Abstract
BACKGROUND:
Previous studies have reported inconsistent results with positive, negative, and J-shaped associations between alcohol consumption and the hazard of aortic aneurysm and dissection (AAD). This study aimed to examine the connections between weekly alcohol consumption and the subsequent risk of AAD.
METHODS:
The UK Biobank study is a population-based cohort study. Weekly alcohol consumption was assessed using self-reported questionnaires and the congenital risk of alcohol consumption was also evaluated using genetic risk score (GRS). Cox proportional hazards models were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the associations between alcohol consumption and AAD. Several sensitivity analyses were performed to assess the robustness of the results.
RESULTS:
Among the 388,955 participants (mean age: 57.1 years, 47.4% male), 2,895 incident AAD cases were documented during a median follow-up of 12.5 years. Compared with never-drinkers, moderate drinkers (adjusted HR: 0.797, 95%CI: 0.646–0.984, P<0.05) and moderate-heavy drinkers (adjusted HR: 0.794, 95%CI: 0.635–0.992, P<0.05) were significantly associated with a decreased risk of incident AAD. Interaction-based subgroup analysis revealed that the protective effect of moderate drinking was reflected mainly in participants younger than 65 years and women.
CONCLUSION:
Our findings support a protective effect of moderate alcohol consumption on AAD, but are limited to participants younger than 65 years and women.
KEYWORDS: Alcohol consumption, Aortic aneurysm and dissection, Genetic risk score, Cohort study, UK Biobank
INTRODUCTION
Aortic aneurysm and dissection (AAD) is a severe macrovascular disorder caused by the inability of the vascular wall to withstand high intraluminal pressures, resulting in dilation of the aortic wall (aneurysm), a tear between the intima and media (dissection), in the worst cases, rupture of aneurysm and sudden death.[1,2] The prevalence of thoracic aortic aneurysms (TAAs) and abdominal aortic aneurysms (AAAs) was 0.16% and 1.0%–8.9%, respectively.[3-5] The global aortic aneurysm death rate per 100 000 population in 1990 was 2.49 (95% CI: 1.78–3.27) in 1990 and 2.78 (95%CI: 2.04 –3.62) in 2010.[6] Identifying risk factors for AAD is particularly important to the early prevention of the disease. In a previous study, advancing age, male sex, and smoking are considered as definite risk factors for AAD.[7] However, more modifiable risk factors remain to be explored in order to apply targeted interventions for the disease.
In the realm of modifiable lifestyle factors, alcohol consumption has been demonstrated to be associated with various clinical conditions. Numerous observational studies have suggested that mild to moderate alcohol consumption is linked to a reduced risk of cardiovascular disease (CVD).[8-11] However, previous studies reported inconsistent results with positive,[12] negative,[8,13] and J-shaped[14] associations between alcohol consumption and AAD. Most studies have primarily focused on exploring the connection between alcohol consumption and AAA, with limited attention given to TAA, dissection, or rupture. Additionally, previous studies have not adequately accounted for the competing risks of death. Therefore, conducting a thorough analysis of the impact of alcohol consumption on the development of AAD within a large represented population is essential.
In this study, our approach involved several stages. First, we carried out a series of epidemiological analyses using data from participants in the UK Biobank. Our goal was to examine both the linear and non-linear connections between weekly alcohol consumption and the subsequent risk of AAD. To address potential confounding effects, we took a step further and substituted the observed alcohol consumption with its genetic predisposition to validate our findings. By combining evidence from both observational and genetic perspectives within a large population cohort, our aim was to untangle the intricate relationship between alcohol consumption and the occurrence of AAD. This comprehensive approach allows us to gain deeper insights into how alcohol consumption may influence the risk of these vascular conditions.
METHODS
Study population
UK Biobank is a prospective study that recruited more than 500,000 community-dwelling participants, aged 37–73 years, across the United Kingdom between 2006 and 2010 (www.ukbiobank.ac.uk).[15] Participants were invited to attend one of 22 assessment centers across England, Scotland, and Wales, where a touchscreen questionnaire was completed, a nurse-led interview was performed, and physical measurements were taken. UK Biobank has approval from the North West Multicenter Research Ethics Committee (MREC), and all participants provided informed consent.
A flowchart of the inclusion and exclusion criteria of this study is presented in Figure 1. Of all 502,627 participants eligible at baseline, we first excluded participants without valid genetic data (n=15,221). Then, we excluded those with missing data on alcohol consumption (n=92,766), those with a history of AAD at baseline (n=374), and those with weekly alcohol consumption beyond the range of (Q1–3IQR[1.86 g/week], Q3+3IQR [624 g/week]) (n=5,311). Eventually, a total of 388,955 participants were enrolled in this cohort study. Participants were followed up until November 22, 2021, and were allowed to accrue follow-up time from baseline until the date of AAD diagnosis, death, or study ended, whichever occurred first.
Figure 1.
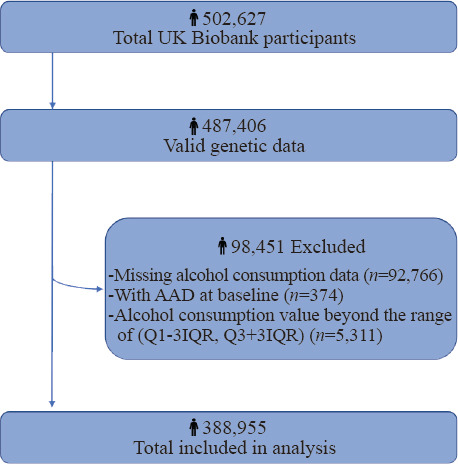
Flowchart of participant selection. AAD: aortic aneurysm and dissection; IQR: interquartile range.
Ascertainment of AAD
The follow-up of the cohort was conducted through the linkage of routinely available national health records such as hospital inpatient episodes and death registrations. The primary outcome of this study was the incidence of AAD recorded as the International Classification of Disease 10th Revision (ICD 10) code I71 in the hospital admission or death record up to October 26, 2022. We subtyped AAD as TAA (ICD 10 code: I712), AAA (ICD 10 code: I714), rupture or dissection (ICD 10 code: I710, I711, I713, I715, I718).
Assessment of alcohol consumption
At baseline, participants were asked about their drinking status and were asked to report their average weekly or monthly consumption of different types of alcoholic beverages. These measures were then converted into standard UK units and summed to obtain an average alcohol consumption in units per week, where one unit contains 8 g of ethanol[16] and is equivalent to half a pint of beer/lager/cider, half a glass of wine/champagne, one measure of spirits, or one glass of fortified wine.[17] Alcopops and other forms of alcohol count is 1.5 units.[18] We separated former drinkers from never-drinkers and used never drinkers as the reference group for analyses. Current drinkers were categorized into four groups: mild drinkers (≤100 g per week), moderate drinkers (>100 to 200 g per week), moderate-heavy drinkers (>200 to <300 g per week), and heavy drinkers (≥300 g per week). Field ID and categories of alcohol consumption are shown in Supplementary Table 1.
Assessment of the genetic risk score
The genome-wide association study (GWAS) conducted by GWAS and Sequencing Consortium of Alcohol and Nicotine use (GSCAN) identified 99 independent single nucleotide polymorphisms (SNPs) associated with alcohol consumption (log-transformed alcoholic drinks per week) at the genome-wide significance threshold (P<5×10-8) in 941,280 individuals of European ancestry.[19] A total of 96 SNPs were available for generating genetic risk scores (GRSs) in our population. We employed the following formula to calculate the GRS: weighted genetic risk score = (β1 × Single Nucleotide Variant 1(SNV1) + β2 ×SNV2 + … + βn× SNVn) × (n/sum of the β coefficients). The GRS was then analyzed in tertiles in our study, which were categorized as low (GRS < 121.573), intermediate (121.573 ≤ GRS ≤ 126.796), and high levels (GRS > 126.796) of genetic predisposition to alcohol consumption.
Assessment of covariates
We considered the following characteristics as the potential covariates: age (<65/≥65 years), sex (female/male), ethnicity (white/not white), Townsend deprivation index (continuous), education level (college or university degree/any other qualification/no qualification), smoking status (never/former or current), regular physical activity,[20] systolic blood pressure (SBP, continuous), diastolic blood pressure (DBP, continuous), body mass index (BMI, <25/≥25 to <30/≥30 kg/m2), cholesterol-lowering medication use, baseline diabetes and , baseline CVD (heart attack, angina, and stroke). Field ID and categories of the covariates are shown in Supplementary Table 1.
Statistical analysis
Baseline characteristics are summarized as proportions for categorical variables and mean ± standard deviation for continuous variables. Missing data on covariates were multiply imputed using the “mice” R package to minimize the potential for inferential bias. To investigate the associations of different drinking categories and genetic risk of alcohol consumption with incident AAD, we used Cox proportional-hazards models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Basic adjustments were made for age, sex, and smoking status in Model 1. In Model 2, further adjustments were made for ethnicity, education level, Townsend deprivation index, regular physical activity, BMI, SBP, DBP, cholesterol-lowering medication use, baseline diabetes and baseline CVD. The proportional hazards assumptions was verified using graphical inspection of log-minus-log plots, which revealed no substantial departures.[21] Once interactions between alcohol consumption or genetically predicted alcohol consumption and specific covariates using the variable cross-product term were observed, stratified analyses according to the status of covariates were performed.
To test for possible linear and non-linear relationships, a restricted cubic spline with 3 knots at fixed percentiles (10th, 50th, 90th) was performed using the “rcssci” R package. Alcohol consumption of 0 g per week was used as the reference.
Sensitivity analyses were conducted to assess the robustness of the results. First, we repeated major analyses after excluding never and former drinkers. Second, we restricted the sample to participants without CVDs at baseline. To minimize the potential influence of reverse causality, we then performed time-lag analyses by excluding cases of AAD occurring within the first 3 years of enrollment. Finally, Fine-Gray subdistribution hazard model was performed to control for potential bias from the competing risk of death.
All statistical analyses were conducted with R software version 4.2.3. One way analysis of variance (ANOVA) was used for multi-group comparisons. And two-sided values of P<0.05 were considered statistically significant for all analyses.
RESULTS
Baseline characteristics of the study population
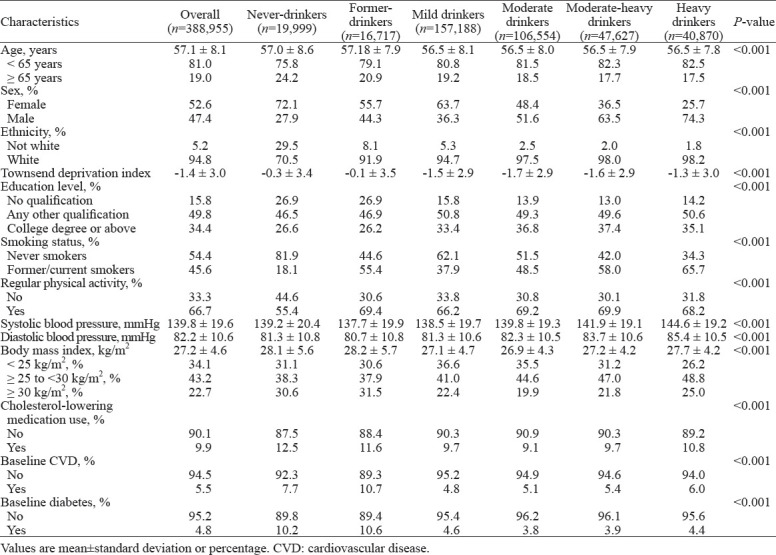
Among 388,955 participants who contributed 4,559,058 person-years during a median follow-up of 12.6 (IQR: 11.9–13.3) years, 2,895 participants developed AAD (mean age: 62.5 years, 79.9% male, Supplementary Table 2), including 719 TAAs, 1,580 AAAs, and 484 dissections or ruptures. Baseline characteristics stratified by alcohol consumption are shown in Table 1. For the drinking status, 90.6% of the participants were current drinkers. Compared with never-drinkers, current drinkers tended to be younger, more frequently male, of white ethnicity, former or current smokers, possess higher educational levels, engage in more physical activity, and have a lower prevalence of high BMI (≥30 kg/m²). Additionally, current drinkers had lower occurrences of cholesterol-lowering medication usage, CVDs, and diabetes at baseline. Furthermore, participants who consumed more alcohol tended to have higher SBP and DBP.
Table 1.
Baseline characteristics stratified by alcohol consumption
Associations between weekly alcohol consumption and incident AAD
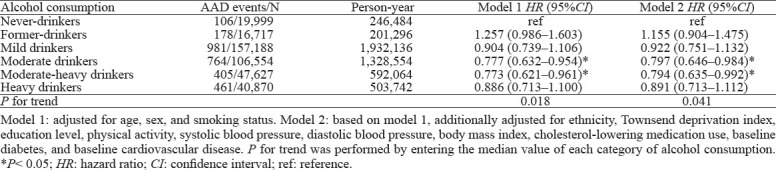
Associations of weekly alcohol consumption with AAD hazard are reported in Table 2. After adjusting for age, sex, and smoking status (Model 1), we observed a classical U-shaped curve association (P for trend = 0.018). Compared with never-drinkers, moderate or moderate-heavy drinkers had a significantly decreased risk, however, there was no statistically significant difference in the risk among mild drinkers, heavy drinkers, or former drinkers. After additional adjustment for ethnicity, Townsend deprivation index, educational level, physical activity, SBP, DBP, BMI, cholesterol-lowering medication use, baseline diabetes, and CVD (Model 2), the associations of alcohol consumption with AAD remained robust (P for trend=0.041). Among all the participants, both moderate drinkers (adjusted HR: 0.797, 95%CI: 0.646–0.984, P<0.05) and moderate-heavy drinkers (adjusted HR: 0.794, 95%CI: 0.635–0.992, P<0.05) had the lowest risk of incident AAD.
Table 2.
Associations of weekly alcohol consumption with hazard of aortic aneurysm and dissection (AAD)
When restricted to TAA, AAA, dissection or rupture, similar findings were observed. Although not statistically significant, the results showed U-shaped trends in TAA and AAA, with moderate and moderate-heavy drinkers having a decreased risk compared with never-drinkers. No significant dose-gradient associations were observed in dissection or rupture (Supplementary Table 3).
There was an interaction between alcohol consumption and age for subsequent AAD risk (P-interaction=0.035), with current drinkers from mild to heavy consumption having a significantly decreased risk among participants < 65 years old. Conversely, current drinkers appeared to have an increased risk among those ≥ 65 years old, although the associations were not statistically significant. In addition, interactions between alcohol consumption and sex were observed (P-interaction=0.03), with the beneficial effects of moderate alcohol consumption being more apparent in women (Figure 2).
Figure 2.
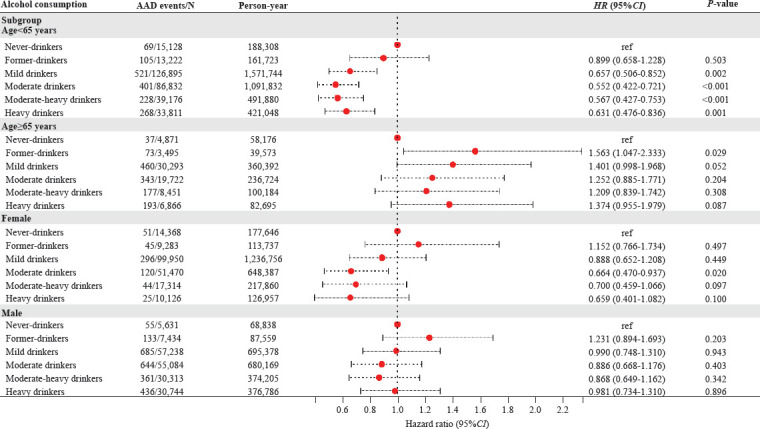
Associations of alcohol consumption with hazard of aortic aneurysm and dissection (AAD) stratified by age and sex. HR were adjusted for age, sex, smoking status, ethnicity, Townsend deprivation index, education level, physical activity, systolic blood pressure, diastolic blood pressure, body mass index, cholesterol-lowering medication use, baseline diabetes, and baseline cardiovascular disease. HR: hazard ratio; CI: confidence interval; ref: reference.
To investigate the dose-response relationship between weekly alcohol consumption and AAD hazard, alcohol consumed per week among participants was modeled with the use of restricted cubic splines (Figure 3A). The hazard decreased with increasing consumption up to ≈ 207 g per week, after which a slow increase in hazard was observed (P-nonlinear < 0.001). Compared with zero alcohol consumption, moderate alcohol consumption of 207 g per week was associated with a 30% (95%CI: 21.3%–37.7%) decrease in hazard. In sensitivity analyses, this trend did not change when never- and former-drinkers at zero consumption were excluded (Figure 3B, Supplementary Table 4). Importantly, we observed that the relationship varied across the spectrum of alcohol consumption, with a consistent inverse relationship for mild to heavy alcohol intake among participants younger than 65 years (Figure 3C). Conversely, among individuals aged 65 years and older, we found that only mild to moderate drinkers presented a slightly lower risk (Figure 3D). When stratified by sex, similar as model 2, a protective effect of moderate alcohol consumption was observed, but was more pronounced in females (Figure 3E, 3F).
Figure 3.
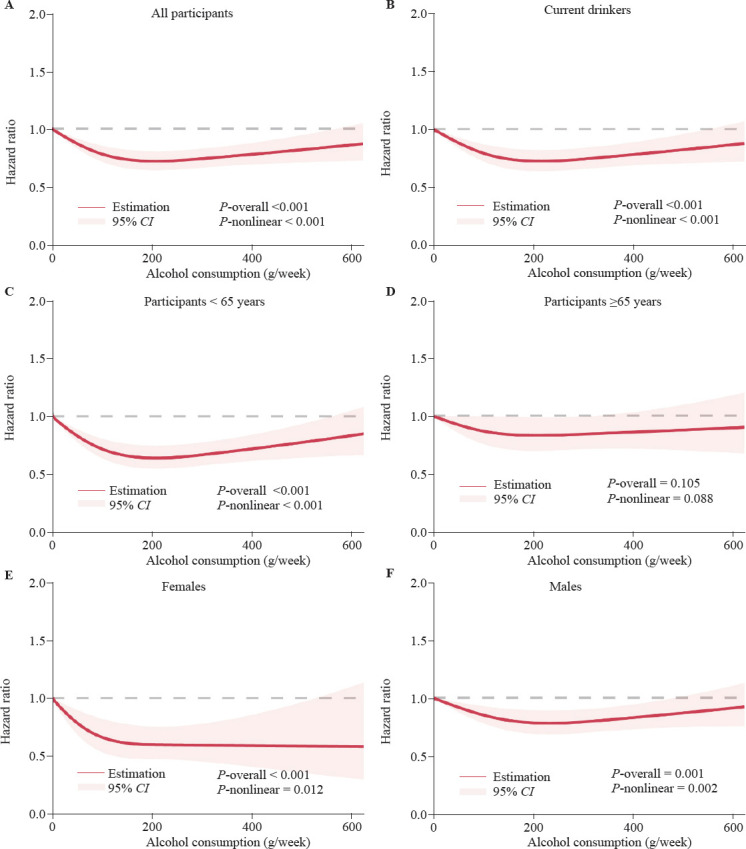
Dose-response hazard ratio of aortic aneurysm and dissection by alcohol consumption (g/week). Data were fitted with the use of a Cox proportional hazards model with restricted cubic splines with 3 knots (10th, 50th, 90th). Estimates were adjusted for the same variables as in model 2 in Table 2. Shaded areas represent 95% confidence intervals. A: for all participants; B: for current drinkers; C: for participants < 65 years; D: for participants ≥65 years; E: for females; F: for males.
Associations between GRS of alcohol consumption and incident AAD
To eliminate residual confounding, we constructed GRS of alcohol consumption derived from a large population-based GWAS analysis for each individual. Consistently, we observed that only participants with intermediate GRSs had a decreased risk (HR: 0.883, 95%CI: 0.807–0.966, P<0.01) of AAD (Table 3). Subgroup analysis (Figure 4) revealed that participants with intermediate GRSs were associated with decreased risk of AAD among those < 65 years old (P-interaction= 0.648) and women (P-interaction=0.005).
Table 3.
Associations of GSCAN of alcohol consumption with hazard of aortic aneurysm and dissection
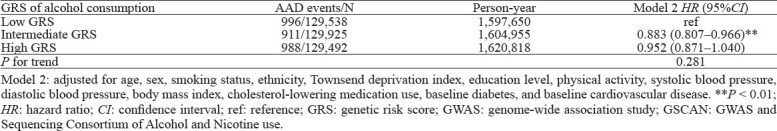
Figure 4.
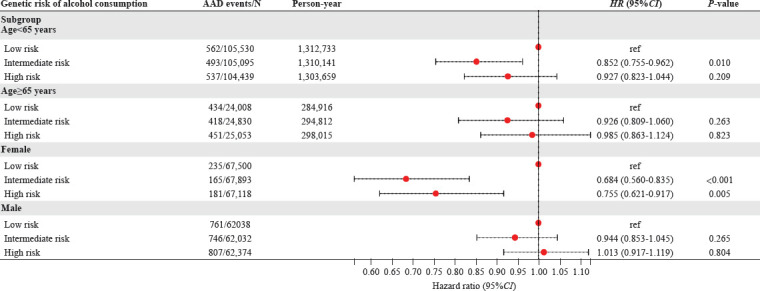
Associations of genetic risk score (GRS) with hazard of aortic aneurysm and dissection (AAD) stratified by age and sex. HR were adjusted for age, sex, smoking status, ethnicity, Townsend deprivation index, education level, physical activity, systolic blood pressure, diastolic blood pressure, body mass index, cholesterol-lowering medication use, baseline diabetes, and baseline cardiovascular disease. HR: hazard ratio; CI: confidence interval; ref: reference.
Sensitivity analyses of associations between alcohol consumption and incident AAD
The major results remained robust in several sensitivity analyses. The associations between alcohol consumption and the occurrence of AAD still displayed U-shaped trends but did not reach statistical significance in three sensitivity analyses, which included the analysis of CVD-free subcohort (Supplementary Table 5), the time-lag analysis conducted by excluding AAD cases that occurred within the first 3 years of follow-up (Supplementary Table 6), and the analysis using Fine-Gray models accounting for a competing risk of death (Supplementary Table 7).
DISCUSSION
In this large population-based cohort from the UK Biobank, we identified a U-shaped association between alcohol consumption and AAD risk, with the lowest risk intervals at 100–200 or 200–300 g/week. Response dose analysis revealed that alcohol consumption had a significant non-linear relationship with the risk of AAD. These results suggest that moderate alcohol consumption may be a protective factor for AAD, which is consistent with previous results from a Swedish cohort.[13] In addition, to further eliminate the influence of confounding factors, we established an individual GRS of alcohol consumption and found that participants with intermediate GRSs were associated with lower AAD risk. The major results were robust in all sensitivity analyses.
A systematic analysis of alcohol consumption for the global burden of disease in 2020[22] pointed out that participants ≥ 40 years without underlying health problems may benefit from mild to moderate alcohol consumption (1–2 standard drinks per day, equal 70–140 g/week). Notably, we observed a significant interaction between alcohol consumption and age, with distinct effects of alcohol consumption on AAD risk at different ages. Among those < 65 years of age, there was a protective effect against AAD, regardless of the amount of alcohol consumed, compared with never drinkers. However, among those who were ≥ 65 years, alcohol consumption tended to increase the risk of AAD, although the P-value was not statistically significant. The results indicated that when conducting such studies, the age at which alcohol intake started and accumulated years of alcohol intake should be considered to account for the impact of long-term exposure. Furthermore, we also identified a notable discovery concerning the variation in alcohol intake between genders concerning AAD incidence. Our observations indicated that the favorable impact of moderate alcohol consumption was primarily observed in females, whereas there was no statistically significant difference observed among males. Currently, there is limited research on how gender differences affect the relationship between alcohol consumption and the risk of AAD. Further investigation is necessary to understand the underlying mechanisms. This could involve female estrogen secretion. In addition, the different compositions of drinking types between males and females may be another reason. However, female drinkers tend to prefer wine, which may have cardiovascular protective effects, whereas male drinkers prefer spirits or beer.[23-25]
The cardiovascular protective role of alcohol consumption has been a controversial issue. Mild to moderate alcohol consumption has been associated with a reduced risk of CVD in previous studies.[8-11] Alcohol consumption may play a protective role by reducing systemic inflammation, reducing oxidative stress and increasing high-density lipoprotein (HDL)content,[11,26,27] which are also important mechanisms for the development of AAD.[28-31] Moreover, a recent study revealed that light to moderate alcohol consumption associates with reduced major adverse cardiovascular events through lowering activity of a stress-related brain network, especially among individuals with prior anxiety.[32] Moderate alcohol consumption may share the similar mechanism for reducing AAD events since anxiety is associated with CVD risk.[33,34]
However, alcohol use is a leading risk factor for global disease and severely impair health, especially in individuals with cancer and alcohol-related diseases.[25,35,36] The Dietary Guidelines for Americans state that women should drink up to 1 standard drink (14 g) of alcohol per day and men should drink no more than 2 standard drinks per day (28 g).[24] Furthermore, multiple population-based cohort studies suggest lower alcohol consumption to reduce the estimated future years of life loss, and the level of consumption that minimizes health loss is zero.[25,37,38] Although our findings support the association of moderate alcohol consumption with a lower risk of AAD, especially among participants <65 years and women, we do not encourage alcohol consumption to prevent AAD development.
There are several limitations to our study. First, the drinking data collected at baseline relied on self-reporting, which introduces potential bias. Additionally, specific types of drinking or drinking patterns were not considered. For example, consuming alcohol multiple times in a week versus engaging in single episodes of heavy drinking. This oversight is important because different drinking habits may have varying effects on CVD events. It has been suggested that the observed effects of specific drinks on health outcomes may be attributed more to residual confounding factors related to socioeconomic status than to a true causal relationship.[39] However, we did take into account variables like educational attainment and the Townsend’s deprivation index to address this concern. Furthermore, our study focused solely on examining the association between baseline alcohol consumption and AAD events. We did not consider potential changes in alcohol consumption during the follow-up period, which may impact the risk of AAD.[12] These limitations should be acknowledged when interpreting the results of our study.
CONCLUSION
The present study suggests that moderate alcohol consumption was associated with a lower hazard of AAD. However, the protective effect was limited to participants younger than 65 years and women.
ACKNOWLEDGEMENTS
We thank the participants of the UK Biobank. This research has been conducted using the UK Biobank Resource under Application Number 88365.
Footnotes
Funding: This study was supported by grants from the Ministry of Science and Technology of the People’s Republic of China, (No. 2020AAA0109605 to XL); grants from the National Natural Science Foundation of China (No. 82272246 and 82072225 to XL); Science and Technology Program of Guangzhou, China (No. 202206010044 to XL); High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (No. DFJHBF202104 to XL).
Ethical approval: UK Biobank has ethics approval from the North West Multi-Centre Research Ethics Committee (11/NW/0382). Appropriate informed consent was obtained from participants and ethical approval was covered by the UK Biobank.
Conflict of interest: The authors declare that they have no competing interests.
Author contribution: YWL, GXZ, and DCW contributed equally to this work and are co-first authors. XL, YH and HLF conceptualized and designed the study. YWL performed the data processing and analysis, and GXZ, DCW, WYZ and JRZ drafted the manuscript. XRH, ML, DCW, CM, FES, YLZ, and JXM contributed to the revision of the manuscript and approved the final draft. XL, YH and HLF were involved in study supervision. All authors contributed to the intellectual content and critical revisions to the drafts of the paper and approved the final version.
All the supplementary files in this paper are available at http://wjem.com.cn.
REFERENCES
- 1.Gao J, Cao H, Hu G, Wu Y, Xu Y, Cui H, et al. The mechanism and therapy of aortic aneurysms. Signal Transduct Target Ther. 2023;8(1):55. doi: 10.1038/s41392-023-01325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossone E, Eagle KA. Epidemiology and management of aortic disease:aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18(5):331–48. doi: 10.1038/s41569-020-00472-6. [DOI] [PubMed] [Google Scholar]
- 3.Gouveia E, Melo R, Silva Duarte G, Lopes A, Alves M, Caldeira D, Fernandes E, Fernandes R, et al. Incidence and prevalence of thoracic aortic aneurysms:a systematic review and meta-analysis of population-based studies. Semin Thorac Cardiovasc Surg. 2022;34(1):1–16. doi: 10.1053/j.semtcvs.2021.02.029. [DOI] [PubMed] [Google Scholar]
- 4.McFarlane MJ. The epidemiologic necropsy for abdominal aortic aneurysm. JAMA. 1991;265(16):2085–8. [PubMed] [Google Scholar]
- 5.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577–89. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 6.Sampson UK, Norman PE, Fowkes FG, Aboyans V, Yanna Song, Harrell FE, Jr, et al. Global and regional burden of aortic dissection and aneurysms:mortality trends in 21 world regions, 1990 to 2010. Glob Heart. 2014;9(1):171–80.e10. doi: 10.1016/j.gheart.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Svensjö S, Björck M, Gürtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124(10):1118–23. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 8.Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases:population based cohort study using linked health records. BMJ Clin Res Ed. 2017;356:j909. doi: 10.1136/bmj.j909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding CY, O'Neill D, Bell S, Stamatakis E, Britton A. Association of alcohol consumption with morbidity and mortality in patients with cardiovascular disease:original data and meta-analysis of 48, 423 men and women. BMC Med. 2021;19(1):167. doi: 10.1186/s12916-021-02040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes:a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millwood IY, Walters RG, Mei XW, Guo Y, Yang L, Bian Z, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology:a prospective study of 500 000 men and women in China. Lancet. 2019;393(10183):1831–42. doi: 10.1016/S0140-6736(18)31772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong DR, Willett WC, Rimm EB. Smoking, hypertension, alcohol consumption, and risk of abdominal aortic aneurysm in men. Am J Epidemiol. 2007;165(7):838–45. doi: 10.1093/aje/kwk063. [DOI] [PubMed] [Google Scholar]
- 13.Stackelberg O, Björck M, Larsson SC, Orsini N, Wolk A. Alcohol consumption, specific alcoholic beverages, and abdominal aortic aneurysm. Circulation. 2014;130(8):646–52. doi: 10.1161/CIRCULATIONAHA.113.008279. [DOI] [PubMed] [Google Scholar]
- 14.Spencer SM, Trower AJ, Jia X, Scott DJA, Greenwood DC. Meta-analysis of the association between alcohol consumption and abdominal aortic aneurysm. Br J Surg. 2017;104(13):1756–64. doi: 10.1002/bjs.10674. [DOI] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank:an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mindell J, Biddulph JP, Hirani V, Stamatakis E, Craig R, Nunn S, et al. Cohort profile:the health survey for England. Int J Epidemiol. 2012;41(6):1585–93. doi: 10.1093/ije/dyr199. [DOI] [PubMed] [Google Scholar]
- 17.Gray L, Batty GD, Craig P, Stewart C, Whyte B, Finlayson A, et al. Cohort profile:the Scottish health surveys cohort:linkage of study participants to routinely collected records for mortality, hospital discharge, cancer and offspring birth characteristics in three nationwide studies. Int J Epidemiol. 2010;39(2):345–50. doi: 10.1093/ije/dyp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knott CS, Coombs N, Stamatakis E, Biddulph JP. All cause mortality and the case for age specific alcohol consumption guidelines:pooled analyses of up to 10 population based cohorts. BMJ. 2015;350:h384. doi: 10.1136/bmj.h384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu MZ, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237–44. doi: 10.1038/s41588-018-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd-Jones DM, Hong YL, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction:the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 21.Kuitunen I, Ponkilainen VT, Uimonen MM, Eskelinen A, Reito A. Testing the proportional hazards assumption in cox regression and dealing with possible non-proportionality in total joint arthroplasty research:methodological perspectives and review. BMC Musculoskelet Disord. 2021;22(1):489. doi: 10.1186/s12891-021-04379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GBD 2020 Alcohol Collaborators. Population-level risks of alcohol consumption by amount, geography, age, sex, and year:a systematic analysis for the Global Burden of Disease Study 2020. Lancet. 2022;400(10347):185–235. doi: 10.1016/S0140-6736(22)00847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castaldo L, Narváez A, Izzo L, Graziani G, Gaspari A, Minno GD, et al. Red wine consumption and cardiovascular health. Molecules. 2019;24(19):3626. doi: 10.3390/molecules24193626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg IJ, Mosca L, Piano MR, Fisher EA. AHA Science Advisory. Wine and your heart:a science advisory for healthcare professionals from the Nutrition Committee, Council on Epidemiology and Prevention, and Council on Cardiovascular Nursing of the American Heart Association. Stroke. 2001;32(2):591–4. doi: 10.1161/01.str.32.2.591. [DOI] [PubMed] [Google Scholar]
- 25.GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016:a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–35. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease:systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease:meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319(7224):1523–8. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao WY, Hong YM, He HW, Han Q, Mao MM, Hu B, et al. MicroRNA-199a-5p aggravates angiotensin II-induced vascular smooth muscle cell senescence by targeting Sirtuin-1 in abdominal aortic aneurysm. J Cell Mol Med. 2021;25(13):6056–69. doi: 10.1111/jcmm.16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Björck M, Wanhainen A. Pathophysiology of AAA:heredity vs environment. Prog Cardiovasc Dis. 2013;56(1):2–6. doi: 10.1016/j.pcad.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Shah PK. Inflammation, metalloproteinases, and increased proteolysis. Circulation. 1997;96(7):2115–7. doi: 10.1161/01.cir.96.7.2115. [DOI] [PubMed] [Google Scholar]
- 31.Adorni MP, Palumbo M, Marchi C, Zimetti F, Ossoli A, Turri M, et al. HDL metabolism and functions impacting on cell cholesterol homeostasis are specifically altered in patients with abdominal aortic aneurysm. Front Immunol. 2022;13:935241. doi: 10.3389/fimmu.2022.935241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mezue K, Osborne MT, Abohashem S, Zureigat H, Gharios C, Grewal SS, et al. Reduced stress-related neural network activity mediates the effect of alcohol on cardiovascular risk. J Am Coll Cardiol. 2023;81(24):2315–25. doi: 10.1016/j.jacc.2023.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batelaan NM, Seldenrijk A, Bot M, van Balkom AJLM, Penninx BWJH. Anxiety and new onset of cardiovascular disease:critical review and meta-analysis. Br J Psychiatry. 2016;208(3):223–31. doi: 10.1192/bjp.bp.114.156554. [DOI] [PubMed] [Google Scholar]
- 34.Nakada S, Ho FK, Celis-Morales C, Jackson CA, Pell JP. Individual and joint associations of anxiety disorder and depression with cardiovascular disease:a UK Biobank prospective cohort study. Eur Psychiatry. 2023;66(1):e54. doi: 10.1192/j.eurpsy.2023.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Im PK, Wright N, Yang L, Chan KH, Chen YP, Guo Y, et al. Alcohol consumption and risks of more than 200 diseases in Chinese men. Nat Med. 2023;29(6):1476–86. doi: 10.1038/s41591-023-02383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smyth A, Teo KK, Rangarajan S, O'Donnell M, Zhang X, PURE Investigators et al. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality:a prospective cohort study. Lancet. 2015;386(10007):1945–54. doi: 10.1016/S0140-6736(15)00235-4. [DOI] [PubMed] [Google Scholar]
- 37.Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group et al. Risk thresholds for alcohol consumption:combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391(10129):1513–23. doi: 10.1016/S0140-6736(18)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S, Yon H, Ban CY, Shin H, Eum S, Lee SW, et al. National trends in alcohol and substance use among adolescents from 2005 to 2021:a Korean serial cross-sectional study of one million adolescents. World J Pediatr. 2023;19(11):1071–81. doi: 10.1007/s12519-023-00715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Castelnuovo A, Bonaccio M, Costanzo S, McElduff P, Linneberg A, Salomaa V, et al. Drinking alcohol in moderation is associated with lower rate of all-cause mortality in individuals with higher rather than lower educational level:findings from the MORGAM project. Eur J Epidemiol. 2023;38(8):869–81. doi: 10.1007/s10654-023-01022-3. [DOI] [PubMed] [Google Scholar]


