle has been isolated from a gizzard muscle filament preparation. 5. The activator was specific for the Mg2+-activated actomyosin ATPase of smooth muscle. 6. The activator required the addition of calmodulin for full effect.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Chacko S., Conti M. A., Adelstein R. S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):129–133. doi: 10.1073/pnas.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S. A simple method of preparing actin-free myosin from smooth muscle. J Biochem. 1976 Jan;79(1):229–231. doi: 10.1093/oxfordjournals.jbchem.a131052. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górecka A., Aksoy M. O., Hartshorne D. J. The effect of phosphorylation of gizzard myosin on actin activation. Biochem Biophys Res Commun. 1976 Jul 12;71(1):325–331. doi: 10.1016/0006-291x(76)90286-2. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Perry S. V., Schaub M. C. A protein factor inhibiting the magnesium-activated adenosine triphosphatase of desensitized actomyosin. Biochem J. 1967 Sep;104(3):907–913. doi: 10.1042/bj1040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh N., Kubo S. Purification and some properties of rabbit stomach myosin. J Biochem. 1977 May;81(5):1497–1503. [PubMed] [Google Scholar]
- Marston S. B., Trevett R. M., Walters M. Calcium ion-regulated thin filaments from vascular smooth muscle. Biochem J. 1980 Feb 1;185(2):355–365. doi: 10.1042/bj1850355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T., Nonomura Y., Hirata M., Ebashi S., Kakiuchi S. Involvement of an acidic protein in regulation of smooth muscle contraction by the tropomyosin-leiotonin system. J Biochem. 1978 Dec;84(6):1633–1636. doi: 10.1093/oxfordjournals.jbchem.a132290. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Toyo-oka T., Nonomura Y., Ebashi S. Essential factor of gizzard "troponin" fraction. A new type of regulatory protein. J Biochem. 1977 Jan;81(1):273–275. doi: 10.1093/oxfordjournals.jbchem.a131447. [DOI] [PubMed] [Google Scholar]
- Moir A. J., Cole H. A., Perry S. V. The phosphorylation sites of troponin T from white skeletal muscle and the effects of interaction with troponin C on their phosphorylation by phosphorylase kinase. Biochem J. 1977 Feb 1;161(2):371–382. doi: 10.1042/bj1610371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M., Perry S. V., Ottaway J. Myosin light-chain phosphatase. Biochem J. 1976 Sep 1;157(3):687–697. doi: 10.1042/bj1570687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K. J., England P. J. Contraction in intact pig aortic strips is not always associated with phosphorylation of myosin light chains. Biochem J. 1980 Dec 15;192(3):967–970. doi: 10.1042/bj1920967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Perry S. V. Calmodulin and myosin light-chain kinase of rabbit fast skeletal muscle. Biochem J. 1979 Apr 1;179(1):89–97. doi: 10.1042/bj1790089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemrick S. M. The phosphorylated L2 light chain of skeletal myosin is a modifier of the actomyosin ATPase. J Biol Chem. 1980 Sep 25;255(18):8836–8841. [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A., Mrwa U., Hartshorne D. J. Effect of phosphorylation on the actin-activated ATPase activity of myosin. Biochem Biophys Res Commun. 1981 Feb 12;98(3):800–805. doi: 10.1016/0006-291x(81)91182-7. [DOI] [PubMed] [Google Scholar]
- Pires E. M., Perry S. V. Purification and properties of myosin light-chain kinase from fast skeletal muscle. Biochem J. 1977 Oct 1;167(1):137–146. doi: 10.1042/bj1670137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The relaxing protein system of striated muscle. Resolution of the troponin complex into inhibitory and calcium ion-sensitizing factors and their relationship to tropomyosin. Biochem J. 1969 Dec;115(5):993–1004. doi: 10.1042/bj1150993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Myosin-linked calcium regulation in vertebrate smooth muscle. J Mol Biol. 1976 Mar 25;102(1):75–92. doi: 10.1016/0022-2836(76)90074-7. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Butler T. M., Bond M., Somlyo A. P. Myosin filaments have non-phosphorylated light chains in relaxed smooth muscle. Nature. 1981 Dec 10;294(5841):567–569. doi: 10.1038/294567a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Walters M., Marston S. B. Phosphorylation of the calcium ion-regulated thin filaments from vascular smooth muscle. A new regulatory mechanism? Biochem J. 1981 Jul 1;197(1):127–139. doi: 10.1042/bj1970127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]