Abstract
Background
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a chronic nasal and sinonasal inflammatory disease. Recently, resident memory B (BRM) cells have been identified in the lungs, although not in the sinonasal mucosa.
Objective
Our aim was to characterize memory B-cell phenotypes with regard to patients with CRSwNP and identify BRM cells in both normal sinonosal mucosa and samples from patients with CRSwNP.
Methods
CD19+ B cells were isolated from patients with CRSwNP and analyzed using flow cytometry and immunohistochemistry.
Results
Although BRM cells were found in the normal sinonasal mucosa, their numbers and frequencies tended to be limited. These findings were confirmed on the basis of immunohistochemical analyses indicating an upregulation of CD69/CD45RB in tissue sections from patients with CRSwNP, although not in normal sinonasal mucosa. Accordingly, BRM cells were established to be enriched in the nasal polyps isolated from patients with CRSwNP.
Conclusion
Our findings in this study reveal that BRM cells can be detected in normal sinonasal mucosa, although they are significantly enriched in nasal polyps derived from patients with CRSwNP. These findings can contribute to gaining a more comprehensive understanding of the immune reactions associated with CRSwNP and facilitate the identification of potential therapeutic targets, such as anti–B-cell therapy.
Key words: Chronic rhinosinusitis with nasal polyps, CRSwNP, nasal polyps, resident memory B cells, memory B cells
Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a chronic nasal and sinonasal inflammatory disease characterized by symptoms that include nasal obstruction, nasal discharge, and olfactory disturbance, which can persist for more than 12 weeks.1 Although the pathophysiology of CRSwNP is heterogeneous and remains incompletely elucidated,2 it has been suggested that immune reactions orchestrated by the adaptive and innate immune systems originating from epithelial cells mount responses targeting exogenous antigenic stimuli.3 Moreover, the findings of previous studies have provided evidence indicating alterations in the naive/memory phenotype of B-cell populations isolated from nasal polyps.4
Recently, resident memory B (BRM) cells have been identified in the lungs of mice subsequent to influenza infection,5 as well as in human barrier tissues, including the lungs, skin, intestines, and female reproductive tract.6 The BRM cells isolated from human lung tissues are characterized by the surface markers CD27+IgD–CD69+CD45RB+CD38–CD83–, with variable CD45RB expression being detected in other barrier tissues (those of the skin, gut, and female reproductive tract).7 In addition to these markers, PD-L2 and CD80 are upregulated in BRM cells.8 The primary function of BRM cells is defense against pathogens following an initial infection,5 and given that the human nasal mucosa is the primary site of antigen encounter, it is assumed that BRM cells would be distributed in the sinonasal mucosa. We accordingly hypothesized that production of BRM cells would increase in patients with CRSwNP.
Results and discussion
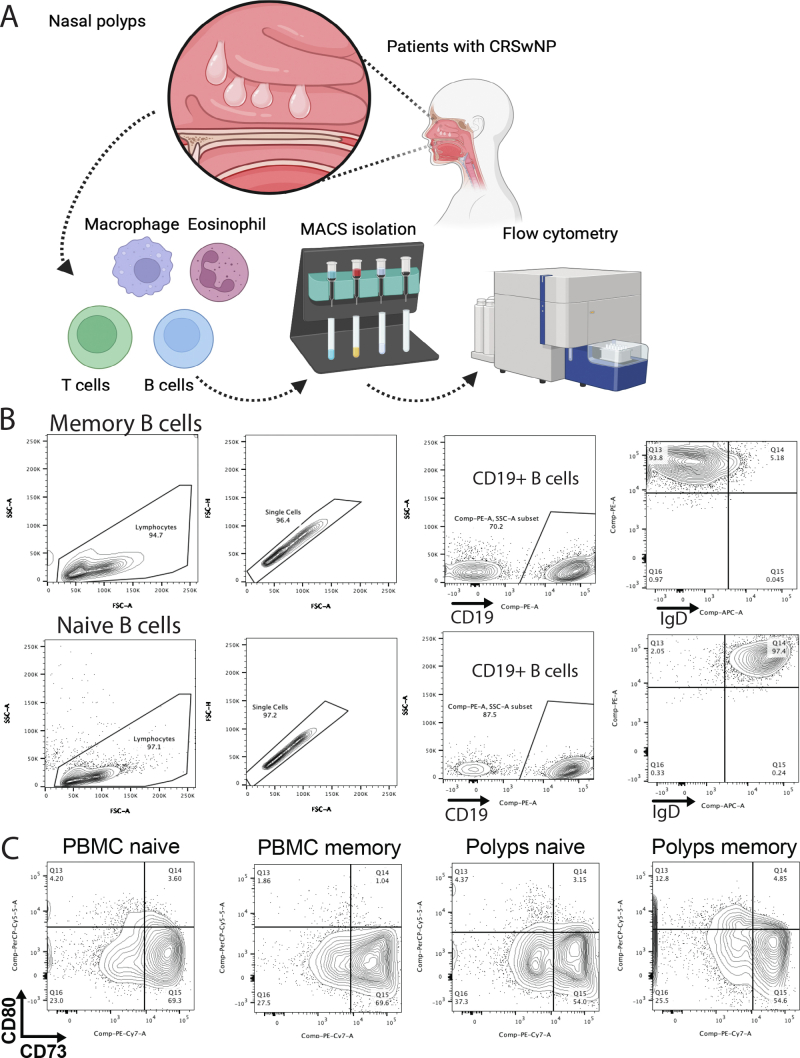
The key mediators of allergic reactions include B cells,9 and to evaluate the B-cell phenotype, we isolated CD19+ B cells from normal sinonasal mucosa during surgical procedures for concha bullosa or inverted papilloma removal (control group [n = 4]) as well as from sinonasal mucosa of patients with CRSwNP (n = 7), the characteristics of which are shown in Table E1 (see the Online Repository at www.jaci-global.org). In addition, PBMCs were isolated from the same patients. The study protocol was approved by the institutional review board of Jikei University School of Medicine (approval no. 33-407(11032)). The study subjects consisted of individuals aged 18 years or older who had undergone endoscopic sinus surgery between June 2022 and March 2023. Written consent was obtained from all subjects, who had been verbally informed regarding the nature of the study. The diagnosis of CRSwNP1 and surgical indications were determined using endoscopic and computed tomography examinations, which confirmed the failure of conservative treatments to achieve healing. Nasal polyp score was evaluated unilaterally on a scale of 0 to 4 points (0-8 points bilaterally),2 whereas computed tomography score was assessed unilaterally on a scale of 0 to 12 points (0-24 points bilaterally).3 For both assessments, a score of 0 indicated the absence of lesions. A definitive diagnosis was established on the basis of postoperative pathologic examination. Control tissue samples were collected from normal sinonasal mucosa during surgical procedures for concha bullosa or inverted papilloma removal, and whole blood samples were collected at the time of surgery. The criteria for exclusion of patients included a history of systemic steroid use within 1 month before surgery, use of immunosuppressive medications, application of biologic agents within 6 months before surgery, and ongoing treatment for malignancy.
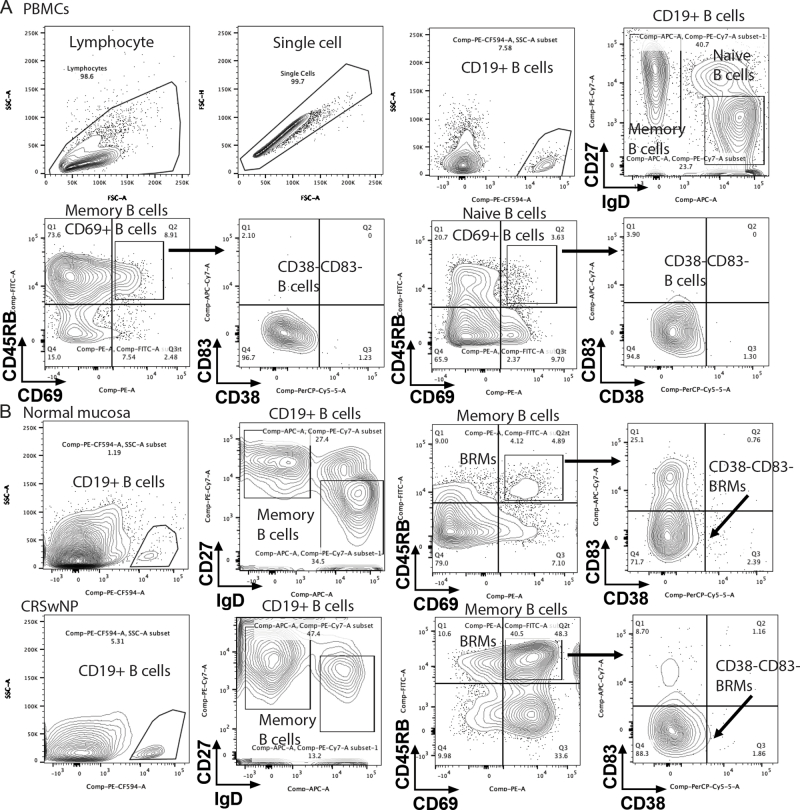
Flow cytometric immunophenotyping identified CD19+ B cells in both control individuals and patients with CRSwNP; the analysis revealed no significant increase in frequency of CD19+ B cells in patients with CRSwNP (Fig 1, A and B and see Fig E1 in the Online Repository at www.jaci-global.org). Furthermore, in both the controls and the patients with CRSwNP, compared with PBMCs, the CD19+ B cells isolated from tissue were found to have shifted to a memory phenotype to the same extent (Fig 1, C), thus indicating that memory B cells are enriched in the sinonasal mucosa of both control individuals and patients.
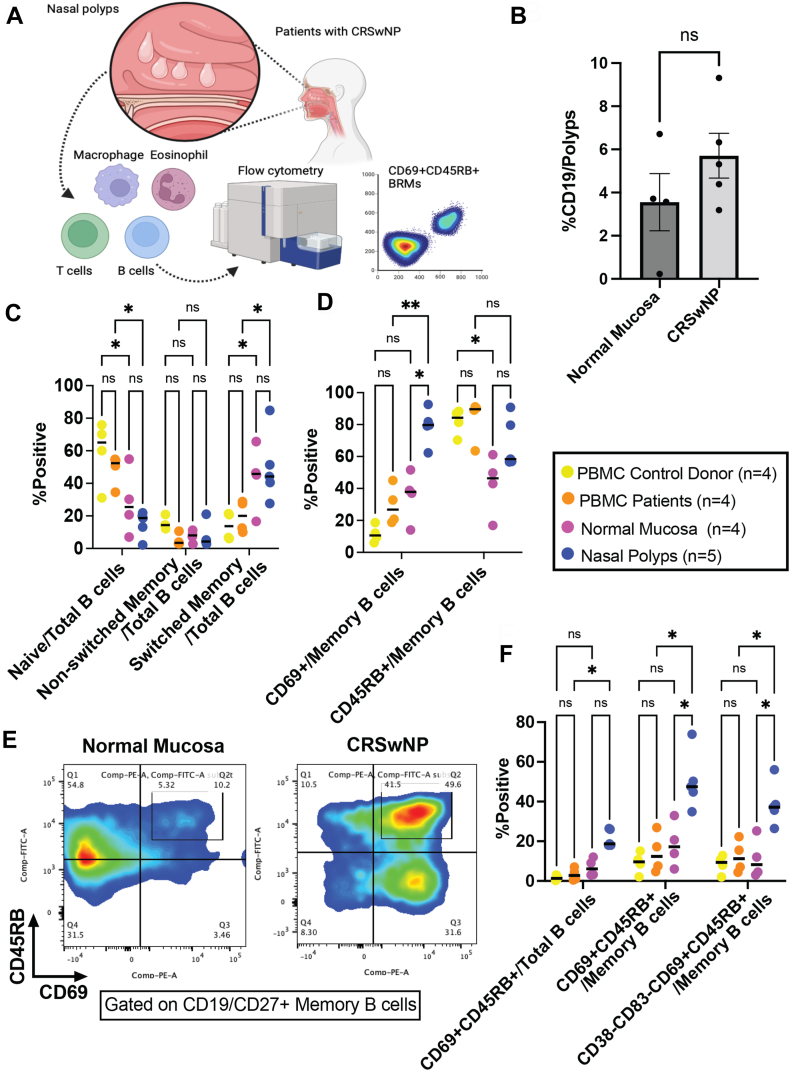
Fig 1.
BRM cells were detected in normal sinonasal mucosa and further enriched in chronic rhinosinusitis with nasal polyps. A, Schematic illustration of the study. B, Frequency of CD19+ B cells in digested normal sinonasal mucosa and tissue from patients with CRSwNP (n = 4 samples of normal sinonasal mucosa and n = 5 samples from patients with CRSwNP). Digested normal sinonasal mucosa and sinonasal mucosa from patients with CRSwNP were analyzed by flow cytometry. C, Naive/memory phenotype of B cells. D, CD69+CD45RB expression in memory B cells. E, Representative dot plots of memory B cells isolated from normal sinonasal mucosa and sinonasal mucosa from patients with CRSwNP. F, Characteristics of CD69+CD45RB+ BRM cells isolated from nasal polyps. Brown-Forsythe ANOVA followed by the Dunnett T3 multiple comparisons post hoc test. ∗P < .05; ∗∗P < .01.
Interestingly, the frequency of CD69+ B cells was higher in the nasal polyps than in the normal sinonasal mucosa and PBMCs isolated from the same patients with CRSwNP (Fig 1, D), whereas we detected a comparable frequency of CD69+ B cells in the PBMCs isolated from the controls and patients with CRSwNP. Although we detected no reduction in CD45RB+ expression in memory B cells isolated from PBMCs and nasal polyps, expression was reduced in the normal sinonasal mucosa versus in the PBMCs isolated from the same controls (Fig 1, D). Furthermore, CD69+CD45RB+ memory B cells were identified in normal sinonasal mucosa, and the frequency of these cells was found to be significantly higher in nasal polyps (Fig 1, E). In addition, the numbers of BRM cells (defined as CD38–CD83–CD69+CD45RB+ memory B cells) isolated from normal sinonasal mucosa and PBMCs were higher than the numbers in the nasal polyps isolated from those same donors (Fig 1, F). Although BRM cells were found in the normal sinonasal mucosa, their numbers and frequencies tended to be limited. These findings were confirmed on the basis of immunohistochemical analyses indicating an upregulation of CD69/CD45RB in tissue sections from patients with CRSwNP, although not in normal sinonasal mucosa (Fig 2, A). Accordingly, BRM cells were established to be enriched in the nasal polyps isolated from patients with CRSwNP.
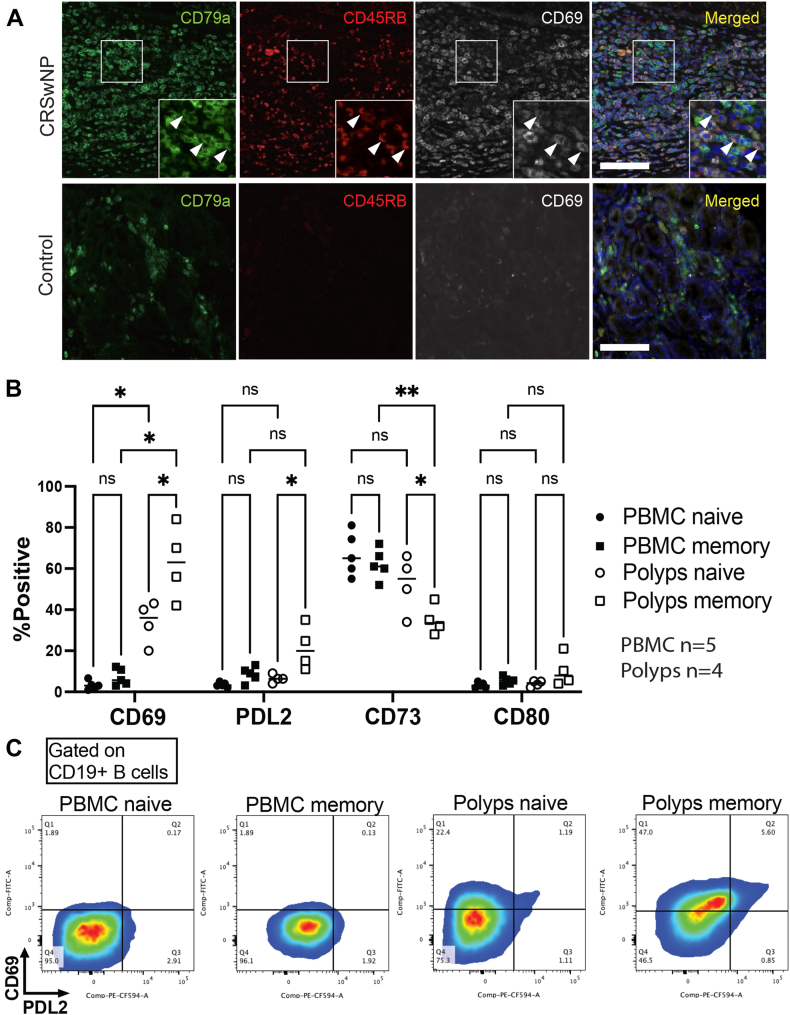
Fig 2.
CD69+CD45RB+ BRM cells detected in patients with CRSNP are characterized by a high expression of PD-L2. A, Immunohistochemistry of normal sinonasal mucosa and sinonasal mucosa from patients with CRSwNP analyzed by fluorescent microscopy. Upper column, CRSwNP; lower column, normal sinonasal mucosa (control). B, BRM cells magnetically isolated from patients with CRSwNP and PBMCs were analyzed using flow cytometry. C, Representative dot plots of memory B cells isolated from CRSwNP and PBMCs with regard to CD69 and PD-L2. ∗P < .05; ∗∗P < .01. Brown-Forsythe ANOVA followed by the Dunnett T3 multiple comparisons post hoc test.
To further delineate the properties of BRM cells, naive B cells (IgD+CD19+) and memory B cells (IgD–CD19+) were isolated by using magnetic separation (Fig 2, B and see Fig E2 in the Online Repository at www.jaci-global.org). Memory B cells extracted from nasal polyps were found to have a phenotype characterized by high levels of CD69, CD80, and PD-L2 and low levels of CD73 (Fig 2, C), which is consistent with the previously described typical characteristics of BRM cells isolated from the lungs.
Our findings in this study reveal that BRM cells can be detected in normal sinonasal mucosa, although they are significantly enriched in nasal polyps derived from patients with CRSwNP. These results can contribute to gaining a more comprehensive understanding of the immune reactions associated with CRSwNP and facilitate the identification of potential therapeutic targets, such as anti–B-cell therapy. A limitation of this study is that we were unable to recruit patients with CRSwNP with aspirin hypersensitivity owing to the relatively low frequency of the disease (affecting approximately 10% of patients with CRSwNP).10 Accordingly, further studies focusing on patients with CRSwNP and aspirin hypersensitivity would be beneficial from the standpoint of discovery of potential therapeutic targets, including anti-IgE antibodies.11
Moreover, the presence of BRM cells in normal sinonasal mucosa indicates that nasal vaccination might provide protection against pathogens, including the influenza virus. According to previous literature on BRM cells in the lung, these cells are responsible for local antibody production against Streptococcus pneumococcus and influenza virus.5,12 Given that these pathogens are also potentially present in the nasal mucosa, further studies to identify target pathogens in the nasal mucosa are warranted. Further studies are warranted to characterize the mucosal immune system in greater depth and thereby facilitate the development of therapeutic interventions for CRSwNP.
Clinical implications.
BRM cells are significantly enriched in patients with CRSwNP. Our findings will facilitate achieving a better understanding of the immune reactions associated with CRSwNP and identifying potential therapeutic targets.
Disclosure statement
Supported by The Jikei University collaborative research fund (to Y.S.).
Disclosure of potential conflict of interest: T. Nakayama receives lecture fees and research grants, and S. Haruna receives lecture fees from Sanofi. The rest of the authors declare that they have no relevant conflicts of interest.
Acknowledgments
We express our gratitude to Dr Saishu Yoshida (Faculty of Science, Toho University) and Dr Kiyotsugu Yoshida (Department of Biochemistry, Jikei University School of Medicine) for granting us access to and providing technical support for the BD Aria III. We gratefully acknowledge Dr Mayumi Tamari (Division of Molecular Genetics, Jikei University School of Medicine) for thoughtful feedback and revision of the article.
Footnotes
Data availability statement: The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available owing to privacy or ethical restrictions.
Supplementary data
Supplementary Fig S1.
Supplementary Fig S2.
References
- 1.Fokkens W.J., Lund V.J., Hopkins C., Hellings P.W., Kern R., Reitsma S., et al. European Position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(suppl S29):1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- 2.Stevens W.W., Lee R.J., Schleimer R.P., Cohen N.A. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015;136:1442–1453. doi: 10.1016/j.jaci.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scadding G.K., Scadding G.W. Innate and adaptive immunity: ILC2 and Th2 cells in upper and lower airway allergic diseases. J Allergy Clin Immunol Pract. 2021;9:1851–1857. doi: 10.1016/j.jaip.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Miljkovic D., Psaltis A., Wormald P.J., Vreugde S. Naive and effector B-cell subtypes are increased in chronic rhinosinusitis with polyps. Am J Rhinol Allergy. 2018;32:3–6. doi: 10.2500/ajra.2018.32.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allie S.R., Bradley J.E., Mudunuru U., Schultz M.D., Graf B.A., Lund F.E., et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol. 2019;20:97–108. doi: 10.1038/s41590-018-0260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C.M., Oh J.E. Resident memory B cells in barrier tissues. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.953088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reusch L., Angeletti D. Memory B-cell diversity: from early generation to tissue residency and reactivation. Eur J Immunol. 2023;53 doi: 10.1002/eji.202250085. [DOI] [PubMed] [Google Scholar]
- 8.Barker K.A., Etesami N.S., Shenoy A.T., Arafa E.I., Lyon de Ana C., Smith N.M., et al. Lung-resident memory B cells protect against bacterial pneumonia. J Clin Invest. 2021;131 doi: 10.1172/JCI141810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen C.D.C. Features of B cell responses relevant to allergic disease. J Immunol. 2022;208:257–266. doi: 10.4049/jimmunol.2100988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oykhman P., Paramo F.A., Bousquet J., Kennedy D.W., Brignardello-Petersen R., Chu D.K. Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: a systematic review and network meta-analysis. J Allergy Clin Immunol. 2022;149:1286–1295. doi: 10.1016/j.jaci.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi H., Fukutomi Y., Mitsui C., Kajiwara K., Watai K., Tomita Y., et al. Omalizumab ameliorates extrarespiratory symptoms in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2023;151:1667–1672.e2. doi: 10.1016/j.jaci.2023.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Tan H.X., Juno J.A., Esterbauer R., Kelly H.G., Wragg K.M., Konstandopoulos P., et al. Lung-resident memory B cells established after pulmonary influenza infection display distinct transcriptional and phenotypic profiles. Sci Immunol. 2022;7 doi: 10.1126/sciimmunol.abf5314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.