Abstract
Purpose
To study corneal epithelial thickness in patients with Dry Eye Disease (DED), according to symptomatology.
Patients and Methods
Cross-sectional study in the outpatient clinic of the Ophthalmology Department of a tertiary hospital in Oporto, Portugal. Adult patients with a clinical diagnosis of dry eye disease were eligible for participation. Each patient underwent corneal epithelial thickness mapping with swept-source optical coherence tomography (SS-OCT, Heidelberg Anterion®) and automated ocular surface analysis (IDRA® Ocular Surface Analyzer SBM Sistemi, Italy). Schirmer’s test, tear film osmolarity (by TearLab® Osmolarity System) and Dry-Eye Related Questionnaire (OSDI-12) were also evaluated. Patients were classified accordingly the severity of symptoms in the OSDI-12 in group 1 (mild disease) and group 2 (moderate to severe disease).
Results
We enrolled 200 eyes (of 100 subjects): 65 in group 1 and 135 in group 2. Median OSDI and Schirmer’s test in group 1 was 7 vs 46 points, p<0.001 and 15 vs 11 mm, p=0.007 in group 2. Eyes from group 2 showed higher mean epithelial thickness (48.4 vs 47.1 µm, p=0.027) and lower mean stromal thickness (522.0 vs 546.6 µm, p<0.001) in comparison with group 1. OSDI score was positively correlated with the mean epithelial thickness (r=0.188, p=0.008) and epithelial variability index (r=0.277, p=0.004) and negatively correlated with the mean stromal thickness (r=−0.313, p<0.001). Patients in group 2 showed higher epithelial variability index (4.5 vs 3.2, p<0.001).
Conclusion
Our study suggests that patients with more severe DED symptoms have thicker corneal epithelia and thinner stroma, which may act as a compensatory response. Epithelial variability index is positively correlated with the OSDI score and may reflect DED severity. This is the first study to report stromal thinning in patients with DED, thereby proving novel information regarding the matter. More studies are needed to confirm these results.
Keywords: dry eye, cornea, epithelium, thickness, anterion
Plain Language Summary
We have conducted original research regarding the effects of Dry Eye Disease on corneal epithelial and stromal thickness, as evaluated by Swept-Source Optical Coherence Tomography (Heidelberg Anterion®). Our study is pioneer in showing that patients with more severe symptoms have thicker epithelia with an apparent compensatory stromal thinning. Besides, corneal epithelium seems to have higher variability index in these patients, which appears to correlate with Dry Eye symptom severity. Our results may be helpful to reveal the role of dry eye disease in corneal structural changes.
Introduction
Dry Eye Disease (DED) is a worldwide pathology, affecting up to 30% of the population aged over 50 years, with a significant burden to patients and healthcare systems.1 It is classified as evaporative (due to tear film instability) and as aqueous deficient (due to insufficient tear film production), although most patients experience mixed characteristics.1–3 There are several identified risk factors, which include increasing age, female gender, meibomian gland dysfunction, and systemic medications (such as antihistamines, anticholinergics, and antidepressants).2
It’s impact in patients’ quality of life has stimulated research and development of innovative treatment modalities, such intense pulsed light.4 Since there is no single adequate test for the diagnosis of DED, its evaluation should be multimodal: including not only clinical findings (tear breakup time, Schirmer’s test, and corneal fluorescein staining) but also recent techniques (such as non-contact meibography, imaging-based ocular surface interferometry, non-invasive tear film breakup time, and measurement of tear film osmolarity).5 Furthermore, there are several subjective questionnaires to standardize patients’ symptoms according to clinical severity, such as the Dry-Eye Related Questionnaire (OSDI-12) score.3,6
The corneal epithelium is the eye’s first and main refracting surface, and as shown to be highly reactive to stromal abnormalities. It is critical in the optimization of corneal optics, which include astigmatism reduction, optimization of asphericity, and reduction of higher order aberrations.7–9 Thus, it is critical to ensure good quality of vision, and can be seen as a marker of ocular surface finesse. Its thickness can be measured with imaging devices, such as in vivo confocal microscopy, high-frequency scanning biomicroscopy and swept-source optical coherence tomography (SS-OCT). SS-OCT is a noncontact technique based on the principle of low-coherence interferometry, which can delineate corneal structure and abnormalities with high precision and reproductivity.8
More recently, its thickness has been studied in DED patients, with few studies reporting these changes, some with contradictory evidence.9–14
Hence, it was postulated that analysis of corneal epithelium and stroma by SS-OCT could provide important information and patterns in patients with DED. Thus, we aimed to study corneal epithelial thickness (CET) and its variability in patients with DED.
Materials and Methods
Study Design and Participants
We conducted a cross-sectional study from the outpatient clinic of the Ophthalmology Department of Unidade Local de Saúde Santo António (ULSSA), Oporto, Portugal, a tertiary hospital. The study was conducted in accordance with the Declaration of Helsinki (1964) and its latest amendment (Brazil, 2013). The study protocol complies with the requirements of the institution’s ethics committee (“Departamento de Ensino, Formação e Investigação”, ID 2021–037[029-DEFI-030-CE]). All data was saved and shared anonymously. All patients gave written informed consent after explanation of the study purpose and design.
We consecutively included patients aged 18 or over with a clinical diagnosis of DED in accordance with the Tear Film & Ocular Society (TFOS) Dry Eye Workshop (DEWS) II report.3 We excluded patients with other ocular surface pathology (eg, ocular burns), recurrent corneal erosion, basal membrane dystrophy, ocular surface neoplasm and history of refractive surgery.
Patients were classified according to OSDI-12 in group 1 (mild disease, score between 13 and 22 points) and group 2 (moderate to severe disease, score with at least 23 points and higher). All patients underwent examination at a fixed time (9–12 AM), and before instilling eye drops.
At each appointment, patient evaluation included slit-lamp biomicroscopy, first without dye instillation (assessing for conjunctival hyperemia, Meibomian gland status, evaluation of lid parallel conjunctival folds), and after fluorescein staining (determining tear break-up time and the presence of corneal epithelial defects). Schirmer’s test was performed by placing a test trip at the lateral third of the lower eyelid and then measuring the moistened portion of the strip.
After this, tear film osmolarity was determined using the point-of-care TearLab Osmolarity System (TearLab, San Diego, CA), from a 50 nL tear sample.15 A full comprehensive automated ocular surface analysis (IDRA® Ocular Surface Analyzer SBM Sistemi, Italy) was performed, which includes non-contact meibography, interferometry, non-invasive tear film break-up time, and blink pattern analysis.
Swept-Source Optical Coherence Tomography (SS-OCT)
In the same visit, each patient underwent corneal epithelial thickness mapping created by swept-source optical coherence tomography (OCT) (by Heidelberg Anterion®). The ANTERION contains two imaging modalities, a scanning OCT and infrared (IR) camera. The OCT modality contains a swept-source laser beam that scans a patient’s eye laterally, and the light reflected from this beam is analyzed by the device, resulting in intensity-based cross-sectional images. The IR camera is used to analyze obvious pathologies and track eye movements. The tracking is based on reflection points to ensure that the image is centered on the corneal vertex. After image acquisition, the software applies automated algorithms to analyze data and produce an epithelial mapping.16
Each scan has an axial resolution of less than 10 μm and we performed 65 radial scan lines with 256 A-scan each centered on the corneal vertex over a 7 mm diameter circle.17
This study considered corneal epithelial thickness and its distribution between 2-, 4-, 6- and 7-mm zones and rings (0–2 mm; 2–4 mm; 4–6 mm; 6–7 mm) centered in the corneal vertex. It also tracks the epithelial thinnest and thickest points, its map position, and displays the mean difference between the inferior and superior region of the epithelium.
Statistical Analysis
Statistical analysis was performed using the SPPS software (SPSS statistics, version 26.0.0 for Mac OS, IBM, Somers, NY). The Kolmogorov–Smirnov test was used to assess normality. Comparison between independent continuous variables was evaluated using the Mann–Whitney U-test and T-Student test. Fisher’s exact test was used for nominal scaled data. Spearman’s bivariate correlation test was applied to study correlations. P values less than 0.05 were considered statistically significant.
Results
This study enrolled 200 eyes (of 100 subjects): 65 in group 1 (mild disease) and 135 in group 2 (moderate to severe disease). We included 100 subjects, with a mean (± SD) age of 66 ± 16 years old, with no significant difference between groups. Fifty-two patients (52.0%) were male. Median (range) OSDI score was 7 (22) in group 1 vs 46 (79) in group 2, p<0.001. Median Schirmer’s test was 15 (25) mm in group 1 vs 11 (34) mm in group 2, p=0.007. No significant differences were found regarding lipid layer thickness, noninvasive tear film break-up time, or tear film osmolarity. Full data is shown in Table 1.
Table 1.
Demographic and Ocular Surface Analysis Data in Eyes with Mild (Group 1) and Moderate to Severe Disease (Group 2)
| Variable | Group 1 | Group 2 | p value |
|---|---|---|---|
| Age, mean (SD) | 62 (18.1) | 60.8 (14.7) | 0.502a |
| Eye blink | 72 (90) | 58 (50) | 0.214b |
| Lipid layer thickness | 74 (59) | 80 (85) | 0.729b |
| Meibography | 15 (89) | 12 (63) | 0.146b |
| Tear film meniscus height | 0.23 (0.51) | 0.29 (0.24) | 0.198b |
| NIBUT | 8.5 (12) | 8.2 (8.6) | 0.106b |
| Tear film Osmolarity | 309 (16.1) | 307 (17.3) | 0.499b |
| Schirmer’s test | 15 (27) | 11 (34) | 0.007b |
| OSDI score | 7 (22) | 46 (79) | <0.001b |
Notes: All values are written as median (interquartile range) unless stated otherwise. aIndependent samples T-test, bMann–Whitney U-test; Bold indicates p value <0.05.
Abbreviation: SD, standard deviation.
Corneal Epithelial and Stromal Mapping
Table 2 shows the quantitative analysis of corneal epithelial and stromal mapping. Eyes from group 2 (moderate to severe disease) showed higher mean epithelial thickness (48.4 vs 47.1 μm, p=0.027) and lower mean stromal thickness (522.0 vs 546.6 μm, p<0.001) in comparison with group 1 (mild disease).
Table 2.
Corneal Epithelial and Stromal Mapping in Eyes with Mild (Group 1) and Moderate to Severe Disease (Group 2)
| Variable | Group 1 | Group 2 | p value |
|---|---|---|---|
| Mean epithelial thickness | 47.1 (4.2) | 48.4 (3.7) | 0.027a |
| Epithelial variability index | 3.2 | 4.5 | 0.001a |
| Epithelial thinnest point | 36.1 (6.6) | 34.5 (8.2) | 0.181a |
| Epithelial thickest point | 58.5 (7.8) | 62.7 (11.5) | 0.009a |
| Epithelial thickness difference (inferior-superior) | 2.6 (2.7) | 3.0 (3.5) | 0.434a |
| Epithelial ring 0–2 mm 2–4 mm 4–6 mm 6–7 mm |
47.5 (4.5) 47.2 (4.5) 47.0 (4.3) 47.2 (4.4) |
48.6 (4.6) 48.5 (3.8) 48.2 (3.5) 47.9 (3.3) |
0.111a 0.032a 0.061a 0.321a |
| Epithelial zone 2 mm 4 mm 6 mm 7 mm |
47.5 (4.5) 47.2 (4.4) 47.1 (4.2) 47.1 (4.7) |
48.6 (4.6) 48.5 (3.7) 48.3 (3.3) 48.1 (2.9) |
0.111a 0.039a 0.038a 0.190a |
| Mean stromal thickness | 546.6 (42.3) | 522.0 (38.5) | <0.001a |
| Stromal variability index | 40.0 | 43.0 | 0.707a |
| Stroma thinnest point | 498.4 (37.4) | 473.9 (43.0) | <0.001a |
| Stroma thickest point | 632.0 (53.5) | 608.9 (43.0) | <0.001a |
| Stromal thickness difference (inferior-superior) | −20.1 (13.3) | −17.7 (14.6) | 0.273a |
| Stromal ring 0–2 mm 2–4 mm 4–6 mm 6–7 mm |
510.0 (39.6) 522.8 (40.7) 550.0 (43.5) 574.3 (40.1) |
484.9 (40.2) 498.5 (39.7) 527.6 (39.3) 553.9 (38.1) |
<0.001a <0.001a <0.001a 0.006a |
| Stromal zone 2 mm 4 mm 6 mm 7 mm |
510.0 (39.6) 520.2 (40.5) 536.7 (42.2) 544.1 (38.8) |
484.9 (40.2) 496.0 (39.6) 514.5 (38.4) 523.4 (38.8) |
<0.001a <0.001a <0.001a 0.005a |
Notes: All values are written as mean (standard-deviation) unless stated otherwise. aIndependent samples T-test; Bold indicates p value <0.05;
The epithelium’s thickest point (“mean maximum epithelial thickness”) was higher in group 2–62.7 vs 58.5 µm in group 1, p=0.009), and seems to be located inferiorly in both groups (mean y position −1.56 vs −1.62 mm, p=0.749).
Regarding epithelial thin point location, we concluded that in both groups, the thinnest point seems to be located superiorly (mean y position 2.38 vs 1.84 mm, p=0.683), which is supported by a positive thickness difference between the inferior and superior epithelial regions (2.2 vs 2.9 μm, p=0.267). There were no significant differences between the two groups regarding the thinnest point thickness (32 vs 37 μm, p=0.071).
We calculated an epithelial variability index, defined as the mean standard deviation of the mean epithelial thickness over a 7 mm area centered in the corneal vertex.
Correlation Between Epithelial and Stromal Mapping and DED Symptoms
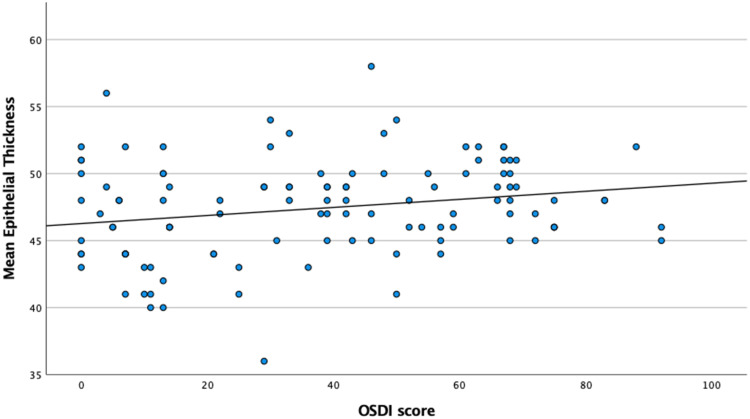
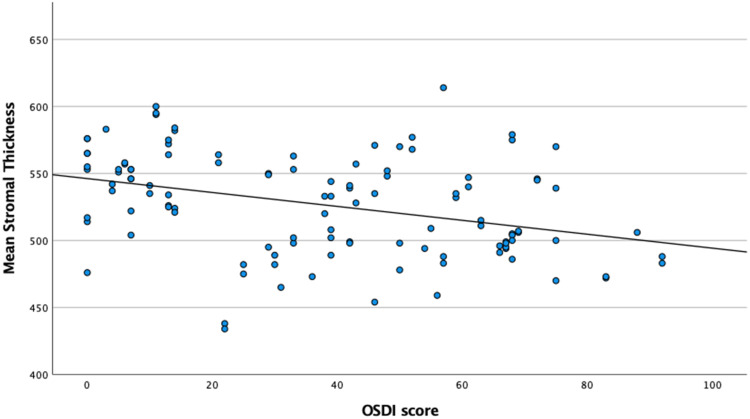
We found the OSDI score to be positively correlated with mean epithelial thickness (r=0.188, p=0.008) and epithelial variability index (r=0.277, p=0.004), and negatively correlated with mean stromal thickness (r=−0.313, p<0.001). Patients in group 2 showed higher epithelial variability index (4.5 vs 3.2 µm, p<0.001). Schirmer’s test was negatively correlated with the difference between the inferior and superior epithelial thickness (r=−0.176, p=0.013). Figures 1 and 2 show corneal epithelial and stromal thickness variation, respectively, according to OSDI-12 score.
Figure 1.
Mean Corneal Epithelial Thickness variation according to OSDI-12 score.
Figure 2.
Mean Corneal Stromal Thickness variation according to OSDI-12 score.
Discussion
Currently, there are few studies comparing corneal epithelial thickness in DED patients, some of them with contradictory evidence, which has motivated our work. Our study showed a real-life application of corneal thickness mapping in DED patients observed at our routine practice from the outpatient clinic of a tertiary hospital.
Firstly, we characterized our study population based on symptom severity. Secondly, we were able to establish both epithelium and stromal thickness and its variability, which adds information that has not yet been reported in patients with DED. Finally, we could correlate the subjective complaints with thickness analysis.
We concluded that patients with more severe disease had thicker epithelia, which is in accordance with some authors,14 but in contradiction with others.18,19 The inflammatory component of DED causes disruption of the epithelial barrier via promotion of cellular apoptosis and production of pro-inflammatory substances, such as matrix metalloproteinases (MMP). These proteolytic enzymes break intercellular junctions and disrupt the epithelial barrier. Epithelial proliferation and cellular size variation seems to be a compensatory mechanism against dry-eye induced apoptosis and epithelial cell damage, which may explain our results.9,20,21
Fabiani et al showed higher epithelial proliferation and morphological cellular changes (hyperplasia with flattened and keratinized apical cells) after desiccating stress in a mouse model.20 The precise mechanisms leading to epithelial hyperplasia are still unknown – probably a combination of hyperosmolar stress, increase in epithelial cell size, and blinking’s microabrasive effect in a fragile epithelium.
There is also reciprocal thinning in stromal thickness, which may be secondary to epithelial imbalance, with the release of proteolytic enzymes and collagen degradation, similar to what seems to occur in eyes with Keratoconus.7,22 On the other hand, increased tear film evaporation in patients with more severe symptoms may also explain stromal thickness reduction. To the best of our knowledge, this is the first work to report stromal thinning in patients with DED. However, it should be noted that we found a negative correlation between severe DED symptoms and corneal stromal thickness, which has not been reported yet, to the best of our knowledge. As we know, the epithelium seems to compensate structural changes in the corneal stroma - we consider this an additional marker of cellular damage and a response to epithelial cell hyperplasia and local disruption.
We also showed that patients with more severe disease seem to present thinner superior corneal epithelium, which correlates with available evidence.9–11 On one hand, this may be explained by an increased blinking to compensate for tear film deficiency, which promotes upper eyelid rubbing on the cornea, hereby thinning the superior region of the epithelium. On the other hand, Rattan et al concluded that dry eye patients had thicker inferior epithelium,11 which seems to be confirmed in our results by a positive difference between inferior and superior epithelial regions – this may translate cellular hyperplasia in response to a deficient tear film.
Francoz et al proposed that epithelial thickness in patients with DED was more altered in the peripheral cornea. However, our results showed that the epithelial and stromal thickness variation seems to be sustained both in the peripheral, paracentral, and central zones of the cornea. However, evidence is still lacking, and more studies are needed to confirm both hypotheses.
We also evaluated the topographic thickness variability index, measured as the mean standard deviation of the mean epithelial thickness over a 7 mm area centered in the corneal vertex. We found that patients with more severe dry eye have higher variability, which was expectable and in line with other studies.10,11,14 We believe this is an additional indicator of an imbalance between cellular proliferation and apoptosis, probably reflecting abnormal cellular shape and size.
Our study provides novel information regarding qualitative and quantitative topographic changes in a large sample size of patients with DED. Furthermore, we characterized a real-life, outpatient setting population, which contributes to our daily practice. We also used an equipment with high axial resolution, which does not include the tear film in the epithelial thickness measurement, therefore improving reproducibility. It should be noted that only exams with high quality were included, with the final values reflecting an average of several measurements.
Despite its strengths, our study has some limitations. Firstly, it is a cross-sectional study, with the inherent disadvantages of its methodology. Secondly, all our patients were being followed for DED, and some had been submitted to different treatment modalities; thus, these groups may not have been perfectly matched regarding previous treatments for DED. Therefore, this may induce some bias when comparing these results. The inclusion of different stages and causes of DED may bias these results, which may also explain why some authors could not find a significant association between CET and DED.9,23,24 Thirdly, our control group included eyes with mild disease, which cannot be generalized to healthy eyes, since it may already show some structural changes. Finally, we were conditioned by the inherent limitations of the software, and therefore could not evaluate different areas (ie, temporal and nasal regions), which may add valuable information regarding topographic variability.
Conclusion
Dry-Eye Disease is a global pathology with dissimilarities between subjective and objective findings. We showed that corneal epithelium seems to be thicker and positively correlated with dry-eye symptom severity. Besides, symptom severity seems to be associated with epithelial irregularity, which may translate a disruption between cellular proliferation and apoptosis. In this regard, epithelial and stromal mapping by SS-OCT may be an important diagnostic tool in the detection of morphological changes associated with DED. To the best of our knowledge, this is the first work to report corneal stromal thinning in patients with DED.
In the future, deep learning analysis of big data and artificial intelligence may develop algorithms to characterize these patterns and introduce novel information.
Disclosure
There are no conflicts of interest in this paper. No specific funding was received from any agency in the public, commercial, or not-for-profit sectors.
References
- 1.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–82. doi: 10.3238/arztebl.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akpek EK, Amescua G, Farid M, et al. Dry eye syndrome preferred practice pattern®. Ophthalmology. 2019;126(1):P286–P334. doi: 10.1016/j.ophtha.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 3.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 4.Barbosa Ribeiro B, Marta A, Ponces Ramalhão J, Marques JH, Barbosa I. Pulsed light therapy in the management of dry eye disease: current perspectives. Clin Ophthalmol. 2022;16:3883–3893. doi: 10.2147/OPTH.S349596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidl D, Schlatter A, Chua J, Tan B, Garhöfer G, Schmetterer L. Novel approaches for imaging-based diagnosis of ocular surface disease. Diagnostics. 2020;10(8):589. doi: 10.3390/diagnostics10080589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi: 10.1097/ICO.0b013e318225415a [DOI] [PubMed] [Google Scholar]
- 7.Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25(7):604–610. doi: 10.3928/1081597X-20090610-06 [DOI] [PubMed] [Google Scholar]
- 8.Feng Y, Reinstein DZ, Nitter T, et al. Heidelberg anterion swept-source OCT corneal epithelial thickness mapping: repeatability and agreement with optovue avanti. J Refract Surg. 2022;38(6):356–363. doi: 10.3928/1081597X-20220414-01 [DOI] [PubMed] [Google Scholar]
- 9.Levy A, Georgeon C, Knoeri J, et al. Corneal epithelial thickness mapping in the diagnosis of ocular surface disorders involving the corneal epithelium: a comparative study. Cornea. 2022;41(11):1353–1361. doi: 10.1097/ICO.0000000000003012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui X, Hong J, Wang F, et al. Assessment of corneal epithelial thickness in dry eye patients. Optom Vis Sci. 2014;91(12):1446–1454. doi: 10.1097/OPX.0000000000000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rattan SA, Anwar DS. Comparison of corneal epithelial thickness profile in dry eye patients, keratoconus suspect, and healthy eyes. Eur J Ophthalmol. 2020;30(6):1506–1511. doi: 10.1177/1120672120952034 [DOI] [PubMed] [Google Scholar]
- 12.Abou Shousha M, Wang J, Kontadakis G, et al. Corneal epithelial thickness profile in dry-eye disease. Eye. 2020;34(5):915–922. doi: 10.1038/s41433-019-0592-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Wang Y. Detailed distribution of corneal epithelial thickness and correlated characteristics measured with SD-OCT in myopic eyes. J Ophthalmol. 2017;2017:1018321. doi: 10.1155/2017/1018321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanellopoulos AJ, Asimellis G. In vivo 3-dimensional corneal epithelial thickness mapping as an indicator of dry eye: preliminary clinical assessment. Am J Ophthalmol. 2014;157(1):63–68.e2. doi: 10.1016/j.ajo.2013.08.025 [DOI] [PubMed] [Google Scholar]
- 15.Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792–798.e1. doi: 10.1016/j.ajo.2010.10.032 [DOI] [PubMed] [Google Scholar]
- 16.ANTERION - Multimodal imaging platform optimized for the anterior segment | Heidelberg Engineering [homepage on the Internet]. Available from: https://business-lounge.heidelbergengineering.com/gb/en/products/anterion/anterion/downloads/. Accessed February 21, 2023.
- 17.Bille JF, ed. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. Springer; 2019. Available from:. http://www.ncbi.nlm.nih.gov/books/NBK554051/. Accessed February 21, 2023. [PubMed] [Google Scholar]
- 18.El-Fayoumi D, Youssef MM, Khafagy MM, Badr El Dine N, Gaber W. Assessment of corneal and tear film parameters in rheumatoid arthritis patients using anterior segment spectral domain optical coherence tomography. Ocul Immunol Inflamm. 2018;26(4):632–638. doi: 10.1080/09273948.2016.1261165 [DOI] [PubMed] [Google Scholar]
- 19.Edorh NA, El Maftouhi A, Djerada Z, Arndt C, Denoyer A. New model to better diagnose dry eye disease integrating OCT corneal epithelial mapping. Br J Ophthalmol. 2022;106(11):1488–1495. doi: 10.1136/bjophthalmol-2021-318826 [DOI] [PubMed] [Google Scholar]
- 20.Fabiani C, Barabino S, Rashid S, Dana MR. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp Eye Res. 2009;89(2):166–171. doi: 10.1016/j.exer.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abtahi MA, Beheshtnejad AH, Latifi G, et al. Corneal epithelial thickness mapping: a major review. J Ophthalmol. 2024;2024:6674747. doi: 10.1155/2024/6674747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ. The pathogenesis of keratoconus. Eye. 2014;28(2):189–195. doi: 10.1038/eye.2013.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francoz M, Karamoko I, Baudouin C, Labbé A. Ocular surface epithelial thickness evaluation with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(12):9116–9123. doi: 10.1167/iovs.11-7988 [DOI] [PubMed] [Google Scholar]
- 24.Liang Q, Liang H, Liu H, Pan Z, Baudouin C, Labbé A. Ocular surface epithelial thickness evaluation in dry eye patients: clinical correlations. J Ophthalmol. 2016;2016:1628469. doi: 10.1155/2016/1628469 [DOI] [PMC free article] [PubMed] [Google Scholar]