Abstract
Lung diseases are associated with high morbidity and mortality rates, thereby jeopardizing human health and imposing a great burden on society. Currently, lung diseases are mainly treated with medications, oxygen therapy and mechanical ventilation, but these approaches are unable to effectively reduce the mortality rate. Therefore, lung transplantation remains the ultimate treatment for various chronic lung diseases, but this treatment is also hindered by the limited availability of lung sources, immature technology and a low survival rate after transplantation. With constant changes in the environment, pathogens, type and amount of harmful substances and the prevalence of respiratory diseases, there is an urgent need to identify alternative treatment methods. Research on stem cell therapy has been very successful in recent years, and mesenchymal stem cells (MSCs), together with their secretory bodies, play a significant therapeutic role. Extracellular vesicles of MSCs (MSC-EVs) are also major components of the paracrine secretion of MSCs, including exosomes, microvesicles, and apoptotic bodies, among which exosomes are the most typical. MSC-EVs are believed to be present in various tissues of the human body where they can carry proteins, DNA, RNA and biologically active factors, just to name a few. They can also transmit various biological signals to participate in different biological activities, including the maintenance of homeostasis within the tissue. Several studies have further demonstrated that MSCs and their generated extracellular vesicles play an important role in the treatment of diseases. In this paper, the origin, properties and roles of MSCs and MSC-EVs are reviewed, the mechanisms of different lung diseases, the limitations of current therapeutic options and the roles of MSC-EVs in Chronic Obstructive Pulmonary Disease, asthma, infectious lung disease, lung cancer, pulmonary fibrosis, pulmonary arterial hypertension, and acute lung injury/ acute respiratory distress syndrome are also discussed (Figure 1). In addition, the current limitations and possible future research directions are also discussed in view of providing new ideas for the role of MSC-EVs in the treatment of lung diseases.
Keywords: mesenchymal stem cells, exosomes, lung disease, immunomodulation, epithelial mesenchymal transition (EMT), MSC-EVs
Introduction
The lungs, being highly vulnerable organs, are susceptible to injury and various chronic diseases caused by pathogens, harmful substances and environmental changes. Indeed, lung infection is a major cause of acute lung injury/ acute respiratory distress syndrome (ALI/ARDS) which represent two stages of a critically severe and highly mortal disease.1 Similarly, smoking, air pollution and other factors can induce conditions such as emphysema, interstitial lung disease and lung cancer which can ultimately lead to respiratory failure to death.2,3 Recently, a large-scale study showed that in 2020–2021, COVID-19 led to a 1.6% decline in the average life expectancy of the global population, with 15.9 million people dying from the pandemic. COVID-19, therefore, posed a serious threat to human health and was a significant burden to healthcare systems.4 In addition, the outbreak brought respiratory diseases into the limelight, causing widespread fear among people while also generating significant interest among researchers. Conventional treatments based on the use of surfactants, artificial respiratory support, mechanical ventilation and antibiotics/anti-inflammatory drugs only provide symptomatic relief and prolong prognosis. However, they have limited effects on damaged airways, on the clearance of alveolar fluids as well as on other harmful effects resulting from inflammatory reactions.5 Hence, there is an urgent need to search for new therapeutic options.
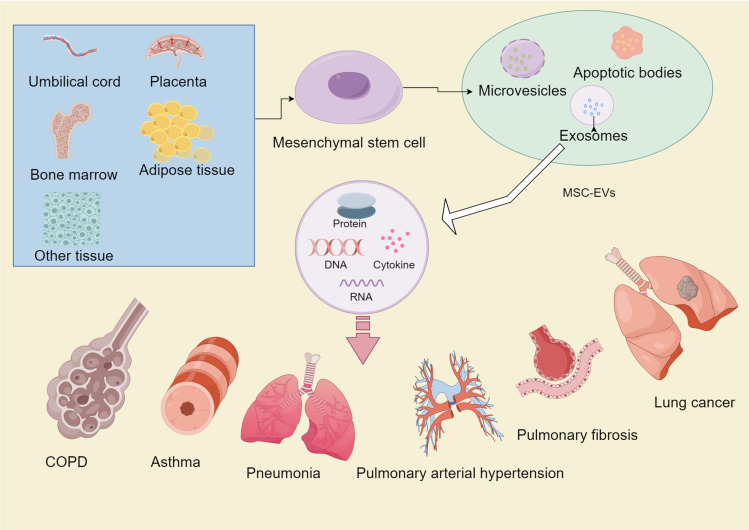
Figure 1.
The origin, types, components, and roles of MSC-EVs in various lung diseases.
Note: This figure is originally drawn by Figdraw platform (www.figdraw.com).
MSCs represent a type of mesoderm-derived multipotent adult stem cells which, compared to human induced multipotent stem cells or multipotent embryonic stem cells, exhibit a relatively weaker capacity for differentiation and proliferation.6 MSCs have demonstrated immunomodulatory roles,7 anti-apoptotic effects8 and potential as novel targets in fibrotic diseases,9 thereby making them a central point for research on lung diseases. Initially, MSCs were believed to home damaged tissues and exert therapeutic effects,10 but more recent studies suggested that their paracrine function was the primary mechanism of their action,11–13 with EVs.14 Besides, due to the lower risk of immune rejection, tumorigenicity, and pulmonary embolism of MSC-EVs15, MSCs are gradually being replaced in medical research. Several studies have demonstrated that MSC-EVs can be involved in the treatment of pulmonary diseases through different mechanisms, and the atomized administration of MSC-EVs has a unique advantage of its action in pulmonary diseases, which has led more and more scholars to focus their attention on it. This article mainly introduces the sources of MSC-EVs, their functions and the current research progress of MSC-EVs in various respiratory diseases, which provides a reference for future research.
Mesenchymal Stem Cells and Their Extracellular Vesicles
MSCs are multipotent cells derived from various tissues, including pancreas, thymus, adipose, lung, kidney, liver, spleen, brain, bone and muscle. These cells possess self-renewal as well as multidirectional differentiation properties and can transform into at least three different cell types, namely adipocytes, chondrocytes and osteoblasts.16 They can also differentiate into vascular cell types, endothelial cell and the smooth muscle cells of macrovessels,17 just to name a few. MSCs have an ability to maintain the growth, viability and multipotential state of certain cells as well as mitochondrial transfer, as they have been found to secrete pro-stromal factors and cell growth factors.18 MSCs also have anti-inflammatory and immunomodulatory, promote tissue repair and regeneration, as well as antioxidant, anti-apoptotic, etc),9,19,20 have become a highly popular type of stem cells for use in regenerative medicine,21 involved in the treatment of various diseases.
Different sources of MSCs are known to exhibit different properties, and currently bone marrow, adipose and umbilical cord, and placenta-derived stem cells are most commonly used in research. An earlier study comparing the differences between MSCs from different sources found that Wharton’s Jelly-derived MSCs had the strongest inhibitory effect on T cell proliferation, the fastest growth rate, the strongest osteogenic differentiation ability, ADMSCs had the strongest adipogenic ability, and among the immune-related genes, the MHC class II genes, TLR 4, TLR 3, JAG 1, NOTCH 2, and NOTCH 3 were the weakest expressed in Wharton’s Jelly-derived MSCs.22 It has also been demonstrated that UCMSCs have a higher rate of proliferation and are able to secrete high concentrations of growth factors, pro-inflammatory proteins and chemokines. On the other hand, BMSCs secrete detectable amounts of VEGF-D, while ADMSCs exhibit better pro-angiogenic properties and secrete more extracellular matrix components and metalloproteinase.23,24 MSCs markers are also expressed differently in vivo and in vitro as well as across species.9 However, MSCs of different origins may still share similar phenotypic profiles,25 such as CD73, CD105, CD90 positivity or CD19, CD79, CD11b, CD14, CD34, CD45 and HLA-DR negativity. These markers facilitate the identification of MSCs, especially when used as a screening condition to distinguish human-derived ones,26 thereby making them accessible for experimental studies. However, despite being promising, the application of MSCs still have drawbacks such as genetic instability, tumorigenicity,27 transmission of cytomegalovirus and herpes simplex virus28 as well as host rejection,29 all of which limit their practical applications in clinical practice. As a result, the discovery of MSC-EVs is gradually replacing the use of MSCs themselves.
Secretory bodies from MSCs include soluble components and encapsulated MSC-EVs which are present in different body fluids such as saliva, cerebrospinal fluid, breast milk, semen, urine and blood.30 Specifically, these MSC-EVs consist of apoptotic vesicles, microvesicles and exosomes.14 Apoptotic vesicles are the largest (>1000 nm) and are formed during apoptosis, while microvesicles are nano-sized (100–1000 nm) and are formed from plasma membrane outgrowths. Similarly, exosomes are the smallest (30–200 nm), originating from the inward outgrowths of endosomal late membranes in multivacuolloid bodies, and they can carry lipids, proteins, genetic material as well as multiple molecular components to fulfill biological functions.31 According to reports, MSCs exert their beneficial effects mainly through EVs, with exosomes being the most characterized MSC-EVs. Furthermore, MSC-EVs possess similar therapeutic effects to MSCs32,33 but with lower risks of immune rejection, tumorigenicity and pulmonary embolism.15
MSC-EVs exhibit a homing effect, making them a promising cell-free therapeutic modality that can help in repairing kidney injury, cardiovascular system damage as well as liver and bone injury through their regenerative function.34 In ischemic tissues, MSC-EVs can also exert pro-lymphangiogenic and pro-angiogenic properties through the transfer of various miRNAs and proteins.35 In other cases, they can activate autophagy and/or inhibit oxidative stress, necrosis and apoptosis in injured intestinal, lung, retinal and neuronal cells as well as in hepatocytes and renal epithelial cells by transferring mRNA and miRNA, hence promoting their survival and regeneration. In addition, MSC-EVs may play a role in inflammatory and autoimmune diseases through various other mechanisms.36 PPARγ is an important transcription factor involved in regulating lipid and glucose metabolism, inflammation and oxidative stress. Studies have shown that MSC-EVs may up-regulate PPARγ’s mRNA expression, exerting anti-inflammatory and antioxidant effects to attenuate pulmonary cystic fibrosis.37 MSC-EVs can also cross the blood-brain barrier easily, hence suggesting that they play a significant role in neurological diseases.38 Altogether, these findings not only demonstrate that MSC-EVs could be studied as a potential novel therapeutic modality but also highlight their function in respiratory diseases.
Role of MSC-EVs in Various Lung Diseases
The main mechanisms of lung disease include disruption of the alveolar endothelial and epithelial barriers, reduced clearance of alveolar fluids, release of associated cytokines, chronic inflammation, inflammatory cell infiltration and remodeling of the airways.39 It has been shown that MSC-EVs can play a significant role in lung diseases through various mechanisms. For instance, they can ameliorate Chronic Obstructive Pulmonary Disease (COPD) by inhibiting airway inflammation40 as well as through immunomodulatory effects on T-cells, macrophages and B-cells.41 In addition, Xiaowei X et al found that the nebulized inhalation of MSC-EVs in mice could inhibit allergic airway inflammation and airway remodeling more effectively than intravenous injection.42 Recent in vivo and in vitro experiments, preclinical studies and clinical trials have demonstrated the considerable therapeutic effects of MSC-EVs, but their effectiveness, safety and more individualized selectivity still requires further investigation. Table 1 summarizes the specific roles of MSC-EVs in different lung diseases to help understand the molecular mechanisms of MSC-EVs therapy.
Table 1.
Summary of the Specific Role of MSC-EVs in Different Lung Disease
| Disease | Species Sex | Damage | Cell Source | Diameter (nm) | EV Treatment Group (Method/Dose) | Therapy Time | Therapy Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|
| COPD | SD male rats | Cigarette smoke | Human-UCMSC | 153nm | I.t/150-μL vehicle (isolated from 2.5×106 hUC-MSCs) | Single after injure | Anti-inflammation in part by the expression of PRKCZ, and NF-κβ subunits p65 and p50 | [40] |
| COPD | SD male rats | Papain-induced | Human -UCMSC | 30–200 nm | i.v/200 ug | Single after injure | Activated VEGF-VEGFR2-mediated AKT pathway and MEK/ERK pathway | [43] |
| COPD | mt-Keima mice | Cigarette smoke | Mouse-BMSC | 115±5 nm | i.p/~15μg | Daily after injure | Reduce mitochondrial dysfunction | [44] |
| Asthmatic | C57BL/6 female mice | OVA | Human - ADMSC | 114–508 | i.v/37ug (released by 105 ADMSC) | One day after injure | Anti-inflammation, modulated airway remodeling | [45] |
| Asthmatic | BALB/c mice | OVA | Human-BMSC | 30–120 | i.v/ 20 μg/mL | Week 7 to week 10 after injure. | Suppress proliferation of airway smooth muscle cells through the miR-188/JARID2/Wnt/β-catenin axis | [46] |
| Asthmatic | BALB/c female mice | OVA/ cfa | Human-UCMSC | 127.7 | i.t/100 μg in 50 μL PBS | 21th day when injure | Reshaping macrophage polarization by inhibition of TRAF1 | [47] |
| Pneumonia | Male C57BL/6 mice | E. coli K1 strain | Human-BMSC | 200 | i.t/30 or 60 mL or i. v/90 mL | 4h after injure | Enhanced phagocytosis of bacteria by Human monocytes | [48] |
| Pneumonia | Male C57BL/6 mice | MDR-PA strain | Human-ADMSC | 50–400 | i.t/ 5.5×107 particles/mL (40ul) | 4h after injure | Regulation macrophage polarization by TIRAP-MyD88-NFκB axis. | [49] |
| Pneumonia | Female C57BL/6 mice | Klebsiella pneumoniae | Mouse-ADSC | – | i.t/70 μg in 100μL PBS | 6h after injure | Targeting STAT3 signaling by miR-181a-5p | [50] |
| Lung cancer | BALB/c male nude mice | A549 cells | Human-BMSC | 60 ~ 260 | i.h/200 μg | Once every 2 days for a total of 10 times. | Blocking the EZH2/PI3K/AKT axis | [51] |
| Lung cancer | BALB/c male nude mice | A549 cells | Human-BMSC | 40–150 | i.h /10 µL EV (1 × 109 EV/mL) | Pretreated for 96 h before ih | Inhibit THBS2 by miR-598 | [52] |
| Lung cancer | BALB/c female nude mice | H1299 cells | Human-UCMSC | 60–450 | i.h /6 × 109 (200μg) | Pretreated for 12 h before i.h. | Inhibited the expression of PTEN. By miR-410 | [53] |
| Lung fibrosis | C57BL/6 mice | Radiation-induced | Mouse-BMSC | 90–150 | i.v/200 μg | 2h before radiation, then repeated once two weeks until sacrifice | Inhibiting AKT/GSK3β pathway via c-MET | [54] |
| Lung fibrosis | SD male rats | Silica-induced | Mouse-BMSC | 30–150 | i.v/1mL (20μg/mL) | 2th day after injure | Attenuating Wnt/β-catenin signaling | [55] |
| Lung fibrosis | Wild type C57 mice | Radiation-Induced | Human-PMSCs | 120 | i.v/(100μg in 100 μL PBS) | At day 0, 3 and 7 after irradiation. | Reduced the levels of DNA damage by downregulating ATM/P53/P21 signaling. | [56] |
| Lung fibrosis | C57BL/6 mice | HOCl | Mouse-MSC | – | i.v/250 ng(or 1500 ng) | Single after injure | Anti-inflammatory and anti-fibrotic markers. | [57] |
| Lung fibrosis | C57BL/6 mice | Bleomycin-induced | Human-BMSC | 35–150 | i.v/200 μL; dose, 5×106 hDF equivalents; ~9.2 ×108 particles) | Single Concurrent with injure | Systemic modulation of monocyte phenotypes. | [58] |
| ALI/ARDS | C57BL/6 mice | LPS | Mouse-BMSC | 50–200 | i.v/70 μg | 4h after injure | Inhibiting NF-κB and hedgehog pathways by miR−23a−3p and miR−182−5p | [59] |
| ALI/ARDS | C57BL/6 male mice | LPS | Mouse-BMSC | 150 | i.v / 10 nmol/20 g | 48h before injure | Epithelial cell apoptosis by upregulating SIRT1 expression, via miR−181 | [60] |
| ALI/ARDS | SD male rats | LPS | Mouse-BMSC | 90–100 | i.t/ 50ul | 4h after injure | miR−384−5p resulted in relieving autophagy disorder in alveolar macrophages by targeting Beclin−1 | [61] |
| ARDS | C57BL/6 male and female mice | LPS | Human-PMSC | 133 | i.p/1.2–1.3 × 1010 particles | 4h after injure | Anti-inflammatory | [62] |
| ALI/ARDS | C57BL/6 female mice | LPS | Human-ADMSC | 50–150 | i.v/ 200 μL (10 μg/mL) | 4h after injure | Transferring mitochondrial component to improve homeostasis of alveolar macrophages | [63] |
| PAH | Wistar male rats | Monocrotaline-induced | Human-UCMSC | 50–150 | i.p/ 25 µg | 3 weeks after injure | Prevent PAH vascular remodelling via regulation of Wnt5a/BMP signalling pathway. | [64] |
| PAH | SD male -rats | Monocrotaline-induced | Mouse-BMSC | 40–300 | i.v/ 30 µg/100 µL | 3 weeks after injure, once/2 d for 2 weeks | Attenuate mPAP and mRVP, reduce RV hypertrophy and pulmonary remodeling | [65] |
| Bronchopulmonary Dysplasia | – | Hyperoxia-induced | Human WJ-MSC and BMSC | 30–150 | i.v/- | Signal at Postnatal day | Modulation of lung macrophage phenotype. | [66] |
| PAH | C57BL/6mouse | Hyperoxia-induced | Human-UCMSC | 30–150 | I.v/50ug (total of 250ug) | One day before injure and after 1, 3, 5, 9 | Promote M2 macrophage polarization, inhibit IL−33/ST2 axis expression. | [67] |
MSC-EVs and COPD
COPD became the third main cause of death globally by 2019, as reported by the World Health Organization.68 Current treatments primarily consist of antibiotics, bronchodilators, glucocorticoids and respiratory support, but these have limited effectiveness. In addition, acute exacerbation can further deteriorate lung function, ultimately leading to death. The main pathological features of COPD include small airway lesions and emphysema, with the pathogenesis being influenced by genetics, age, airway inflammation, protease-antiprotease imbalance, airway remodeling and oxidative stress.69
Research has shown that the application of MSC-EVs can reduce airway inflammation, decrease immune cell infiltration, reduce mucus production and prevent emphysema formation.40 Lung tissue apoptosis is an important mechanism in the development of COPD, especially in emphysema formation,70 with the VEGF/VEGFR2 signaling pathway possibly playing a key role.71 For example, human UCMSC-EXO was found to significantly inhibit papain-induced apoptosis in emphysema, possibly through the ERK pathway and the VEGF/VEGFR2-mediated AKT pathway. Furthermore, miRNA, such as hsa-miR-146a-5p and hsa-miR-10a-5p, which were differentially expressed in UCMSC-EXO, could also have been important components of the observed functions.43 Cigarette smoking, a major cause of COPD, leads to emphysema and airway inflammation through different mechanisms, including apoptosis inhibition, inflammatory responses and oxidative stress, amongst others.72 In this context, Krishna Prahlad Maremanda et al demonstrated that cigarette smoke affected some of the early mitochondrial genes involved in the fission/fusion process, increased the level of cytokines in the lungs of mice and exacerbated the injury response. On the other hand, an MSC+exosome combination treatment improved mitochondrial dysfunction in mouse lungs, thereby reducing lung inflammation and injury.44
The above findings clearly indicate that MSC-EVs are effective at reducing the occurrence and development of COPD by controlling oxidative stress as well as through anti-inflammatory, immune suppression and anti-apoptosis effects, but the precise mechanisms involved remain unclear, thus warranting further experiments.
MSC-EVs and Bronchial Asthma
Asthma, another common chronic lung disease beside COPD, is mainly characterized airway hyperresponsiveness as well as chronic inflammation of the airways, but unlike COPD, the airflow limitation is reversible during the early stages of the disease. In this case, the main pathological mechanisms include increased mucus secretion in the airways, increased vascularity, extravasation of plasma and inward flow of multiple inflammatory cells, hyperplasia of the airway’s smooth muscle, goblet cell proliferation, reduced integrity of the epithelium and cartilage as well as excessive subepithelial collagen deposition. Altogether, these different features lead to airway narrowing and thickening of the airway walls.73 Corticosteroids are central to asthma treatment, but they are not without various side effects. Furthermore, many patients tend to be Corticosteroids-dependent or even resistant. In this context, the development of new biological agents can be promising for treating severe asthma, but these therapeutic approaches are still in the preliminary stages and have limited efficacy.74 Therefore, there is an urgent need to develop new therapeutic approaches.
Previous studies have demonstrated that placental MSCs ameliorate airway hyperresponsiveness and inflammation in asthmatic rats by modulating Th 17/Treg balance.75 Whereas Ligia Lins de Castro et al found in a mouse model of asthma induced with OV that ADMSC-EVs decreased collagen fiber deposition in the lung parenchyma and airways, TGF-β levels in lung tissues, and total cell counts and eosinophil counts as well as in the lung tissue as well as in the airways equally as did ADMSCs, both compared to systemic administration of ADMSCs reduced CD4+CD25+Foxp3+ regulatory T-cell counts and reduced IL-5, IL-13 and eotaxin levels, whereas ADMSC-EVs significantly reduced eosinophil counts and decreased CD3+CD4+ T-cell counts, IL-4 and IL-5 levels in lung tissues. This suggests that ADMSC-EVs can exert similar therapeutic effects of ADMSCs while the specific mechanisms involved are different, and still need to be further studied by me in the future.45
Airway smooth muscle cells which represent major components of the airway, are involved in asthma progression. In particular, ADMSC-EXO were found to be effectively internalized by airway smooth muscle cells, while miR-301a-3p in ADMSC-EXO significantly inhibited the proliferation and migration of airway smooth muscle cells, stimulated by PDGF-BB (platelet-derived growth factor-BB). Additionally, in the latter case, enhanced apoptosis and reduced secretion of inflammatory factors were also noted.76 Airway remodeling, the main cause of asthma’s chronicity and a decline in lung function, can be induced by TGF-β through multiple mechanisms.77 For instance, TGF-β1 has been used in some studies for simulating an asthma-like state in vitro as well as an ovalbumin (OVA)-induced asthma model in mice. In this context, it was found that BMSC-EXO prevented Bronchial smooth muscle cells from significantly proliferating and migrating abnormally, while decreasing mucus production and collagen deposition in the lung tissues of mice, with these effects potentially attributed to the miR188/JARID2/Wnt/β-catenin axis.46
The immune system is largely involved in asthma, and MSC-EXO can regulate various immune cells involved in its pathogenesis.78 Severe steroid-resistant asthma is different from the ordinary one in terms of its severity and difficulty to treat.79 However, a study based on a mouse model of Severe steroid-resistant asthma found that MSC-EXO was effective at regulating macrophage polarization and reducing the inflammatory cascade response, possibly by targeting TRAF1 to regulate the activation of NF-κB and PI3K/AKT signaling pathways. It should nevertheless be noted that systemic depletion of macrophages weakened the therapeutic effects of MSC-EXO,47 thus providing a basis for further studies on controlling ordinary asthma and treating steroid-resistant ones.
MSC-EVs and Infectious Lung Diseases
Pneumonia refers to infectious lesions in the lungs caused by various pathogens, such as viruses, bacteria, and fungi, and it can be classified as hospital-acquired pneumonia or community-acquired pneumonia.80 Besides pulmonary symptoms, pneumonia can also lead to sepsis, cardiovascular diseases and neurological symptoms.81 Advances in diagnostic techniques, new antimicrobial therapies and prophylactic measures have changed the epidemiology of pneumonia, with a gradual increase in viral and atypical pathogen infections.81 At the same time, bacterial resistance remains a significant challenge. Previous studies have highlighted the pro-inflammatory, anti-inflammatory and antiviral effects of EVs in pneumonia, along with their potential as diagnostic biomarkers and partial therapeutic agents.82 Therefore, MSC-EVs play an important role in pneumonia.
Unlike community-acquired pneumonia, hospital-acquired pneumonia involves pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus spp, Escherichia coli and multidrug-resistant bacteria which are critical components of severe and highly lethal pneumonia.80 MSC-derived microvesicles (MSC-MVs) have been found to reduce lung inflammation, protein permeability and histological severity, while decreasing bacteremia incidence, increasing the phagocytosis of E. coli by monocytes and improving mouse survival by secreting keratinocyte growth factor in E. coli-induced acute lung injury mice.48. In addition, MSC-EVs significantly increased macrophage phagocytosis and decreased the mortality of mice with multidrug-resistant Pseudomonas aeruginosa-induced pneumonia, with miR-466 in MSC- EVs possibly playing an essential role for this effect.49 Similar effects were observed in an E. coli-induced model of human lung pneumonia.83 Ren-Jing Hu et al found that ADMSC-EXO-derived miR-181a-5p could not only inhibit bacterial growth and dissemination in the lungs of mice infected with Klebsiella pneumoniae, but also suppress macrophage infiltration in lung tissues as well as inhibit the expression of STAT3 at the post-transcriptional level, possibly through NLRP3-associated pyroptosis.50 Furthermore, nebulized human MSC-EVs also improved the survival rate of Pseudomonas aeruginosa-induced lung injury in a mouse model to 80% at 96 h, and all volunteer subjects tolerated the AMSC-EVs nebulized inhalation well, with no serious adverse events observed.84 This suggests that nebulized inhalation may be particularly effective for MSC-EVs in lung disease treatment.
Viruses are common pathogens causing respiratory infections, but the lack of specific drugs for many viral infections often results in significantly higher mortality rates among immunocompromised individuals. The sudden outbreak of COVID-19 was a severe blow to humanity,4 and the role of BMSC-EXO in treating neocoronaviral infection has attracted great interest from researchers. In April 2020, 24 patients with moderate to severe COVID-19 were treated with BMSC- EXO at a hospital center. The patients were given a single dose of exosomes (15 mL) intravenously, with safety and efficacy subsequently assessed from day 1 to day 14 after treatment. Overall, survival was 83%, with the cases being as follows: 17 of the 24 patients (71%) recovered, 3 (13%) were stable but in critical conditions and 4 (16%) died of causes unrelated to treatment. In addition, all safety endpoints were met since after treatment administration, no adverse events were observed within 72 hours. Therefore, a single treatment session improved the clinical status and oxygenation of the patients, with the mean arterial oxygen pressure to fraction of inspired oxygen (PaO2/FiO2) ratio being also higher by 192%. These findings highlighted BMSC-EXO’s potential in the treatment of severe COVID-19.85 Meiping et al included seven patients with COVID-19 pneumonia in their study. The patients were given nebulized treatment with UCMSC-EXO, with the results confirming that the nebulized administration was safer and did not cause acute allergic or secondary allergic reactions. Although, in this case, the sample size was insufficient and the difference in efficacy was not statistically significant, the treatment still promoted the resorption of lung lesions and shortened COVID-19 pneumonia. Furthermore, for patients presenting mild COVID-19 pneumonia, the hospitalization time was shortened.86 Zhu et al also conducted related clinical experiments. They selected 7 patients with severe COVID-19-related pneumonia and administered adipose-derived mesenchymal stem cell-derived exosomes (2.0 × 108 nano vesicles) via nebulization daily for 5 consecutive days. All patients tolerated the treatment well, with no evidence of clinical instability or dose-related toxicity. This further verifies the safety of MSC-EVs nebulization therapy.87
The above studies demonstrated the therapeutic potential of MSC-EVs in pulmonary infectious diseases, while highlighting the importance of miRNAs delivered by MSC-EVs. However, the data and study types are still insufficient, and are currently limited to COVID-19 as well as a few bacterial pneumonias, However, diseases like tuberculosis, Staphylococcus aureus, Aspergillus and H. influenzae have not been mentioned. Therefore, the roles and detailed mechanisms are yet to be explored.
MSC-EVs and Lung Cancer
Lung cancer includes primary and secondary types, with the former being further divided into either non-small cell lung cancer (NSCLC) which accounts for around 80–85% of cases or small cell lung cancer.88 Treatment currently includes targeted therapy, immunotherapy, chemotherapy, radiotherapy or even surgery.89 Despite the availability of a wide range of treatment options, the mortality rate is gradually on the rise, with a 2021 survey even placing lung cancer as the leading cause of cancer mortality rate globally.90 Such a high mortality rate for malignant tumors is largely due to their invasive and metastatic ability as well as the risk of recurrence and chemotherapy resistance even after early surgery. Therefore, finding ways to control tumor progression and recurrence is crucial.
The growth, differentiation, invasion and metastasis of lung cancer involve multiple signaling pathways, including the Wnt/β-catenin, the JAK/STAT, the PI3K/AKT/mTOR and the TGF-β/Smad signaling pathways.91,92 Current research has found that MSCs play a dual role in tumors,93,94 whereas studies on the effects of MSC-EVs on lung cancer are very limited. Unlike MSCs, MSC-EVs almost always play a tumor-suppressive role in current studies, except for hypoxia-induced conditions, where MSC-EVs may promote lung cancer growth/invasion and metastasis, mostly through their secreted miRNA and circRNA.
It has been demonstrated that a state of EMT reduces cell-cell adhesion, increases cell mobility, improves their invasiveness and exhibit both stem cell-like properties as well as resistance to apoptosis. As such, EMT can play a significant role in tumors.95 One study confirmed that after hypoxic BMSC-EVs could transfermiR-193a-3p, miR-210-3p and microRNA-5100 into lung cancer cells and activate STAT3-induced EMT, thereby promoting metastasis of lung cancer cells. In addition, they can serve as markers to distinguish cancer patients from non-cancer control ones, or even to distinguish metastatic lung cancer patients from non-metastatic lung cancer patients.96 This provides a new approach for the diagnosis of cancer, especially to assess the presence or absence of lung metastasis. In addition, it has been demonstrated that increased expression of miR-21-5p by MSC-EVs after hypoxic stimulation promotes the development of non-small cell lung cancer by reducing apoptosis and promoting macrophage M2 polarization.97
In addition to the above miRNAs, various MSC-EVs-derived miRNAs have been shown to exert inhibitory effects on NSCLC. In this context, Tong Wu et al demonstrated that BMSC-EXO-derived miR-30b-5p could promote NSCLC cell apoptosis and inhibit tumor growth in nude mice by inhibiting the EZH2/PI3K/AKT axis.51 In contrast, BMSC-EXO-carrying miRNA-204 inhibited the migration and invasion of NSCLC cells in vitro via the KLF7/AKT/HIF-1α axis.98 Yuan Liang et al further found that the up-regulation of BMSC-EXO-derived miR-144 inhibited NSCLC cell proliferation, colony formation as well as the number of S-phase-blocked cells, with these different characteristics confirmed through in vivo experiments.99 The low expression of miRNA-598 in NSCLC has been linked to poor prognosis of NSCLC patients, but given that MSCS-EVs transduces miR-598 within NSCLC cells to inhibit their proliferation, migration and invasion, it is likely that they may have played this role by directly interacting with their target gene THBS2.52 UCMSC-EXO harboring miR-320 a may inhibit lung cancer cell growth through SOX 4/Wnt/b-linker protein axis.100 BMSC-EVs-derived let-7i has an inhibitory effect in lung cancer progression through the KDM 3A/DCLK 1/FXYD 3 axis.101 The investigators found that UCMSC-EVs co-implanted mice showed a similar trend of tumor growth as UCMSC co-implanted mice, both of which promoted the growth of lung adenocarcinoma cells, which may be related to the fact that the BMSC-EVs-derived miR-410 translocated to lung adenocarcinoma cells to directly inhibit the expression of PTEN.53
Chemotherapy represents an effective treatment modality for non-small cell lung cancer, but it can also increase the proportion of polyploid cancer cells in vivo and even induce chemotherapy resistance in lung cancer. For example, Lili Wang et al found that the present MSC conditioned medium, MSCs and MSC-EXO had no effects on the proliferation of polyploid A549 and H1299 cells, but could significantly promote their EMT through the AMPK pathway, cell migration, anti-apoptosis and autophagy.102 These results present new insights into the mechanism of tumor metastatic recurrence. So far, the study of MSC-EVs in lung cancer is limited, and whether it plays a promotional or inhibitory role still needs to be further explored.
MSC-EVs and Pulmonary Fibrosis
Pulmonary fibrosis (PF), a common end stage of many interstitial lung diseases, is influenced by genetics, drugs, radiotherapy, connective tissue disease as well as organic and inorganic dust, amongst other factors. Idiopathic pulmonary fibrosis is the most common type of this condition, with the average life expectancy after diagnosis being only 3–5 years. In addition, a significant number of COVID-19 patients also die of PF during the latter stages of the disease. As far as treatment options for PF are concerned, they tend to be very limited, with lung transplantation being the ultimate choice. However, lung sources are also limited, while the success and survival rates are generally low.103,104 Therefore, there is an urgent need to identify novel and effective treatment modalities. In recent years, through continuous research, it was found that the treatment of idiopathic pulmonary fibrosis using MSC-EXO resulted in inhibited proliferation and activation of fibroblasts, reduced collagen overexpression, inhibited EMT process, inhibited release of inflammatory factors as well as the modulation of immune responses.105
EMT is an important mechanism for PF, and existing evidence points out that damaged alveolar epithelial cells undergo the EMT process after radiation therapy. This can further be accompanied by the accumulation of collagen and extracellular matrix proteins which ultimately leads to PF.106 In this context, Yi L et al experimentally demonstrated that mouse MSC-EXO could reduce alveolar EMT in vitro and attenuate lung injury in vivo. Additionally, miR-466f-3p in mouse MSC-EXO could have been dependent on the AKT/GSK3β pathway targeting C-MET (mesenchymal epidermal transition factor) to eliminate radiation-induced EMT and attenuate PF after radiation.54 Moreover, BMSC-EXO also attenuated silica-induced PF in rats, possibly by inhibiting the expression of the Wnt/β-catenin pathway.55
The maturation of myofibroblasts represents an important process in PF development,107 and with damaged alveolar epithelial cells undergo the EMT process being the main source of such myofibroblasts.108 In particular, those of type 2 alveolar epithelial cell senescence tend to be critical for the pathogenesis of PF.109 For instance, Xudan Lei et al found that MSC-EVs could downregulate the level of ATM/P53/P21 signaling by miR-214-3p to reduce radiation-induced DNA damage and attenuate radiation-induced pulmonary vascular injury, inflammation and fibrosis. At the same time, the MSC-EVs inhibited the development of senescence-associated secretory phenotype and attenuated radiation-induced endothelial cell damage.5 It has also been demonstrated that UCMSC-EVs could attenuate silica-induced lung inflammation and fibrosis in mice through the circPWWP2A/miR-223-3p /NLRP3 axis.110 Yaoying Long et al used lentiviruses loaded with CD38 antigen receptor - CD8 transmembrane fragment fusion plasmid to transfect MSCs and construct CD38 antigen receptor membrane-modified MSCs-EVs (CD38- arm - MSCs - ev). In this case, it was found that the CD38- arm - msc-ev was effective in natural aging mice in vitro while its intraperitoneal administration showed higher expression of the CD38 antigen receptor. Similarly, the anti-fibrotic miRNA in a natural aging mouse model after drug administration and targeted senescent type 2 alveolar epithelial cells with high CD38 expression, and was effective in restoring NAD+ levels in vitro. This approach also reversed the EMT phenotype, and rejuvenated the senescent A549 cells, thereby alleviating various age-associated phenotypes and attenuating the PF in senescent mice.109 This provides us with a novel approach for applying MSC-EVs in disease research.
Finally, Pauline Rozier et al found that interferon gamma induction enhanced the therapeutic efficacy of MSC-EVs to improve PF in mice with systemic sclerosis.57 MSC-EXO also modulated the lung macrophage phenotype by shifting the ratio of lung pro-inflammatory/classical and non-classical monocytes as well as alveolar macrophages towards the monocyte/macrophage profile in control mice. The transplantation of bone marrow-derived monocytes, pretreated with MSC-EXO, further attenuated the core features of PF and lung inflammation, suggesting that the beneficial effects of MSC-EXO may be mediated through a systemic regulation of the monocyte phenotype.58
MSC-EVs with ALI/ARDS
ALI refers to acute interstitial lung lesions and pulmonary edema caused by various infections, trauma, shock, amongst others. In this case, the inability to quickly control the condition can lead to ARDS whose clinical manifestations include progressive hypoxemia and respiratory distress.111 The main pathological features involve excessive inflammation in the lungs, capillary endothelial cell injury and apoptosis of alveolar epithelial cells.112 Furthermore, according to a big data in 2016, the overall mortality rate of ARDS reached 40%, while that of severe ARDS was as high as 46.1%.113 These figures underscore the need to identify new and effective treatments.
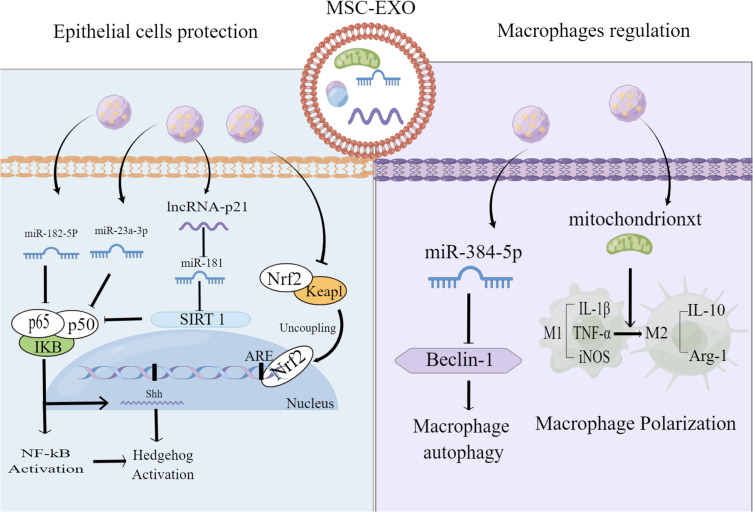
Research has found that BMSC-EXO could reverse ALI through the NRF-2/ARE and NF-κB signaling pathways.114 Sui et al found that LncRNA-pretreated BMSC-EXO inhibited the apoptosis of epithelial cells by up-regulating SIRT1 expression, thereby atenuting LPS-induced lung tissue injury.60 Similarly, MiR-182-5p and MiR-23a-3p, derived from BMSC-EXO, attenuated LPS-induced lung epithelial cell injury in mouse lungs by negatively regulating the Usp5/Ikbkb axis.59 In addition, it was also found that BMSC-EXO could prevent alveolar macrophage injury, while miR-384-5p in BMSC-EXO could target Beclin-1 to alleviate autophagic stress in alveolar macrophages, thereby protecting against LPS-induced ALI in vitro and in vivo.61 Xia, Liangjun et al further found that ADMSC-EXO increased OXPHOS activity, ATP production, the mtDNA levels of macrophages and the membrane potential of mitochondria in a mouse model of LPS-induced lung injury. The treatment also alleviated LPS-induced mROS stress in macrophages and promoted the shift of macrophages to an anti-inflammatory phenotype by restoring mitochondrial integrity which, in turn, attenuated their lung inflammation and injury.63 The above studies have confirmed that BMSC-EXO can alleviate acute lung injury by modulating different signaling pathways through the delivery of miRNA and LncRNA mitochondria (Figure 2). Current research primarily uses BMSC-EXO, and it is unclear whether exosomes from different cell sources or other EVs components have the same effect. Further research is needed to explore deeper mechanisms and effects in the future.
Figure 2.
Mechanisms of MSC-EXO in treating ALI/ARDS. MSC- EXO Inhibit alveolar epithelial apoptosis, improve macrophage damage, and regulate macrophage polarization state.
Note: This figure is originally drawn by Figdraw platform (www.figdraw.com).
Besides, Using a mouse model of LPS-induced ALI conventional MSC-MVs and MSC-MVs lacking Ang-1, Tang et al successively administered mRNA in the trachea and found that the latter led to the progression of lung inflammation and failure of pulmonary capillary permeability restoration. Thus, it was concluded that MSC-MVs could exert a therapeutic effect on ALI via Ang-1 mRNA.115 Through intranasal injection of lipopolysaccharides, Paulius Valiukeviˇcius et al induced ARDS-like lung injury in mice and found that human placental MSCs as well as their EVs therapy could improve the integrity of alveolar barriers and accelerate inflammatory regression in lung tissues, with that EVs therapy being more effective than human placental MSCs.62 In another study, 43 patients with COVID-19 along with ARDS were given MSCs and MSC-EVs in groups. Overall, they were found to significantly reduce the levels of serum inflammatory markers in COVID-19 patients but without serious adverse events.116 Although a comprehensive understanding and the underlying mechanism remain unclear, this work provides a reference for MSC-EVs in clinical trials.
MSC-EVs and Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) encompasses both primary and secondary changes and can arise from various chronic lung diseases. Other etiologies include pulmonary embolism, bronchopulmonary dysplasia, heart failure and metabolic disorders which are characterized by mitochondrial metabolic dysfunction, immune-inflammatory dysregulation, pulmonary vascular remodeling and pulmonary vasoconstriction.117 At the present time, the main therapeutic agents include calcium channel blockers, endothelin receptor antagonists, prostacyclin analogs, and phosphodiesterase inhibitors-5, which are not yet effective, and the newest tyrosine kinase inhibitors, imatinib and activin signaling inhibitors, sotericin, are believed to be effective in lowering PAH and improving pulmonary vascular remodeling,118 however, the long-term safety and efficacy of their use in the clinic still need to be further verified. Therefore, more effective and safer therapies should be explored, many studies have reported the protective effects of MSC-EVs against PAH.
For instance, Hong Liu et al demonstrated that human UCMSC-EXO could inhibit hypoxia-induced proliferation of pulmonary artery smooth muscle cells and ameliorate hypoxia-induced pulmonary arterial hypertension by promoting M2 macrophage polarization and preventing the expression of the IL-33/ST2 axis.67 MSC-EXO can also exhibit a protective effect on PAH by regulating the Wnt5a/BMP signaling pathway,64 while Jian-ying Chen et al used intravenous injections of rat BMSC-MVs or MSCs to significantly improve the mean right ventricular pressure and the mean PAH while significantly reducing the thickness index, small pulmonary artery area index and right ventricular hypertrophy of PAH rats.65 In a different study, researchers induced a bronchopulmonary dysplasia model by exposing neonatal mice to hyperoxia (HYRX; 75% O2) prior to treatment using MSC-EXO. The latter was found to improve lung function and PAH, while modulating macrophage phenotypic branching and reducing fibrosis and pulmonary vascular remodeling. An enhanced anti-inflammatory “m2-like” state inhibition as well as a pro-inflammatory “M1” state could be the likely mechanisms of the observed therapeutic effects.66 These suggest that macrophage activation is critical in PAH and the protective effect of MSC-EVs on PAH involves signaling pathways such as Wnt5a/BMP and IL-33/ST2, which is an important entry point in our study.
Limitations and Improvements of MSC-EVs in Clinical Applications
MSC- EVs are affected by a variety of factors during intracellular production, release, extraction, and experimentation, but there are still many limitations both at the animal and cellular level and in clinical research.
Firstly, it is difficult to generate a completely homogeneous population of EVs during their preparation, and effective separation, purification and quantification is an ongoing challenge, thus new techniques to improve the separation, purity and stability of EVs are essential. Currently, the most commonly used method is differential (ultra) centrifugation, and others include tangential flow filtration, density gradient/padding, size exclusion chromatography, fluid flow-based separations, separations based on charge and molecular separation, kits, etc.119 In addition, innovative techniques for isolation of EVs such as fluorescent labeling and subsequent analysis using high-resolution flow cytometry, specialized flow cytometry, and the use of laser tweezers and Raman spectroscopy can be helpful in improving exosome concentration and purification.16 Each of the above methods has its own advantages and disadvantages. Even though some efforts have been made by researchers for this purpose, for example, Ashley G. et al compared the effect of ultracentrifugation time on the quality and quantity of MSC-EVs and found that 1-hour differential ultracentrifugation was optimal for isolation of high-quality and high-quantity MSC- EVs from MSCs medium, and that freshly isolated EVs were more efficiently taken up by cells than frozen EVs.120 Another new method, immunoaffinity superparamagnetic nanoparticles were shown to have significantly higher particle-to-protein masses and ratios than those isolated by conventional ultracentrifugation, PEG-based precipitation PEG-based precipitation, and commercial Kit commercial Kit (3.2 ± 0.8×109).120 However, there is still no single method that can perfectly obtain the target EVs, and a combination of different methods can be tried in the future, which may lead to better results.
Secondly, increasing exosome production is another challenge as clinical translation requires large-scale production of MSC-EVs. Current approaches to stimulate exosome production include two main levels: (1). genetic manipulation based on exon biogenesis and cycling pathways (2); different environmental stimulants or media compositions,121 such as adjustments cell inoculation density,122 triple-D culture,123 hypoxia and ischemia exposure,124 as well as a variety of genetic modulations, use of hollow fiber systems, different liposome stimulation, etc.121 In addition to regulating various reaction processes in organisms by serving as carriers to deliver proteins, DNA, miRNA, LncRNA, circRNA and mRNA, exosomes can also play an important role in disease treatment by being engineered to carry drugs, antibodies, genes and to participate in targeted therapy.125,126 Specific signals, such as IFN-γ, TNF-A and hypoxia can enhance MSCs’ functionality.127 the extracellular matrix stiffness could regulate the sorting of functional cargoes into MSC-EVs, thus affecting their secretion and macrophage uptake. Therefore, by varying the stiffness of the extracellular matrix, different phenotypes of MSC-EVs could be rationally prepared.128 Recent experiments have explored new screening methods and primers for the large-scale production of MSC-EVs in bioreactor manufacturing in view of enhancing their function.129 The shelf life of MSC-EXO also represents a challenge, and in this case, the lyophilization of MSC-EXO or the addition of stabilizers, such as sugar or polyethylene glycol, may be an effective approach to overcome the issue.130 Finally, in some experiments, hydrogels were shown to be effective in improving the survival time and the therapeutic effects of exosomes due to their excellent biocompatibility.131,132
In addition to this, the functional differences of MSC-EVs from different sources, the biodistribution after action, the optimal dose, the optimal time of action, the appropriate mode of administration and the possible side effects are still unclear to us and have been explored by some researchers. As with MSCs, the phenotype and function of MSC-EVs and therapeutic effects can vary depending on their origin.125 It has been found that the proliferative and apoptotic effects on keratoblastoma by BMSC-EVs, UCMSC-EVs, and ADMSC-EVs differently,133 ADMSC-EVs had higher proliferation rates and lower senescence.134 Exosomes, the most studied EVs, have been shown to enhance the growth of neural synapses in neurodegenerative lesions more strongly in menstrual-derived exosomes relative to UCMSCS, BMSCs, and choriocapillaris stem cells-derived exosomes.135 Personalized selection of MSC-EVs will also become necessary in future studies, however, there are limited relevant studies at present.
Some studies have summarized the biodistribution of EVs after administration in animals and found that regardless of the source or size of EVs or the receptor species to which EVs are administered, they are mainly distributed in the liver, lungs, kidneys, and spleens after injection, but different modes of injection may produce differences in the concentration and time of distribution of EVs in organs.136 However, there are no studies related to the biodistribution of MSC-EVs after nebulized administration and comparison of the same disease using different MSC-EVs administration methods. Therefore, future studies are essential to better standardize the treatment of MSC-EVs and individualize the use of MSC-EVs in disease. The cellular origin of EVs, labeling techniques, and animal models can influence the observed biodistribution, and efficient magnetic labeling allows MRI to track the homing of stem cell-derived extracellular vesicles after systemic delivery.137 Zheng Han et al developed a novel labeling strategy to prepare magnetic EVs superparamagnetic iron oxide nanoparticles coated with a polyhistidine tag, to efficiently separate magnetic EVs from unencapsulated superparamagnetic iron oxide particles, thus allowing sensitive and specific MRI tracking of systemically injected therapeutic EVs.138 This adopted a new approach for biodistribution studies of MSC-EVs.
Detection in the liver and kidney peaked 1 hour after intravenous injection of small EVs, while distribution in the lungs and spleen peaked at 2–12 hours. Large EVs was most abundant in the lungs, reaching a local peak in the first hour after administration and decreasing between 2–12 hours.136 Thus the intravenous mode of administration as well as small EVs may be more advantageous in liver disease, whereas large EVs may be more suitable for lung disease due to their pulmonary distribution advantage. However, it has recently been demonstrated that large UCMSC-EVs activate the coagulation cascade response in a dose- and TF/CD 142 -dependent manner, which leads to intravascular coagulation, pulmonary embolism, and acute death in mice, which has not been previously reported, and it has further been found that modification of the infusion regimen (by slowing down the rate of infusion and by the use of heparin as an anticoagulant) can be effective in preventing the adverse effects of UCMSC-EVs infusion.139. In addition, the homing effect of MSC-EVs may target the site of inflammation and injury. It has been found that MSC-EVs will exhibit greater pulmonary targeting in acute lung injury in a state of increased pulmonary vascular permeability and inflammation.140 In recent years, some clinics have also used nebulized drug delivery to achieve certain effects, and significant adverse effects have been found. This property of targeting the site of injury and the feasibility of nebulized treatment may partially overcome the limitations of tissue and organ distribution of MSC-EVs, thus greatly improving their application in lung diseases.
Summary and Future Outlook
Lung diseases represent a serious threat to human health, MSC-Evs play an important role in the treatment of lung disease, but the clinical translation process of MSC-EVs is still very challenging. The majority of studies have been conducted at the cellular and animal levels, and a few clinical studies have been conducted in the specific context of the new coronavirus outbreak, which have confirmed the role of MSC-EVs in lung disease, however, the data are limited. How to obtain more standardized MSC-EVs in the future, how to choose the right source, the optimal dose, the duration of action, and the mode of administration are still challenges we need to solve. In addition to the direct application of the original MSC-EVs, through different engineering modifications, different biological effects can be exerted, and their ability to carry targeted drugs can be enhanced, thus playing a unique role in the treatment of lung disease. In conclusion, the use of MSC-EVs is valuable and promising, and they may become a new and effective modality for the treatment of various lung diseases.
Funding Statement
This review was supported by Zhejiang Medical and health science and technology plan project of provincial and municipal co-construction project (No. WKJ-ZJ-2450), Zhejiang public welfare fund research project experimental animal project (No. LGD22H030007), Jinhua Municipal Science and Technology Bureau Key Project (No. 2023-3-091) and Jinhua Municipal Central Hospital Science and Technology Project (No. JY2023-1-04).
Abbreviations
MSCs, mesenchymal stem cells; EVs, extracellular vesicles; MSC-EVs, extracellular vesicles of MSCs; MSC-EXO,mesenchymal stem cell-derived exosomes; MSC-MVs, MSC-derived microvesicles; UCMSCs, umbilical cord-derived mesenchymal stem cells; BMSCs, bone marrow mesenchymal stem cells;ADMSCs, adipose-derived mesenchymal stem cells; COPD, Chronic Obstructive Pulmonary Disease; ALI/ARDS, acute lung injury/ acute respiratory distress syndrome; EMT, Epithelial mesenchymal transition; NSCLC, non-small cell lung cancer; PF, pulmonary fibrosis; PAH, Pulmonary arterial hypertension; i.p, intraperitoneal; i.h, subcutaneous injection; i.t, intratracheal administration; i.v, intravenous injection.
Data Sharing Statement
There are no data and no material associated with this manuscript.
Ethics Approval and Consent to Participate
There is no human subject, and this is a review, so there is no need for ethical approval and consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interest in this work.
References
- 1.Charles-Edouard L. et al. Pulmonary infections complicating ARDS. Intensive Care Med. 2020;46(12):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christine DB, et al. Air Pollution and Lung Cancer: a Review by International Association for the Study of Lung Cancer Early Detection and Screening Committee. J Thorac Oncol. 2023;18(10):1. [DOI] [PubMed] [Google Scholar]
- 3.Alexandra S, et al. Nationwide indoor smoking ban and impact on smoking behaviour and lung function: a two-population natural experiment. Thorax. 2022;78(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schumacher AE. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950-2021, and the impact of the COVID-19 pandemic: a comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;2024:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoang D, Pham PT, Bach TQ, et al. Stem cell-based therapy for human diseases. Signal Transduc Target Ther. 2022;7(1):272. doi: 10.1038/s41392-022-01134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv F, Tuan RS, Cheung KMC, et al. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–1419. doi: 10.1002/stem.1681 [DOI] [PubMed] [Google Scholar]
- 7.Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41(9):653–664. doi: 10.1016/j.tips.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Jiang X, Ren L. Optimization of the adipose-derived mesenchymal stem cell delivery time for radiation-induced lung fibrosis treatment in rats. Sci Rep. 2019;9(1):5589. doi: 10.1038/s41598-019-41576-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akasaka Y. The Role of Mesenchymal Stromal Cells in Tissue Repair and Fibrosis. Adv Wound Care. 2022;11(11):561–574. doi: 10.1089/wound.2021.0037 [DOI] [PubMed] [Google Scholar]
- 10.Zhao S, Wehner R, Bornhäuser M, et al. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Develop. 2010;19(5):607–614. doi: 10.1089/scd.2009.0345 [DOI] [PubMed] [Google Scholar]
- 11.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nature Med. 2005;11(4):367–368. doi: 10.1038/nm0405-367 [DOI] [PubMed] [Google Scholar]
- 12.Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303(11):L967–77. doi: 10.1152/ajplung.00144.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. JASN. 2009;20(5):1053–1067. doi: 10.1681/ASN.2008070798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrell C, et al. Molecular Mechanisms Responsible for Anti-inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv Exp Med Biol. 2019;1084:187–206. [DOI] [PubMed] [Google Scholar]
- 15.Peng H, Ji W, Zhao R, et al. Exosome: a significant nano-scale drug delivery carrier. J Mater Chem B. 2020;8(34):7591–7608. doi: 10.1039/D0TB01499K [DOI] [PubMed] [Google Scholar]
- 16.Zhu L, Wang Q, Guo M, et al. Mesenchymal Stem Cell-Derived Exosomes in Various Chronic Liver Diseases: hype or Hope? J Inflamm Res. 2024;17:171–189. doi: 10.2147/JIR.S439974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozito T, Kuo CK, Taboas JM, et al. Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J Cell Biochem. 2009;107(4):714–722. doi: 10.1002/jcb.22167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spees J, Lee R, Gregory C. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. doi: 10.1186/s13287-016-0363-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Xu Y, Zhong Z, et al. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: paracrine Activity Without Remuscularization. Circulation Res. 2016;118(6):970–983. doi: 10.1161/CIRCRESAHA.115.307516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guillamat-Prats R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells. 2021;10(7):1729. doi: 10.3390/cells10071729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: an Update. Cell Transpl. 2016;25(5):829–848. doi: 10.3727/096368915X689622 [DOI] [PubMed] [Google Scholar]
- 22.Xiuying L, et al. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34(3):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu L, et al. Differentiation of Wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue engineering. Part A. 2009;15(10):2865–2873. [DOI] [PubMed] [Google Scholar]
- 24.Amable P, Teixeira MVT, Carias RBV, et al. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther. 2014;5(2):53. doi: 10.1186/scrt442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Fu X, Yan Y, et al. In vitro differentiation of rhesus macaque bone marrow- and adipose tissue-derived MSCs into hepatocyte-like cells. Exp Ther Med. 2020;20(1):251–260. doi: 10.3892/etm.2020.8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mabuchi Y, Morikawa S, Harada S, et al. LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Rep. 2013;1(2):152–165. doi: 10.1016/j.stemcr.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Røsland G, Svendsen A, Torsvik A, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69(13):5331–5339. doi: 10.1158/0008-5472.CAN-08-4630 [DOI] [PubMed] [Google Scholar]
- 28.Meier R, Müller YD, Morel P, et al. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013;11(3):1348–1364. doi: 10.1016/j.scr.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Kemeny DM, Heng BC, et al. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Iimmunol. 2006;176(5):2864–2871. doi: 10.4049/jimmunol.176.5.2864 [DOI] [PubMed] [Google Scholar]
- 30.Kim D, Lee J, Kim SR, et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics. 2015;31(6):933–939. doi: 10.1093/bioinformatics/btu741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maguire G. Stem cell therapy without the cells. Commun Integr Biol. 2013;6(6):e26631. doi: 10.4161/cib.26631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbaszadeh H, Ghorbani F, Derakhshani M, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: a novel therapeutic paradigm. J Cell Physiol. 2020;235(2):706–717. doi: 10.1002/jcp.29004 [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaka Y, Yashiro R. Therapeutic Strategy of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles as Regenerative Medicine. Int J Mol Sci. 2022;23(12):6480. doi: 10.3390/ijms23126480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsiapalis D, O’Driscoll L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells. 2020;9(4):991. doi: 10.3390/cells9040991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Łabędź-masłowska A, Vergori L, Kędracka-Krok S, et al. Mesenchymal stem cell-derived extracellular vesicles exert pro-angiogenic and pro-lymphangiogenic effects in ischemic tissues by transferring various microRNAs and proteins including ITGa5 and NRP1. J Nanobiotechnol. 2024;22(1):60. doi: 10.1186/s12951-024-02304-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Lei B, Zhang E, et al. Targeted Therapy for Inflammatory Diseases with Mesenchymal Stem Cells and Their Derived Exosomes: from Basic to Clinics. Int j Nanomed. 2022;17:1757–1781. doi: 10.2147/IJN.S355366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zulueta A, Colombo M, Peli V, et al. Lung mesenchymal stem cells-derived extracellular vesicles attenuate the inflammatory profile of Cystic Fibrosis epithelial cells. Cell. Signalling. 2018;51:110–118. doi: 10.1016/j.cellsig.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 38.David -R-R, et al. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Control Release. 2017;262:1. [DOI] [PubMed] [Google Scholar]
- 39.Duc MH, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noridzzaida R, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD). Stem Cell Res Ther. 2021;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jing-Hua W, et al. Role of mesenchymal stem cell derived extracellular vesicles in autoimmunity: a systematic review. World J Stem Cells. 2020;12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiaowei X, et al. A non-invasive strategy for suppressing asthmatic airway inflammation and remodeling: inhalation of nebulized hypoxic hUCMSC-derived extracellular vesicles. Front Immunol. 2023;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin C, et al. Exosomes derived from human umbilical cord mesenchymal stem cells protect against papain-induced emphysema by preventing apoptosis through activating VEGF-VEGFR2-mediated AKT and MEK/ERK pathways in rats. Regen Ther. 2022;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishna Prahlad M, Isaac Kirubakaran S, Irfan R. Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicol Appl Pharmacol. 2019;385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LC L, et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res Ther. 2017;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lishen S, et al. Human bone marrow-mesenchymal stem cell-derived exosomal microRNA-188 reduces bronchial smooth muscle cell proliferation in asthma through suppressing the JARID2/Wnt/β-catenin axis. Cell Cycle. 2022;21(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bing D, et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res Ther. 2021;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antoine M, et al. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med. 2015;192(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng-Meng S, et al. Role of miR-466 in mesenchymal stromal cell derived extracellular vesicles treating inoculation pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. Clin Transl Med. 2021;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu R, Chen X-C, Xu L, et al. KlebsiellaMiR-181a-5p Delivered by Adipose-Derived Mesenchymal Stem Cell Exosomes Alleviates pneumonia Infection-Induced Lung Injury by Targeting STAT3 Signaling. Mediators Inflammation. 2022;2022:5188895. doi: 10.1155/2022/5188895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tong W, et al. Inhibitory role of bone marrow mesenchymal stem cells-derived exosome in non-small-cell lung cancer: microRNA-30b-5p, EZH2 and PI3K/AKT pathway. J Cell Mol Med. 2023;27(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xuebo L, Fan W. Mesenchymal stem cell-derived extracellular vesicles transfer miR-598 to inhibit the growth and metastasis of non-small-cell lung cancer by targeting THBS2. Cell Death Discov. 2023;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong L, Pu Y, Zhang L, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018;9(2):218. doi: 10.1038/s41419-018-0323-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi L, et al. Mouse mesenchymal stem cell-derived exosomal miR-466f-3p reverses EMT process through inhibiting AKT/GSK3β pathway via c-MET in radiation-induced lung injury. J Exp Clin Cancer Res. 2022;41(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enguo Z, et al. Exosomes derived from bone marrow mesenchymal stem cells reverse epithelial-mesenchymal transition potentially via attenuating Wnt/β-catenin signaling to alleviate silica-induced pulmonary fibrosis. Toxicol Mech Methods. 2021;31(9). [DOI] [PubMed] [Google Scholar]
- 56.Xudan L, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Radiation-Induced Lung Injury via miRNA-214-3p. Antioxid Redox Signal. 2020;35(11). [DOI] [PubMed] [Google Scholar]
- 57.Pauline R, et al. Lung Fibrosis Is Improved by Extracellular Vesicles from IFNγ-Primed Mesenchymal Stromal Cells in Murine Systemic Sclerosis. Cells. 2021;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nahal M, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kun X, et al. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acut lung injury. Cell Death Dis. 2020;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xintong S, Wei L, Zhi L. Exosomal lncRNA-p21 derived from mesenchymal stem cells protects epithelial cells during LPS-induced acute lung injury by sponging miR-181. Acta Biochim Biophys Sin. 2021;53(6). [DOI] [PubMed] [Google Scholar]
- 61.Xuan L, et al. BMSC-Derived Exosomes Ameliorate LPS-Induced Acute Lung Injury by miR-384-5p-Controlled Alveolar Macrophage Autophagy. Oxid Med Cell Longev. 2021;2021(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paulius V, et al. Human Placental Mesenchymal Stem Cells and Derived Extracellular Vesicles Ameliorate Lung Injury in Acute Respiratory Distress Syndrome Murine Model. Cells. 2023;12(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liangjun X, et al. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. 2022;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhaohua Z, et al. The protective effects of MSC-EXO against pulmonary hypertension through regulating Wnt5a/BMP signalling pathway. J Cell Mol Med. 2020;24(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jian-ying C, et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacol Sin. 2014;35(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gareth RW, et al. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med. 2017;197(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong L, et al. Human Umbilical Cord Mesenchymal Stromal Cell-Derived Exosomes Alleviate Hypoxia-Induced Pulmonary Arterial Hypertension in Mice Via Macrophages. Stem Cells. 2023;42(4). [DOI] [PubMed] [Google Scholar]
- 68.Stephanie AC, et al. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342). [DOI] [PubMed] [Google Scholar]
- 69.Romain AP, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5). [DOI] [PubMed] [Google Scholar]
- 70.Ingel KD, et al. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guohua Z, et al. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy. 2010;12(5). [DOI] [PubMed] [Google Scholar]
- 72.Winda S, et al. Smoking from a Younger Age Is the Dominant Factor in the Incidence of Chronic Obstructive Pulmonary Disease: case-Control Study. Int J Environ Res Public Health. 2021;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tadech B, et al. Immunologic mechanisms in asthma. Semin Immunol. 2019;46. [DOI] [PubMed] [Google Scholar]
- 74.Mary Clare M, et al. Role of Biologics in Asthma. Am J Respir Crit Care Med. 2018;199(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yingying L, et al. Placenta‑derived mesenchymal stem cells improve airway hyperresponsiveness and inflammation in asthmatic rats by modulating the Th17/Treg balance. Mol Med Rep. 2017;16(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen-Ye F, et al. Adipose-Derived Mesenchymal Stem Cell-Derived Exosomal miR-301a-3p Regulates Airway Smooth Muscle Cells During Asthma by Targeting STAT3. J Asthma Allergy. 2022;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gang C, Nasreen K. TGF-beta1 increases proliferation of airway smooth muscle cells by phosphorylation of map kinases. Respir Res. 2006;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Na S, Martijn S, Khalid S. Mesenchymal Stem Cell Immunomodulation: mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michael CP, et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol. 2018;143(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Charles WL, Ali MI, David HW. Community-acquired Pneumonia and Hospital-acquired Pneumonia. Med Clin North Am. 2019;103(3). [DOI] [PubMed] [Google Scholar]
- 81.Antoni T, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1). [DOI] [PubMed] [Google Scholar]
- 82.Shadi H, Hani H. Extracellular Vesicles and Their Role in Lung Infections. Int J Mol Sci. 2023;24(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park J, Kim S, Lim H, et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74(1):43–50. doi: 10.1136/thoraxjnl-2018-211576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi M, Yang Q-Y, Monsel A, et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J Extracell Vesicles. 2021;10(10):e12134. doi: 10.1002/jev2.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vikram S, et al. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chu M, Wang H, Bian L, et al. Nebulization Therapy with Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Pneumonia. Stem Cell Rev Rep. 2022;18(6):2152–2163. doi: 10.1007/s12015-022-10398-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y, Shi -M-M, Monsel A, et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study. Stem Cell Res Ther. 2022;13(1):220. doi: 10.1186/s13287-022-02900-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anna MR, Anna D. Multiple primary lung cancer: a literature review. Adv Clin Exp Med. 2018;27(5). [DOI] [PubMed] [Google Scholar]
- 89.Fred RH, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2016;389(10066). [DOI] [PubMed] [Google Scholar]
- 90.Hyuna S, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3). [DOI] [PubMed] [Google Scholar]
- 91.Min Y, Min Y, Sun X, et al. Signal pathways and precision therapy of small-cell lung cancer. Signal Transduct Target Ther. 2022;7(1). doi: 10.1038/s41392-022-01187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhenyi N, et al. Signaling pathways and targeted therapies in lung squamous cell carcinoma: mechanisms and clinical trials. Signal Transduct Target Ther. 2022;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Youssef S, et al. The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: pro-Tumorigenic Effects versus Therapeutic Potential. Int J Mol Sci. 2023;24(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding W, Zhang K, Li Q, et al. Advances in Understanding the Roles of Mesenchymal Stem Cells in Lung Cancer. Cell Reprogramming. 2023;25(1):20–31. doi: 10.1089/cell.2022.0133 [DOI] [PubMed] [Google Scholar]
- 95.Lindsay JT, Syamal DB, Paul KC. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol. 2012;3(2). [PMC free article] [PubMed] [Google Scholar]
- 96.Xina Z, et al. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weihua R, et al. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Cancer Res. 2019;38(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao-Ni L, et al. microRNA-204 shuttled by mesenchymal stem cell-derived exosomes inhibits the migration and invasion of non-small-cell lung cancer cells via the KLF7/AKT/HIF-1α axis. Neoplasma. 2021;68(4). [DOI] [PubMed] [Google Scholar]
- 99.Yuan L, Wu Y, Wang Y, et al. Exosomal microRNA-144 from bone marrow-derived mesenchymal stem cells inhibits the progression of non-small cell lung cancer by targeting CCNE1 and CCNE2. Stem Cell Res Ther. 2020;11(1). doi: 10.1186/s13287-020-01926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie H, Wang J. MicroRNA-320a-containing exosomes from human umbilical cord mesenchymal stem cells curtail proliferation and metastasis in lung cancer by binding to SOX4. J Recept Sign Transduc Res. 2022;42(3):268–278. doi: 10.1080/10799893.2021.1918166 [DOI] [PubMed] [Google Scholar]
- 101.Liu J, Feng Y, Zeng X, et al. Extracellular vesicles-encapsulated let-7i shed from bone mesenchymal stem cells suppress lung cancer via KDM3A/DCLK1/FXYD3 axis. J Cell & Mol Med. 2021;25(4):1911–1926. doi: 10.1111/jcmm.15866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lili W, et al. Mesenchymal Stem Cells and their Derived Exosomes Promote Malignant Phenotype of Polyploid Non-Small-Cell Lung Cancer Cells through AMPK Signaling Pathway. Anal Cell Pathol. 2022;2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paolo S, et al. Idiopathic pulmonary fibrosis: disease mechanisms and drug development. Pharmacol Ther. 2020;222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luca R, Harold R C, Mark G J. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082). [DOI] [PubMed] [Google Scholar]
- 105.Carl Randall H, et al. Molecular Mechanisms Responsible for the Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Lung Fibrosis. Int J Mol Sci. 2024;25(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kevin KK, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anna L, et al. Cytokines and radiation-induced pulmonary injuries. J Radiat Res. 2018;59(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brigham CW, Roland M, Zea B. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yaoying L, et al. Targeting Senescent Alveolar Epithelial Cells Using Engineered Mesenchymal Stem Cell-Derived Extracellular Vesicles To Treat Pulmonary Fibrosis. ACS Nano. 2024;18(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin H, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles alleviated silica induced lung inflammation and fibrosis in mice via circPWWP2A/miR-223-3p/NLRP3 axis. Ecotoxicol Environ Saf. 2023;251. [DOI] [PubMed] [Google Scholar]
- 111.Nuala J, Luciano MG, Carolyn SC. Acute respiratory distress syndrome. Lancet. 2021;398(10300). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daniely Cornélio F, et al. Potential effects of medicinal plants and secondary metabolites on acute lung injury. Biomed Res Int. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giacomo B, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8). [DOI] [PubMed] [Google Scholar]
- 114.Jun L, et al. Mesenchymal stem cell exosomes reverse acute lung injury through Nrf-2/ARE and NF-κB signaling pathways. PeerJ. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiao-Dan T, et al. Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 mRNA. Stem Cells. 2017;35(7). [DOI] [PubMed] [Google Scholar]
- 116.Morteza Z, et al. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: a randomized controlled trial. Stem Cell Res Ther. 2023;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Poch D, Mandel J. Pulmonary Hypertension. Ann Internal Med. 2021;174(4):ITC49–ITC64. doi: 10.7326/AITC202104200 [DOI] [PubMed] [Google Scholar]
- 118.Ghofrani H, Gomberg-Maitland M, Zhao L, et al. Mechanisms and treatment of pulmonary arterial hypertension. Nat Rev Cardiol. 2024. doi: 10.1038/s41569-024-01064-4 [DOI] [PubMed] [Google Scholar]
- 119.Welsh J, Goberdhan DCI, O’Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404. doi: 10.1002/jev2.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao A, Shah K, Cromer B, et al. Comparative analysis of extracellular vesicles isolated from human mesenchymal stem cells by different isolation methods and visualisation of their uptake. Exp Cell Res. 2022;414(2):113097. doi: 10.1016/j.yexcr.2022.113097 [DOI] [PubMed] [Google Scholar]
- 121.Jafari D, Malih S, Eini M, et al. Improvement, scaling-up, and downstream analysis of exosome production. Crit Rev Biotechnol. 2020;40(8):1098–1112. doi: 10.1080/07388551.2020.1805406 [DOI] [PubMed] [Google Scholar]
- 122.Divya BP, et al. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng Transl Med. 2017;2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mijin K, et al. Three-Dimensional Spheroid Culture Increases Exosome Secretion from Mesenchymal Stem Cells. Tissue Eng Regen Med. 2019;15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xuejun S, et al. Hypoxia-Regulated Tumor-Derived Exosomes and Tumor Progression: a Focus on Immune Evasion. Int J Mol Sci. 2022;23(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.María Cecilia S, et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles as Biological Carriers for Drug Delivery in Cancer Therapy. Front Bioeng Biotechnol. 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fang X, et al. Engineered Extracellular Vesicles with SHP2 high Expression Promote Mitophagy for Alzheimer’s Disease Treatment. Adv Mater. 2022;34(49). [DOI] [PubMed] [Google Scholar]
- 127.Dimitrios K, et al. Mesenchymal Stem Cell Functionalization for Enhanced Therapeutic Applications. Tissue Eng B. 2018;25(1). [DOI] [PubMed] [Google Scholar]
- 128.Zhixiao L, et al. ECM stiffness affects cargo sorting into MSC-EVs to regulate their secretion and uptake behaviors. J Nanobiotechnol. 2024;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Andrew ML, et al. High throughput screening of mesenchymal stromal cell morphological response to inflammatory signals for bioreactor-based manufacturing of extracellular vesicles that modulate microglia. Bioact Mater. 2024;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gang G, et al. The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer. Stem Cell Res Ther. 2022;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaiyue Z, et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl Mater Interfaces. 2018;10(36). [DOI] [PubMed] [Google Scholar]
- 132.Chaoyu Z, et al. Combination of lyophilized adipose-derived stem cell concentrated conditioned medium and polysaccharide hydrogel in the inhibition of hypertrophic scarring. Stem Cell Res Ther. 2021;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Andrea DF, et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther. 2014;15(4). [DOI] [PubMed] [Google Scholar]
- 134.Caroline M, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Therapeutic Use and in Bioengineering Applications. Cells. 2022;11(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alejandra MLV, et al. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320. [DOI] [PubMed] [Google Scholar]
- 136.Matthew K, et al. Biodistribution of extracellular vesicles following administration into animals: a systematic review. J Extracell Vesicles. 2021;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zachary TR, et al. Investigating the In Vivo Biodistribution of Extracellular Vesicles Isolated from Various Human Cell Sources Using Positron Emission Tomography. Mol Pharm. 2024;21(9). [DOI] [PubMed] [Google Scholar]
- 138.Zheng H, et al. Highly efficient magnetic labelling allows MRI tracking of the homing of stem cell-derived extracellular vesicles following systemic delivery. J Extracell Vesicles. 2021;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bian-Lei Y, et al. Lethal pulmonary thromboembolism in mice induced by intravenous human umbilical cord mesenchymal stem cell-derived large extracellular vesicles in a dose- and tissue factor-dependent manner. Acta Pharmacol Sin. 2024;2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alvin T, et al. Biodistribution of mesenchymal stromal cell-derived extracellular vesicles administered during acute lung injury. Stem Cell Res Ther. 2023;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data and no material associated with this manuscript.