Abstract
Background
Hepatocellular carcinoma (HCC) with pulmonary metastasis (PM) significantly worsens prognosis, and current treatment options remain limited.
Methods
A retrospective study was conducted on HCC patients treated with sintilimab combined with lenvatinib at three hospitals in China between 2020 and 2021. Progression-free survival (PFS), overall survival (OS), and tumor response based on RECIST 1.1 were compared. Treatment safety was assessed by analyzing treatment-related adverse events (TRAEs).
Results
Among 144 patients, 105 received sintilimab combined with lenvatinib (S+L), while 39 were treated with radiotherapy combined with sintilimab and lenvatinib (RT+S+L). The RT+S+L group showed superior outcomes in OS (25 months vs 16 months, HR = 0.58, 95% CI = 0.35–0.94, P=0.025) and PFS (14 months vs 6 months, HR = 0.61, 95% CI = 0.40–0.94, P=0.022) compared to the S+L group. Similarly, the RT+S+L group exhibited significantly higher objective response rate (ORR) and disease control rate (DCR) compared to the S+L group (61.5% vs 27.6%, P<0.001; 94.9% vs 76.2%, P=0.011). The most common grade 3/4 TRAEs in the RT+S+L group were hypertension, decreased platelet count, elevated total bilirubin, and proteinuria.
Conclusion
Radiotherapy combined with sintilimab and lenvatinib is an effective strategy for treating HCC with pulmonary metastasis. These findings highlight the critical role of radiotherapy in the management of HCC.
Keywords: hepatocellular carcinoma, pulmonary metastasis, radiotherapy, targeted therapy, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common tumor globally and the third leading cause of cancer-related deaths.1 Due to the subtle early symptoms of HCC, many patients are diagnosed at an advanced stage, with major vascular invasion or extrahepatic metastasis (EHM).2 The presence of EHM presents a formidable challenge to the survival of HCC patients, with an expected median survival time of only 6 to 10 months.3 The lungs are the most common site of HCC metastasis, accounting for 51% of all EHMs.4,5 A large-scale population study revealed that the 1-year overall survival rate for patients diagnosed with HCC with pulmonary metastasis (PM) is merely 12.8%.6 Therefore, improving the prognosis for these patients is an urgent issue.
For an extended period, sorafenib or lenvatinib has constituted the standard first-line treatment for patients with advanced HCC.7–10 However, these therapies have consistently failed to meet the prevailing demands for better prognostic outcomes in advanced HCC. In recent years, systemic treatments for HCC have made significant strides, particularly with the combination of anti-angiogenic drugs and immune checkpoint inhibitors (ICIs), which have demonstrated remarkable efficacy.11,12 The most notable example is the IMbrave150 trial. The latest results reveal that the median overall survival (OS) and progression-free survival (PFS) for HCC patients were 19.2 months and 6.9 months, respectively, both superior to sorafenib.13–15 Additionally, positive results were observed with camrelizumab plus rivoceranib and sintilimab plus a bevacizumab biosimilar (IBI305) compared to sorafenib monotherapy.16,17 Consequently, the combination of anti-angiogenic drugs and ICIs has become the standard first-line therapy for advanced HCC.
The evolution of systemic therapy has inspired new approaches, particularly the potential synergy between systemic and local treatments, becoming a focal point of interest.18 Local treatments such as radiofrequency ablation, transcatheter arterial chemoembolization, and radiotherapy induce tumor necrosis and apoptosis, which release cancer antigens and initiate the cancer-immunity cycle.18 This integrated approach of modulating the cancer-immune cycle is crucial for achieving sustained immunotherapeutic effects in most cancer patients.19 Interestingly, radiotherapy can trigger a rare phenomenon known as the abscopal effect, where treating one tumor site leads to the regression of distant, untreated metastatic cancer.20,21 Increasing evidence suggests that combining radiotherapy with immunotherapy enhances the likelihood of the abscopal effect.22 Therefore, we hypothesize that combining radiotherapy with sintilimab plus lenvatinib may offer a promising treatment option for HCC patients with PM.
To validate our hypothesis, we conducted this retrospective study aiming to compare the efficacy and safety of sintilimab plus lenvatinib with or without radiotherapy.
Methods
Patient Population
A retrospective study was conducted on patients with HCC with PM from 2020 to 2021 at Eastern Hepatobiliary Surgery Hospital, Nantong Haimen People’s Hospital, and The First people’s Hospital of Yancheng. Inclusion criteria were: (1) HCC with PM confirmed by preoperative enhanced CT/MRI; (2) age over 18 years; (3) treatment with sintilimab combined with lenvatinib; (4) good general condition and liver function classified as Child-Pugh A or B. Exclusion criteria included: (1) contraindications to sintilimab or lenvatinib; (2) receiving treatments other than radiotherapy; (3) history of other anti-cancer treatments; (4) history of other cancers; (5) incomplete clinical data.
This study adhered to the ethical standards of the Declaration of Helsinki and received approval from the institutional ethics committees of each participating center. Due to strict confidentiality of patient information and the retrospective nature of the study, informed consent was waived by the ethics committees.
Treatment
The treatment plan for each patient was developed by a multidisciplinary team—including liver surgeons, medical oncologists, radiologists, and radiation oncologists—while respecting the patient’s preferences. For patients receiving radiotherapy combined with sintilimab plus lenvatinib, the default radiotherapy regimen was intensity-modulated radiotherapy (IMRT), with radiation doses as previously described.23 The primary gross tumor volume (GTV) was delineated on the planning CT scan, referencing pretreatment multiphasic contrast MRI through an image fusion approach. The clinical target volume included the primary GTV with a 0.5-cm margin in all directions, without an additional margin. To account for respiratory liver motion and setup errors in 4-dimensional CT, the planning target volume (PTV) was defined by expanding the clinical target volume by 0.5 cm in the anterior-posterior and left-right directions and by 1.0 cm in the cranial-caudal direction.24 A minimal number of radiation fields, typically 7 to 9, and optimal radiation beam angles were selected during IMRT planning to minimize the dose and volume of irradiated normal liver tissue. The mean dose to the normal liver (total liver volume minus GTV) was restricted to ≤23 Gy, with the dose-volume histogram for the normal liver maintained within tolerance limits: liver volume receiving ≥5 Gy (V5) was <86%; V10, <68%; V15, <59%; V20, <49%; V25, <35%; V30, <28%; V35, <25%; and V40, <20%. The maximum allowable point dose for the stomach and duodenum was set to <54 Gy, for the colon <55 Gy, and for the spinal cord <40 Gy. The kidney volume receiving ≥20 Gy (V20) was limited to <50%. The planned target area of HCC received a daily dose of 200 cGy over a 28-day radiotherapy cycle, with a planned total dose of 52–56 Gy. Sintilimab plus lenvatinib was initiated at least three days after the completion of the first dose of radiotherapy.
Sintilimab was administered intravenously at a dose of 200 mg every three weeks. In the event of low-grade infusion reactions, the infusion rate was slowed or paused; treatment was resumed once symptoms resolved, with close monitoring. Lenvatinib was taken orally once daily, dosed by body weight at 12 mg (for patients ≥60 kg) or 8 mg (for patients <60 kg). In the case of lenvatinib-related toxicity, the dose was reduced to alleviate symptoms (to 8 mg or 4 mg daily, or 4 mg every other day). Treatment continued until the attending physician determined, based on imaging results, biochemical parameters, and the patient’s clinical status, that unacceptable toxicity or loss of clinical benefit had occurred.
Outcomes and Follow-Up
All patients were followed up in the outpatient clinic every three months. During each follow-up visit, routine physical examinations, laboratory blood tests, and enhanced CT/MRI scans were performed. The primary outcome of this study was PFS, defined as the time from the start of treatment to tumor progression, death from any cause, or the most recent follow-up. Tumor progression was assessed based on the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1),25 including progression of treated lesions and the appearance of new intrahepatic or extrahepatic lesions. Secondary endpoints included OS, objective response rate (ORR), disease control rate (DCR), and treatment-related adverse events (TRAEs). OS was defined as the time from the start of treatment to death from any cause or the date of the most recent follow-up.
Data on TRAEs were collected from clinical follow-up records or medical records. The assessment of TRAEs was based on the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. If multiple instances of the same type of toxicity occurred, the highest grade in each specific category was recorded for each patient.
Statistical Analysis
All clinical data were analyzed using IBM SPSS Statistics 23 or R 4.0 software (http://www.r-project.org/). The comparison of continuous variables was performed using Student’s t-test, and the comparison of categorical variables was performed using the chi-square test or Fisher’s exact probability test. Survival curves were calculated using the Kaplan-Meier method and compared using the Log rank test. Hazard ratios (HR) were calculated using the Cox regression model. Univariate Cox regression analysis was used to evaluate the significance of variables in the entire cohort. All variables associated with PFS/OS (p<0.1) were included in the multivariate Cox regression analysis. P < 0.05 was considered to indicate a significant difference.
To mitigate potential confounders, we utilized propensity score matching (PSM), a statistical technique designed to balance covariates between treatment groups, thereby attributing observed differences in outcomes more directly to the treatments rather than confounding factors. Propensity scores were calculated using logistic regression, incorporating all baseline demographic and clinical characteristics. We employed 1:1 greedy nearest neighbor matching with a 0.2 caliper, utilizing the R package “MatchIt”. The method calculated a distance between units from each group, assigning each unit a control unit as a match in sequence. This “greedy” approach did not optimize an overall criterion; each match was made without considering subsequent pairings.
Results
Patients
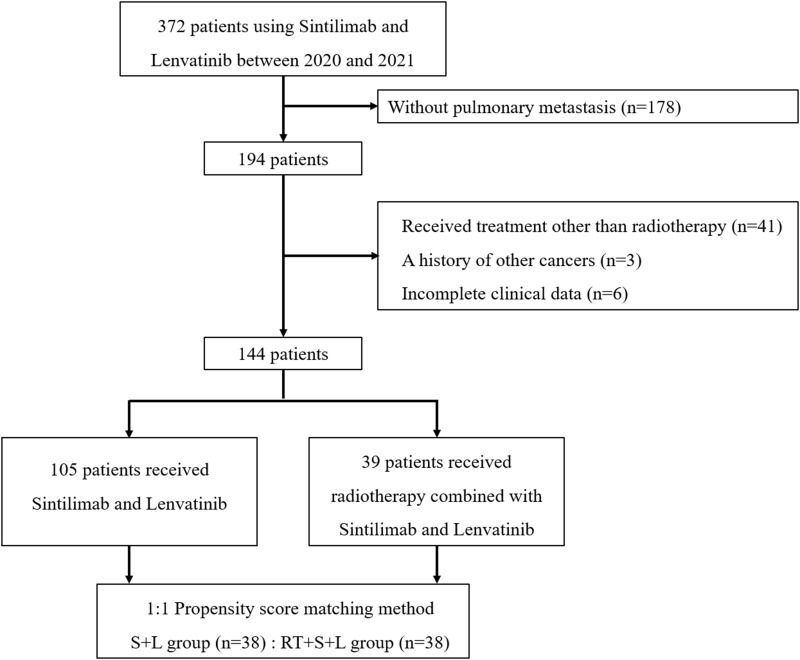
As shown in Figure 1, a total of 144 patients meeting the inclusion and exclusion criteria were included in this study, comprising 105 patients who received only sintilimab and lenvatinib treatment (S+L), and 39 patients who received radiotherapy combined with sintilimab and lenvatinib treatment (RT+S+L). After PSM, each group included 38 patients. The baseline characteristics of the two groups are detailed in Table 1, showing no significant differences between the groups.
Figure 1.
Flow diagram for the present study.
Table 1.
Baseline Covariates Before and After Matching
| Variables | Level | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|---|
| S+L (n=105) | RT+S+L (n=39) | SMD | S+L (n=38) | RT+S+L (n=38) | SMD | ||
| Age (%) | ≤ 60 | 72 (68.6) | 28 (71.8) | 0.072 | 29 (76.3) | 27 (71.1) | −0.117 |
| ˃ 60 | 33 (31.4) | 11 (28.2) | −0.072 | 9 (23.7) | 11 (28.9) | 0.117 | |
| Sex (%) | Female | 18 (17.1) | 7 (17.9) | 0.021 | 10 (26.3) | 7 (18.4) | −0.206 |
| Male | 87 (82.9) | 32 (82.1) | −0.021 | 28 (73.7) | 31 (81.6) | 0.206 | |
| Albumin (%) | ≤ 35 | 23 (21.9) | 5 (12.8) | −0.272 | 6 (15.8) | 5 (13.2) | −0.079 |
| ˃ 35 | 82 (78.1) | 34 (87.2) | 0.272 | 32 (84.2) | 33 (86.8) | 0.079 | |
| HBsAg (%) | Negative | 32 (30.5) | 11 (28.2) | −0.050 | 13 (34.2) | 11 (28.9) | −0.117 |
| Positive | 73 (69.5) | 28 (71.8) | 0.050 | 25 (65.8) | 27 (71.1) | 0.117 | |
| AFP.ng.mL (%) | ≤ 400 | 49 (46.7) | 13 (33.3) | −0.283 | 11 (28.9) | 13 (34.2) | 0.112 |
| ˃ 400 | 56 (53.3) | 26 (66.7) | 0.283 | 27 (71.1) | 25 (65.8) | −0.112 | |
| Alanine aminotransferase (%) | ≤ 44 | 81 (77.1) | 30 (76.9) | −0.005 | 29 (76.3) | 30 (78.9) | 0.062 |
| ˃ 44 | 24 (22.9) | 9 (23.1) | 0.005 | 9 (23.7) | 8 (21.1) | −0.062 | |
| Total bilirubin (%) | ≤ 17.1 | 57 (54.3) | 21 (53.8) | −0.009 | 24 (63.2) | 21 (55.3) | −0.158 |
| ˃ 17.1 | 48 (45.7) | 18 (46.2) | 0.009 | 14 (36.8) | 17 (44.7) | 0.158 | |
| Platelet (%) | ≤ 100 | 13 (12.4) | 4 (10.3) | −0.070 | 4 (10.5) | 4 (10.5) | 0.000 |
| ˃ 100 | 92 (87.6) | 35 (89.7) | 0.070 | 34 (89.5) | 34 (89.5) | 0.000 | |
| Tumor number (%) | Multiple | 54 (51.4) | 16 (41.0) | −0.211 | 14 (36.8) | 16 (42.1) | 0.107 |
| Single | 51 (48.6) | 23 (59.0) | 0.211 | 24 (63.2) | 22 (57.9) | −0.107 | |
| Maximum tumor diameter (%) | ≤ 5 | 45 (42.9) | 22 (56.4) | 0.273 | 22 (57.9) | 21 (55.3) | −0.053 |
| ˃ 5 | 60 (57.1) | 17 (43.6) | −0.273 | 16 (42.1) | 17 (44.7) | 0.053 | |
| Number of lung metastatic lesions (%) | 1 | 36 (34.3) | 8 (20.5) | −0.341 | 5 (13.2) | 8 (21.1) | 0.196 |
| 2 | 9 (8.6) | 5 (12.8) | 0.127 | 7 (18.4) | 5 (13.2) | −0.157 | |
| 3 | 4 (3.8) | 2 (5.1) | 0.060 | 1 (2.6) | 2 (5.3) | 0.119 | |
| ˃3 | 56 (53.3) | 24 (61.5) | 0.169 | 25 (65.8) | 23 (60.5) | −0.108 | |
| PVTT (%) | With | 43 (41.0) | 16 (41.0) | 0.001 | 17 (44.7) | 16 (42.1) | −0.054 |
| Without | 62 (59.0) | 23 (59.0) | −0.001 | 21 (55.3) | 22 (57.9) | 0.054 | |
| Child-Pugh classification (%) | A5 | 54 (51.4) | 25 (64.1) | 0.264 | 21 (55.3) | 24 (63.2) | 0.165 |
| A6 | 41 (39.0) | 12 (30.8) | −0.179 | 14 (36.8) | 12 (31.6) | −0.114 | |
| B7 | 10 (9.5) | 2 (5.1) | −0.199 | 3 (7.9) | 2 (5.3) | −0.119 | |
| ECOG performance status score (%) | 0 | 83 (79.0) | 24 (61.5) | −0.360 | 26 (68.4) | 24 (63.2) | −0.108 |
| 1 | 22 (21.0) | 15 (38.5) | 0.360 | 12 (31.6) | 14 (36.8) | 0.108 | |
| Other extrahepatic spread (%) | Bone | 11 (10.5) | 4 (10.3) | −0.007 | 5 (13.2) | 4 (10.5) | −0.087 |
| Lymph node | 18 (17.1) | 8 (20.5) | 0.083 | 8 (21.1) | 7 (18.4) | −0.065 | |
| None | 76 (72.4) | 27 (69.2) | −0.068 | 25 (65.8) | 27 (71.1) | 0.114 | |
Abbreviations: RT+S+L, radiotherapy combine with sintilimab plus lenvatinib; S+L, sintilimab plus lenvatinib; SMD, Standardized Mean Difference; HBsAg, hepatitis B surface antigen; AFP, alpha fetoprotein; ECOG, Eastern Cooperative Oncology Group; PVTT, portal vein tumor thrombus.
The median follow-up time for the RT+S+L group was 20 months (range 4–44 months), and for the S+L group, it was 16 months (range 3–44 months). Throughout the entire cohort, a total of 96 patients died during the follow-up period, with 21 from the RT+S+L group and 75 from the S+L group.
Efficacy
Table 2 summarizes the best tumor responses of patients assessed according to RECIST version 1.1. Before PSM, the ORR for the RT+S+L group was 61.5%, significantly higher than the 27.6% in the S+L group (P<0.001). The DCR in the RT+S+L group was 94.9%, also significantly higher than the 76.2% in the S+L group (P=0.011). After PSM, the ORR and DCR in the RT+S+L group remained higher than in the S+L group. The ORR was 60.5% in the RT+S+L group compared to 21.1% in the S+L group (P = 0.003), and the DCR was 94.7% versus 68.4%, respectively (P = 0.003).
Table 2.
Best Tumor Response According to RECIST v1.1
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| RT+S+L (n=39) | S+L (n=105) | P value | RT+S+L (n=38) | S+L (n=38) | P value | |
| Radiation target | ||||||
| CR | 1 (2.6%) | 2 (1.9%) | 1 (2.6%) | 1 (2.6%) | ||
| PR | 23 (59.0%) | 27 (25.7%) | 22 (57.9%) | 7 (18.4%) | ||
| SD | 13 (33.3%) | 51 (48.6%) | 13 (34.2%) | 16 (42.1%) | ||
| PD | 2 (5.1%) | 25 (23.8%) | 2 (5.3%) | 12 (34.2%) | ||
| Non-radiation target | ||||||
| CR | 1 (2.6%) | 2 (1.9%) | 1 (2.6%) | 1 (2.6%) | ||
| PR | 13 (33.3%) | 24 (22.9%) | 12 (31.6%) | 6 (15.8%) | ||
| SD | 20 (51.3%) | 60 (57.1%) | 20 (52.6%) | 23 (60.5%) | ||
| PD | 2 (5.1%) | 12 (11.4%) | 2 (5.3%) | 5 (13.2%) | ||
| NE | 3 (7.7%) | 7 (6.7%) | 3 (7.9%) | 3 (7.9%) | ||
| ORR | 24 (61.5%) | 29 (27.6%) | <0.001 | 23 (60.5%) | 8 (21.1%) | 0.003 |
| DCR | 37 (94.9%) | 80 (76.2%) | 0.011 | 36 (94.7%) | 26 (68.4%) | 0.003 |
Abbreviations: RT+S+L, radiotherapy combine with sintilimab plus lenvatinib; S+L, sintilimab plus lenvatinib; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, Not Evaluable; ORR, objective response rate; DCR, disease control rate.
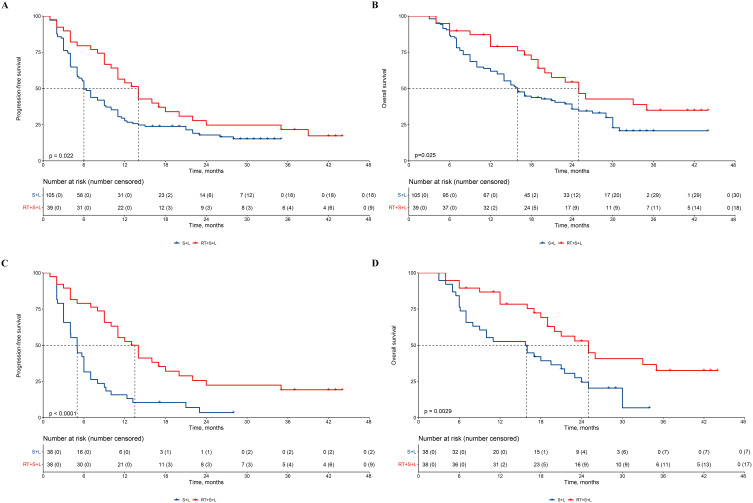
Before PSM, the median PFS for the RT+S+L group was 14 months (95% CI = 11–22) compared to 6 months (95% CI = 5–9) for the S+L group, with RT+S+L significantly prolonging PFS (Figure 2A, HR = 0.61, 95% CI = 0.40–0.94, P=0.022). Univariate Cox regression results indicated that, besides treatment grouping, the number of tumors and the number of lung metastatic lesions were also potential factors affecting PFS. After adjusting for these factors, RT+S+L still significantly extended PFS (Supplementary Table 1, HR = 0. 49, 95% CI = 0.31–0.78, P=0.002). The median OS for the RT+S+L group was 25 months (95% CI = 19-not reached) compared to 16 months (95% CI = 14–24) for the S+L group, with RT+S+L significantly prolonging OS (Figure 2B, HR = 0.58, 95% CI = 0.35–0.94, P=0.025). Univariate Cox regression results showed that, besides treatment grouping, the number of tumors and portal vein tumor thrombus were also potential factors affecting OS. After adjusting for these factors, RT+S+L still significantly extended OS (Supplementary Table 2, HR = 0.53, 95% CI = 0.32–0.87, P=0.013).
Figure 2.
Kaplan–Meier-estimated PFS and OS curves in HCC patients with PM receiving different therapies. (A) PFS before PSM; (B) OS before PSM; (C)PFS after PSM; (D)OS after PSM.
Abbreviations: PFS, progression-free survival; OS, overall survival; HCC, hepatocellular carcinoma; PM, pulmonary metastases; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; S+L, sintilimab combined with lenvatinib; RT+S+L, radiotherapy combined with sintilimab and lenvatinib.
After PSM, the median PFS for the RT+S+L group was 25 months (95% CI = 19–20) compared to 15.9 months (95% CI = 4–7) for the S+L group, with RT+S+L significantly prolonging PFS (Figure 2C, HR = 0.37, 95% CI = 0.22–0.62, P < 0.0001). The median OS for the RT+S+L group was 25 months (95% CI = 19-not reached) compared to 15.9 months (95% CI = 8–21.5) for the S+L group, with RT+S+L significantly prolonging OS (Figure 2D, HR = 0.44, 95% CI = 0.25–0.77, P=0.025).
Treatment on Progression
Throughout the cohort, 117 patients experienced progression during follow-up, including 30 from the RT+S+L group and 87 from the S+L group, with progression details summarized in Supplementary Table 3.
Supplementary Table 4 summarizes the treatment on progression. In the RT+S+L group, among the patients with progression, 10 received regorafenib, 7 received apatinib, 6 received atezolizumab plus bevacizumab, 5 received donafenib, and 2 received supportive care. In the S+L group, among the patients with progression, 31 received regorafenib, 16 received apatinib, 23 received atezolizumab plus bevacizumab, 12 received donafenib, 4 received supportive care, and 1 refused further treatment.
Safety
Table 3 details the TRAEs for both groups, with no treatment-related deaths (Grade 5 adverse events) reported. The incidence of Grade 3/4 adverse events was 43.6% in the RT+S+L group, with the most common being hypertension, decreased PLT, elevated TB, and proteinuria. In the S+L group, the incidence of Grade 3/4 adverse events was 40.0%, with the most common being hypertension, decreased PLT, and proteinuria.
Table 3.
Treatment Emergent Adverse Events
| Adverse Events | RT+S+L (n = 39) | S+L (n = 105) | ||
|---|---|---|---|---|
| Grade 1/2.n (%) | Grade 3/4. n (%) | Grade 1/2.n (%) | Grade 3/4. n (%) | |
| Any treatment-emergent adverse event | 21 (53.8%) | 17 (43.6%) | 51 (48.6%) | 42 (40.0%) |
| Elevated TB | 15 (38.5%) | 4 (10.3%) | 31 (29.5%) | 8 (7.6%) |
| Fatigue | 15 (38.5%) | 1 (2.6%) | 35 (33.3%) | 1 (1.0%) |
| Elevated AST | 14 (35.9%) | 3 (7.7%) | 29 (27.6%) | 7 (6.7%) |
| Proteinuria | 14 (35.9%) | 4 (10.3%) | 28 (26.7%) | 9 (8.6%) |
| Decreased PLT | 13 (33.3%) | 5 (12.8%) | 28 (26.7%) | 10 (9.5%) |
| Elevated ALT | 12 (30.8%) | 2 (5.1%) | 27 (25.7%) | 7 (6.7%) |
| Hypertension | 9 (23.1%) | 7 (17.9%) | 24 (22.9%) | 16 (15.2%) |
| Pyrexia | 9 (23.1%) | 1 (2.6%) | 20 (19.0%) | 4 (3.8%) |
| Hypoalbuminaemia | 8 (20.5%) | 1 (2.6%) | 17 (16.2%) | 2 (1.9%) |
| Bleeding (gingiva) | 7 (17.9%) | 0 (0.0%) | 16 (15.2%) | 0 (0.0%) |
| Diarrhea | 5 (12.8%) | 1 (2.6%) | 14 (13.3%) | 2 (1.9%) |
| Anaemia | 5 (12.8%) | 3 (7.7%) | 14 (13.3%) | 5 (4.8%) |
| Hypothyroidism | 4 (10.3%) | 0 (0.0%) | 13 (12.4%) | 0 (0.0%) |
| Gastrointestinal hemorrhage | 3 (7.7%) | 1 (2.6%) | 7 (6.7%) | 1 (1.0%) |
| Hand-foot skin reaction | 3 (7.7%) | 1 (2.6%) | 7 (6.7%) | 1 (1.0%) |
| Elevated creatinine | 3 (7.7%) | 1 (2.6%) | 7 (6.7%) | 0 (0.0%) |
| Rash | 3 (7.7%) | 0 (0.0%) | 6 (5.7%) | 0 (0.0%) |
Abbreviations: RT+S+L, radiotherapy combine with sintilimab plus lenvatinib; S+L, sintilimab plus lenvatinib; PLT, platelet; AST, aspartate transaminase; ALT, alanine aminotransferase; TB, total bilirubin.
Discussion
This study compares the efficacy and safety of sintilimab plus lenvatinib with or without radiotherapy in patients with advanced HCC with PM. The results indicate that RT+S+L significantly prolongs PFS and OS compared to S+L. Additionally, the combination therapy exhibited higher ORR and DCR. No unexpected TRAEs were observed with the addition of radiotherapy, confirming its acceptable safety profile. In summary, we consider RT+S+L as a potential treatment option for patients with advanced HCC with PM.
In current clinical practice, PD-1/PD-L1 inhibitors combined with anti-VEGF agents have been widely recognized as first-line treatments for advanced HCC.11,14,16,17 However, the ORR for this combination is below 30%, necessitating further research for improvement. Data on radiotherapy in HCC, especially in conjunction with systemic treatments, are encouraging. Mechanistically, RT enhances immune cell activation and infiltration into tumors. Conversely, radiotherapy induces the expression of immune checkpoint molecules and VEGF, which, when targeted by specific drugs, further amplify the effects of radiotherapy.26 A recent prospective single-arm clinical trial demonstrated an ORR of 58.7% for sintilimab plus bevacizumab combined with radiotherapy in patients with advanced HCC.27
IMRT is an advanced high-precision radiotherapy technique that uses computer-controlled linear accelerators to deliver precise radiation doses to malignant tumors or specific areas within the tumor.28 This technique allows the radiation dose to conform more precisely to the tumor’s three-dimensional shape, minimizing exposure to surrounding normal structures. IMRT is utilized in various malignancies, including prostate cancer, head and neck cancers, gastrointestinal cancers, gynecologic cancers, and brain tumors.29–31 In each case, IMRT has proven effective by improving tumor control and reducing treatment-related morbidity. A prospective observational study found that IMRT combined with atezolizumab and bevacizumab achieved an impressive ORR of 76.6% in patients with advanced HCC. These findings underscore the critical role of IMRT in enhancing tumor control.
However, the aforementioned studies were single-arm with historical controls, making it difficult to draw definitive conclusions. A limited retrospective propensity score-matched study indicated that combining PD-1 inhibitors with anti-angiogenic therapy and radiotherapy was more effective than PD-1 inhibitors with anti-angiogenic therapy, with no significant difference in treatment-related adverse events between the two groups.32 This aligns with our study’s results, where the RT+S+L group had an ORR of 61.5%, higher than the S+L group’s 27.6%. The S+L group’s ORR was similar to the IMBRAVE 150 and ORIENT-32 trials,15,17 further validating our findings’ robustness. Notably, the ORR rate of the non-radiotherapy target in the RT+S+L group was 34.2%, higher than the 18.4% in the S+L group, potentially due to the induction of an abscopal response. As previously explained, when tumors are exposed to radiation, cellular stress or damage within the tumor might lead to the release of neoantigens or tumor-specific antigens in the context of necrotic and apoptotic tumor cells. In this case, the addition of immunotherapy to RT enhances this effect. The RT+S+L group had a PFS of 14 months, comparable to Zhu et al’s report on sintilimab plus bevacizumab with radiotherapy,27 and longer than Wang et al’s results, possibly due to the latter’s inclusion of more advanced patients with main trunk tumor thrombus.23 Compared to the S+L group’s 6 months, the addition of radiotherapy significantly prolonged PFS, indicating that radiotherapy combined with systemic treatment benefits not only liver-confined HCC but also patients with PM, though caution is needed in interpreting results from this retrospective study. This suggests that future clinical trials in HCC treatment should consider incorporating radiotherapy as an effective option.
One reason for the limited adoption of radiotherapy in HCC is potential liver damage. In terms of safety, no unexpected toxicity was observed in the RT+S+L group. The incidence of Grade 3/4 adverse events was 43.6% in the RT+S+L group, with the most common being hypertension, decreased platelet count, elevated total bilirubin, and proteinuria. These adverse events were mostly manageable, with no treatment-related deaths reported.
Our study has limitations. First, being retrospective, it is inherently susceptible to selection and confounding biases. Additionally, although our regular follow-up interval was three months, actual patient follow-up times may have varied, potentially affecting PFS measurement accuracy. Furthermore, the relatively small sample size may reduce statistical power, necessitating future prospective studies to further verify the efficacy of combination therapy.
In conclusion, this study suggests that RT+S+L combination therapy may offer enhanced efficacy compared to S+L in managing HCC patients with lung metastases, with manageable safety concerns. These findings contribute to the growing body of evidence supporting comprehensive treatment strategies in advanced hepatocellular carcinoma, though further studies are warranted to confirm these results.
Funding Statement
This work is supported by the National Natural Science Foundation of China (No.82073293); Naval Medical University Youth Initiation Fund (No.2022QN097).
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author (Dr. Jie Shi, docshijie@aliyun.com) on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki and the Eastern Hepatobiliary Surgery Hospital (EHBH) Clinical Research Ethics Committee approved this retrospective study. The requirement for informed consent was waived due to the retrospective nature of the study, and no personal information was disclosed.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Authors declare no Conflict of Interests for this article.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanda M, Tateishi R, Yoshida H, et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008;28(9):1256–1263. doi: 10.1111/j.1478-3231.2008.01864.x [DOI] [PubMed] [Google Scholar]
- 4.Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13(3):414–420. doi: 10.3748/wjg.v13.i3.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. doi: 10.1148/radiology.216.3.r00se24698 [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Ren X, Zhang Q. Incidence, risk factors, and prognosis in patients with primary hepatocellular carcinoma and lung metastasis: a population-based study. Cancer Manag Res. 2019;11:2759–2768. doi: 10.2147/CMAR.S192896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong SW, Jang JY, Shim KY, et al. Practical effect of sorafenib monotherapy on advanced hepatocellular carcinoma and portal vein tumor thrombosis. Gut Liver. 2013;7(6):696–703. doi: 10.5009/gnl.2013.7.6.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi X, Guo X. Sorafenib for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis: a systematic review of comparative studies. Prz Gastroenterol. 2015;10(3):142–147. doi: 10.5114/pg.2015.52470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14(1):84. doi: 10.1186/1471-230X-14-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 11.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singal AG, Llovet JM, Yarchoan M, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78(6):1922–1965. doi: 10.1097/HEP.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 14.Galle PR, Finn RS, Qin S, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(7):991–1001. doi: 10.1016/S1470-2045(21)00151-0 [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi: 10.1016/j.jhep.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 16.Qin S, Chan SL, Gu S, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402(10408):1133–1146. doi: 10.1016/S0140-6736(23)00961-3 [DOI] [PubMed] [Google Scholar]
- 17.Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2-3 study. Lancet Oncol. 2021;22(7):977–990. doi: 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi: 10.1038/s41575-020-00395-0 [DOI] [PubMed] [Google Scholar]
- 19.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 20.Craig DJ, Nanavaty NS, Devanaboyina M, et al. The abscopal effect of radiation therapy. Future Oncol. 2021;17(13):1683–1694. doi: 10.2217/fon-2020-0994 [DOI] [PubMed] [Google Scholar]
- 21.Chen AC, Butler EB, Lo SS, Teh BS. Radiotherapy and the abscopal effect: insight from the past, present, and future. J Radiat Oncol. 2015;4(4):321–330. doi: 10.1007/s13566-015-0223-6 [DOI] [Google Scholar]
- 22.Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39(8):644–655. doi: 10.1016/j.it.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Xiang YJ, Yu HM, et al. Intensity-modulated radiotherapy combined with systemic atezolizumab and bevacizumab in treatment of hepatocellular carcinoma with extrahepatic portal vein tumor thrombus: a preliminary multicenter single-arm prospective study. Front Immunol. 2023;14:1107542. doi: 10.3389/fimmu.2023.1107542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao YT, Liu ZK, Wu QW, et al. Observation of different tumor motion magnitude within liver and estimate of internal motion margins in postoperative patients with hepatocellular carcinoma. Cancer Manag Res. 2017;9:839–848. doi: 10.2147/CMAR.S147185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 26.Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational immunotherapy for hepatocellular carcinoma: radiotherapy, immune checkpoint blockade and beyond. Front Immunol. 2020;11:568759. doi: 10.3389/fimmu.2020.568759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu M, Liu Z, Chen S, et al.Sintilimab plus bevacizumab combined with radiotherapy as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a multicenter, single-arm, phase 2 study. Hepatology. 2024;2024:10-–1097 [DOI] [PubMed] [Google Scholar]
- 28.Teh BS, Woo SY, Butler EB. Intensity modulated radiation therapy (IMRT): a new promising technology in radiation oncology. Oncologist. 1999;4(6):433–442. [PubMed] [Google Scholar]
- 29.Bortfeld T. IMRT: a review and preview. Phys Med Biol. 2006;51(13):R363–379. doi: 10.1088/0031-9155/51/13/R21 [DOI] [PubMed] [Google Scholar]
- 30.Hatano K, Tohyama N, Kodama T, Okabe N, Sakai M, Konoeda K. Current status of intensity-modulated radiation therapy for prostate cancer: history, clinical results and future directions. Int J Urol. 2019;26(8):775–784. doi: 10.1111/iju.14011 [DOI] [PubMed] [Google Scholar]
- 31.Thariat J, Hannoun-Levi JM, Sun Myint A, Vuong T, Gérard JP. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev Clin Oncol. 2013;10(1):52–60. doi: 10.1038/nrclinonc.2012.203 [DOI] [PubMed] [Google Scholar]
- 32.Su K, Guo L, Ma W, et al. PD-1 inhibitors plus anti-angiogenic therapy with or without intensity-modulated radiotherapy for advanced hepatocellular carcinoma: a propensity score matching study. Front Immunol. 2022;13:972503. doi: 10.3389/fimmu.2022.972503 [DOI] [PMC free article] [PubMed] [Google Scholar]