Abstract
BACKGROUND:
Trauma-induced coagulopathy (TIC) due to serious injuries significantly leads to increased mortality and morbidity among elderly patients. However, the risk factors of TIC are not well elucidated. This study aimed to explore the risk factors of TIC in elderly patients who have major trauma.
METHODS:
In this retrospective study, the risk factors for TIC in elderly trauma patients at a single trauma center were investigated between January 2015 and September 2020. The demographic information including gender, age, trauma parts, injury severity, use of blood products, use of vasopressors, need of emergency surgery, duration of mechanical ventilation, length of stay in the intensive care unit (ICU) and hospital, and clinical outcomes were extracted from electric medical records. Multivariate logistic regression analysis was performed to differentiate risk factors, and the performance of the model was evaluated using receiver operating characteristics (ROC) curves.
RESULTS:
Among the 371 elderly trauma patients, 248 (66.8%) were male, with the age of 72.5 ± 6.8 years, median injury severity score (ISS) of 24 (IQR: 17–29), and Glasgow coma score (GCS) of 14 (IQR: 7–15). Of these patients, 129 (34.8%) were diagnosed with TIC, whereas 242 (65.2%) were diagnosed with non-TIC. The severity scores such as ISS (25 [20–34] vs. 21 [16–29], P<0.001) and shock index (SI), (0.90±0.66 vs. 0.58 ± 0.18, P<0.001) was significantly higher in the TIC group than in the non-TIC group. Serum calcium levels (1.97±0.19 mmol/L vs. 2.15±0.16 mmol/L, P<0.001), fibrinogen levels (1.7±0.8 g/L vs. 2.8±0.9 g/L, P<0.001), and base excess (BE, -4.9±4.6 mmol/L vs. -1.2 ± 3.1 mmol/L, P<0.001) were significantly lower in the TIC group than in the non-TIC group. Multivariate logistic regression analysis revealed that ISS>16 (OR: 3.404, 95%CI: 1.471–7.880; P=0.004), SI>1 (OR: 5.641, 95%CI: 1.700–18.719; P=0.005), low BE (OR: 0.868, 95%CI: 0.760–0.991; P=0.037), hypocalcemia (OR: 0.060, 95%CI: 0.009–0.392; P=0.003), and hypofibrinogenemia (OR: 0.266, 95%CI: 0.168–0.419; P<0.001) were independent risk factors for TIC in elderly trauma patients. The AUC of the prediction model included all these risk factors was 0.887 (95%CI: 0.851–0.923) with a sensitivity and specificity of 83.6% and 82.6%, respectively.
CONCLUSION:
Higher ISS (more than 16), higher SI (more than 1), acidosis, hypocalcemia, and hypofibrinogenemia emerged as independent risk factors for TIC in elderly trauma patients.
KEYWORDS: Trauma, Elderly patients, Trauma-induced coagulopathy, Hypocalcemia
INTRODUCTION
Trauma represents a significant global health concern, resulting in 4–6 million fatalities annually.[1-3] Uncontrolled bleeding remains the primary cause of potentially avoidable death among individuals with severe injuries, with one-third of such patients experiencing coagulopathy upon hospital admission.[4-6]Throughout the course of severe trauma, trauma-induced coagulopathy (TIC) manifests across a spectrum from reduced blood clotting to excessive clotting, and is influenced by factors such as extensive tissue damage and severe hemorrhagic shock.[1,7-9] Patients with TIC are at greater risk of multiple organ failure, elevated rates of morbidity and mortality compared with trauma patients without coagulation disorders.[1,7,10,11] Additionally, advanced age is a significant predictor of worse outcomes following severe trauma.[12,13] Elderly individuals are more susceptible to coagulation abnormalities due to age-related physiological changes, severe injuries, comorbidities, and less effective therapeutic interventions post-trauma.[12,14] Consequently, elderly trauma patients, particularly those with TIC, experience poorer outcomes. [9,13,15,16] While conventional coagulation tests (CCTs) and viscoelastography tests (VETs) are commonly used for TIC assessment, there remains a need for rapid and convenient indicators to facilitate effective resuscitation strategies. [3,17] Despite extensive research on coagulopathy resulting from severe hemorrhagic shock and tissue injury, there is still no unified definition of TIC. Risk factors such as serum calcium play a crucial role in traumatic coagulation abnormalities;[18,19] however, little information exists regarding the relationships between these factors and TIC in elderly trauma patients.
Therefore, we conducted a retrospective study at a single center to investigate the risk factors associated with TIC in elderly individuals with major trauma. Our findings aim to increase the accuracy of diagnosis and the timely initiation of treatment for elderly patients with TIC.
METHODS
Study design
A retrospective, observational, single-center study was conducted on elderly trauma patients admitted to the Second Affiliated Hospital of Zhejiang University School of Medicine between January 2015 and September 2020.
The inclusion criteria were as follows: patients older than 65 years, with major trauma requiring intensive care unit (ICU) hospitalization. The exclusion criteria were as follows: (1) use of anticoagulants or anti-platelet medication before the traumatic incident; (2) coagulation disorders resulting from pre-existing medical conditions; (3) cardiac arrest and death within 3 h of admission; (4) malignant tumor; (5) incomplete medical records; (6) transfusion of blood products prior to admission.
Demographic information, including patient gender, age, trauma parts, injury severity, therapeutic interventions, and clinical outcomes, such as the use of blood products, vasopressors, acute surgeries, duration of mechanical ventilation, length of stay in the ICU and hospital, and in-hospital mortality, were extracted.
Data were retrieved from electronic medical records of our hospital. The Institutional Review Board approved the study and waived the requirement for informed consent from patients ([2020] IRB-976) and their legal representatives (ClinicalTrials.gov, NCT05530161).
Definition of TIC
In this study, TIC was defined as a history of trauma and an international normalized ratio (INR) > 1.2. For additional analysis, the TIC group was categorized into severe TIC (INR > 1.5) and non-severe TIC.[4, 20-23]
Statistical analysis
Statistical analysis was conducted via SPSS, version 26.0 (IBM, USA). The normality of continuous data was assessed using the Kolmogorov-Smirnov test. Normally distributed continuous data are presented as mean ± standard deviation, while non-normally distributed data are presented as medians and interquartile ranges. Nominal variables are reported as numbers and proportions (%). Categorical variables were analyzed using the Chi-square test or Fisher’s exact test, and continuous variables were analyzed using the Mann-Whitney U-test or Student’s t-test, as appropriate. Multivariate logistic regression analysis was performed to identify risk factors, and receiver operating characteristics (ROC) curves were utilized to assess the predictive ability of the model. A two-sided P-value < 0.05 was considered statistically significant for all tests.
RESULTS
General information
A total of 371 elderly trauma patients were included in the study, comprising 248 (66.8%) male individuals, with a mean age of 72.5 ± 6.8 years. The median injury severity score (ISS) was 24, and the median Glasgow coma score (GCS) score was 14. All patients were admitted to the ICU, with 59.8% requiring mechanical ventilation. Within the cohort, 129 (34.8%) patients were in the TIC group, while 242 (65.2%) were in the non-TIC group. The TIC group was subsequently categorized into severe and non-severe TIC groups based on a cutoff value of INR at 1.5. There were 38 (29.5%) patients in the severe TIC group, and 91 (70.5%) were in the non-severe TIC group (Figure 1).
Figure 1.
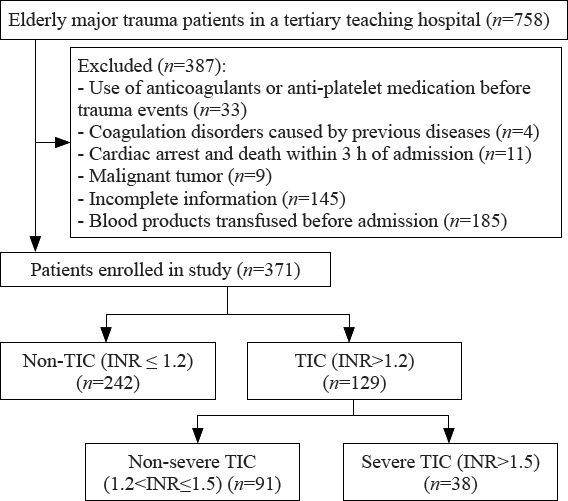
Flowchart of the study. TIC: trauma-induced coagulopathy; INR: international normalized ratio.
Characteristics of the elderly trauma patients
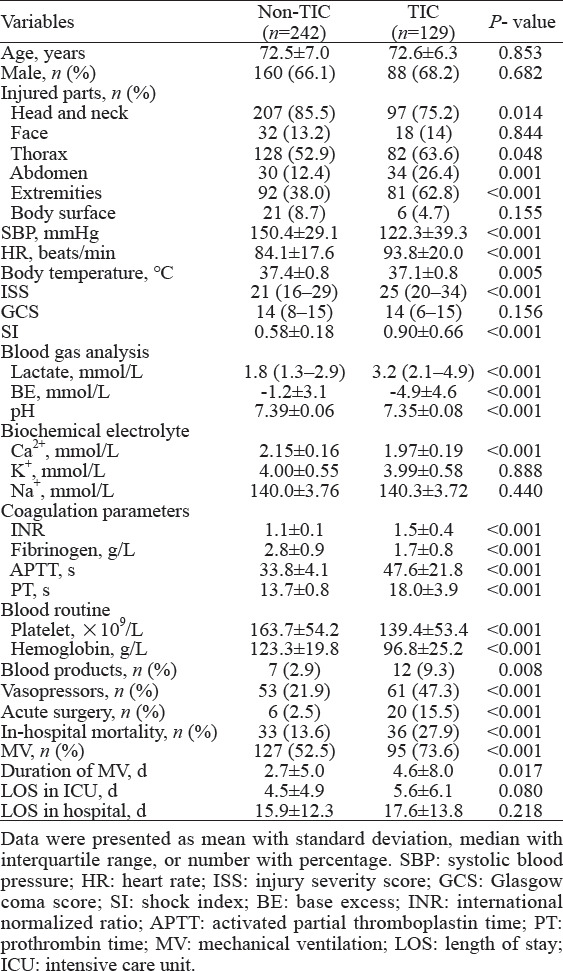
In our cohort, the heart rate (HR), ISS, and shock index (SI) in the TIC group were significantly higher than those in the non-TIC group (P<0.001). Additionally, the TIC group presented significantly higher serum lactate levels, activated partial thromboplastin time (APTT), and PT than the non-TIC group (P<0.001). Conversely, BE, pH, serum calcium, fibrinogen, platelets, hemoglobin, initial systolic blood pressure (SBP), and body temperature were significantly lower in the TIC group (P<0.001). Furthermore, the TIC group had a significantly higher ratio of blood product transfusions, vasopressor use, acute surgeries, in-hospital death, and duration of mechanical ventilation (MV) compared to the non-TIC group (P<0.001) (Table 1).
Table 1.
Comparison between elderly trauma patients with and without trauma-induced coagulopathy (TIC)
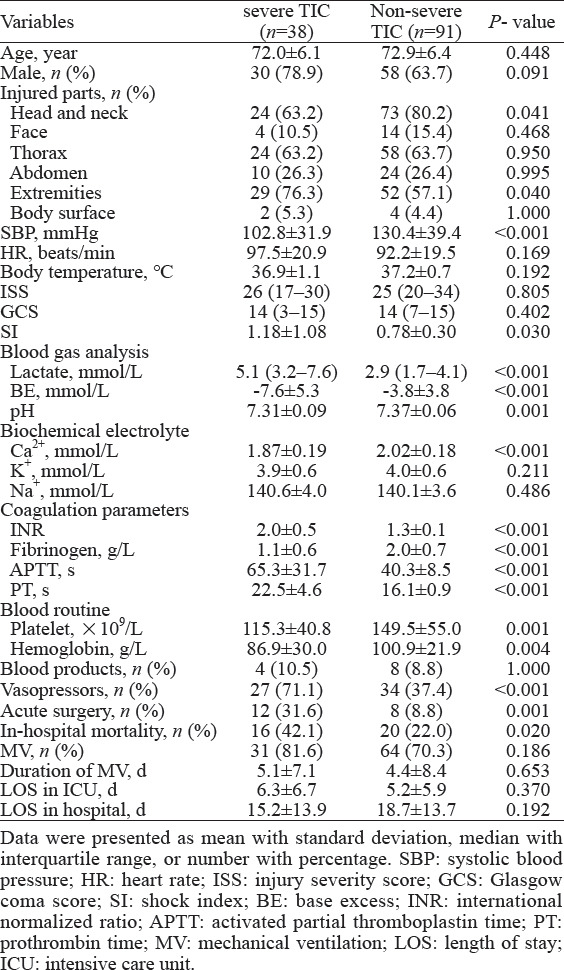
For further analysis, the TIC group was categorized into the severe and non-severe TIC groups. Compared with patients with non-severe TIC, patients with severe TIC were more critical, especially in terms of hemodynamics and acidosis, required more vasopressors and surgical support, and have a higher in-hospital mortality (22.0% vs. 42.1%, P=0.020, Table 2).
Table 2.
Comparison of elderly trauma patients with and without severe trauma-induced coagulopathy (TIC)
Risk factors for the elderly TIC patients
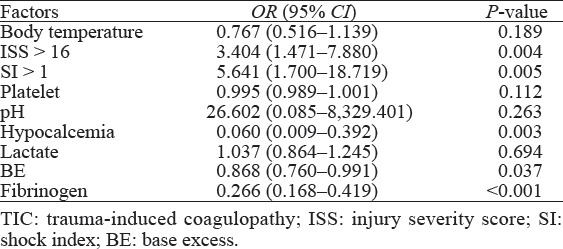
Multivariate logistic regression analysis revealed that ISS>16 (OR: 3.404, 95%CI: 1.471–7.880; P=0.004), SI>1 (OR: 5.641, 95%CI: 1.700–18.719; P=0.005), low BE (OR: 0.868, 95%CI: 0.760–0.991; P=0.037), hypocalcemia (OR: 0.060, 95%CI: 0.009–0.392; P=0.003), and hypofibrinogenemia (OR: 0.266, 95%CI: 0.168–0.419; P<0.001) were independent risk factors for TIC in elderly trauma patients (Table 3).
Table 3.
Risk factors for TIC in elderly trauma patients
The AUC of the prediction model was 0.887 (95%CI: 0.851–0.923). The Youden’s index was maximized with a sensitivity and specificity of 83.6% and 82.6%, respectively (Figure 2).
Figure 2.
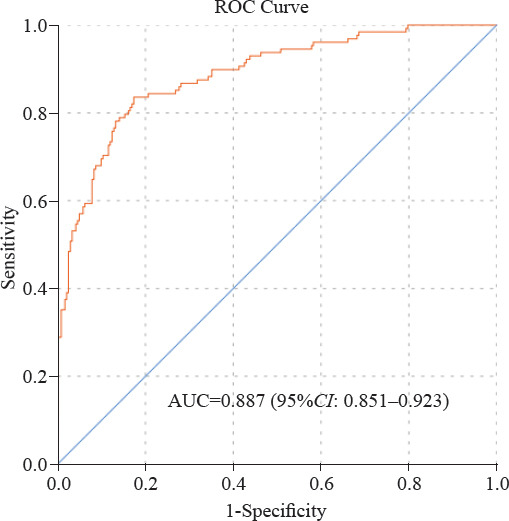
ROC curve of regression model for predicting trauma-induced coagulopathy. The regression model included ISS, SI, serum calcium, base excess and fibrinogen as risk factors.
DISCUSSION
As the global population ages, the annual incidence of trauma among older patients is rising markedly.[12] Unlike adult patients, elderly trauma patients experience distinct clinical outcomes due to physiological decline, pre-existing conditions, and less effective therapeutic interventions following severe trauma.[13-15,24,25] Severe coagulation disorders resulting from significant hemorrhage are common among severely injured elderly patients. However, few studies have investigated the risk factors of poor outcomes in elderly patients with TIC.[3,4,6] Our findings revealed that elderly TIC patients exhibited significantly higher values of ISS, SI, lactate, APTT, and PT compared to non-TIC patients. Additionally, the levels of BE, pH, fibrinogen, platelets, and hemoglobin were notably lower in the TIC group, indicating traumatic acidosis and substantial blood component loss. Consequently, the administration of blood transfusions, vasopressors, and emergency surgeries is necessary for the effective management of elderly patients with major trauma. Compared with non-TIC patients, elderly TIC patients also required prolonged mechanical ventilation. Therefore, there is a critical need for prompt evaluation and treatment of elderly patients with TIC.
In this study, we confirmed that an ISS > 16 (indicative of major trauma), SI > 1 (indicating shock), low BE (indicating metabolic acidosis), hypocalcemia, and hypofibrinogenemia were independent risk factors of TIC in elderly trauma patients. Previous studies have consistently identified severe tissue injury, shock, and metabolic acidosis as risk factors for TIC.[1,4] Comparing risk factors for TICs between elderly and young adult populations is difficult due to the absence of age stratification in previous studies. Both the ISS and the SI reflect the severity of injury in trauma patients and indicate the occurrence of coagulation dysfunction and metabolic acidosis. Patients with severe injuries and ongoing bleeding or profound hemorrhagic shock usually have a poor prognosis.[1] Prompt management of hemorrhage and appropriate resuscitation are crucial measures for rectifying coagulation disorders.
TIC is characterized by hypofibrinogenemia and often elevated fibrinolytic activity.[4,26,27] Effective cessation of bleeding primarily depends on the formation of stable clots, which relies on adequate fibrinogen and platelet levels rather than the speed of coagulation initiation.[27,28] Khan et al[29] found that neither lactate levels nor any viscoelastic parameter exhibited improvement in patients who received mixed transfusion packages with delayed or no fibrinogen supplementation. In addition to early administration of tranexamic acid, early fibrinogen administration is crucial, ideally guided by a fibrinogen concentration < 1.5 g/L or viscoelastic evidence of functional fibrinogen deficiency.[4]
Previous studies has thoroughly investigated variations in serum calcium levels as an indicator for evaluating coagulation function in trauma patients.[30-33] However, the relationship between hypocalcemia and the “lethal triad symptoms” has been poorly elucidated in prior research. Early prevention of hypocalcemia may offer benefits for elderly patients with TIC. Therefore, it is essential to closely monitor and maintain normal serum calcium levels in elderly TIC patients.[18, 33-35] However, the identification and treatment algorithms for hypocalcemia in TIC patients remain limited.[31, 32]
Several limitations should be acknowledged in this study. First, it was a single-center retrospective study, potentially introducing selection bias. Multicenter prospective studies are required for further evaluate risk factors for TIC in elderly patients. Second, the lack of data in this study, such as ionized calcium levels, serum protein levels, calcium supplementation, and trauma mechanisms, hindered further analysis of the correlation between calcium levels and TIC in elderly patients.
CONCLUSION
In conclusion, higher ISS (>16), higher SI (>1), acidosis, hypocalcemia, and hypofibrinogenemia were identified as independent risk factors for TIC in elderly trauma patients. Prompt and effective correction of shock, acidosis, hypocalcemia, and hypofibrinogenemia in elderly TIC patients could significantly improve clinical outcomes.
Footnotes
Funding: This work was supported by National Natural Science Foundation of China (81571916), Key Research and Development (R&D) Program of Zhejiang Province (2024C03186) and Major Project of National-Zhejiang Provincial Administration of Traditional Chinese Medicine (GZY-ZJ-KJ-24030).
Ethical approval: This study was approved by the Second Affiliated Hospital of Zhejiang University School of Medicine. The institutional review board waived the requirement for informed consent ([2020] IRB-976) from patients and their relatives, given the observational nature of the study.
Conflicts of interest: The authors have no conflicts of interest related to this study.
Author contributions: YBK, QY, and HBD contributed equally to this paper and are co-first authors. YBK and YAX conceived the study and carried out the study design. YBK, JSS, QY, YFH, YCJ, SXX, HBD, FR, BJC, YPF and LBJ participated in the data acquisition. YBK, LBJ, GRW and YAX interpreted the data. YBK edited the manuscript. All the authors read and approved the final version of the manuscript.
REFERENCES
- 1.Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primers. 2021;7(1):30. doi: 10.1038/s41572-021-00264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savioli G, Ceresa IF, Caneva L, Gerosa S, Ricevuti G. Trauma-induced coagulopathy:overview of an emerging medical problem from pathophysiology to outcomes. Medicines (Basel) 2021;8(4):16. doi: 10.3390/medicines8040016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cap A, Hunt B. Acute traumatic coagulopathy. Curr Opin Crit Care. 2014;20(6):638–45. doi: 10.1097/MCC.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 4.Rossaint R, Afshari A, Bouillon B, Cerny V, Cimpoesu D, Curry N, et al. The European guideline on management of major bleeding and coagulopathy following trauma:sixth edition. Crit Care. 2023;27(1):80. doi: 10.1186/s13054-023-04327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davenport RA, Guerreiro M, Frith D, Rourke C, Platton S, Cohen M, et al. Activated protein C drives the hyperfibrinolysis of acute traumatic coagulopathy. Anesthesiology. 2017;126(1):115–27. doi: 10.1097/ALN.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liras IN, Caplan HW, Stensballe J, Wade CE, Cox CS, Cotton BA. Prevalence and impact of admission acute traumatic coagulopathy on treatment intensity, resource use, and mortality:an evaluation of 956 severely injured children and adolescents. J Am Coll Surg. 2017;224(4):625–32. doi: 10.1016/j.jamcollsurg.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 7.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 8.Floccard B, Rugeri L, Faure A, Saint Denis M, Boyle EM, Peguet O, et al. Early coagulopathy in trauma patients:an on-scene and hospital admission study. Injury. 2012;43(1):26–32. doi: 10.1016/j.injury.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Song JC, Yang LK, Zhao W, Zhu F, Wang G, Chen YP, et al. Chinese expert consensus on diagnosis and treatment of trauma-induced hypercoagulopathy. Mil Med Res. 2021;8(1):25. doi: 10.1186/s40779-021-00317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma:hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–7. doi: 10.1097/TA.0b013e318169cd3c. discussion1217. [DOI] [PubMed] [Google Scholar]
- 11.Cap A, Hunt BJ. The pathogenesis of traumatic coagulopathy. Anaesthesia. 2015;70(Suppl 1):96–101. doi: 10.1111/anae.12914. e32-4. [DOI] [PubMed] [Google Scholar]
- 12.Dinh MM, Roncal S, Byrne CM, Petchell J. Growing trend in older patients with severe injuries:mortality and mechanisms of injury between 1991 and 2010 at an inner city major trauma centre. ANZ J Surg. 2013;83(1-2):65–9. doi: 10.1111/j.1445-2197.2012.06180.x. [DOI] [PubMed] [Google Scholar]
- 13.Hukkelhoven CW, Steyerberg EW, Rampen AJJ, Farace E, Habbema JD, Marshall LF, et al. Patient age and outcome following severe traumatic brain injury:an analysis of 5600 patients. J Neurosurg. 2003;99(4):666–73. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- 14.Dinh MM, McNamara K, Bein KJ, Roncal S, Barnes EH, McBride K, et al. Effect of the elderly and increasing injury severity on acute hospital resource utilization in a cohort of inner city trauma patients. ANZ J Surg. 2013;83(1-2):60–4. doi: 10.1111/j.1445-2197.2012.06177.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakae R, Fujiki Y, Takayama Y, Kanaya T, Igarashi Y, Suzuki G, et al. Age-related differences in the time course of coagulation and fibrinolytic parameters in patients with traumatic brain injury. Int J Mol Sci. 2020;21(16):5613. doi: 10.3390/ijms21165613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folkerson LE, Sloan D, Cotton BA, Holcomb JB, Tomasek JS, Wade CE. Predicting progressive hemorrhagic injury from isolated traumatic brain injury and coagulation. Surgery. 2015;158(3):655–61. doi: 10.1016/j.surg.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann J, Walsh M, Grisoli A, Thomas AV, Shariff F, McCauley R, et al. Diagnosis and treatment of trauma-induced coagulopathy by viscoelastography. Semin Thromb Hemost. 2020;46(2):134–46. doi: 10.1055/s-0040-1702171. [DOI] [PubMed] [Google Scholar]
- 18.Giancarelli A, Birrer KL, Alban RF, Hobbs BP, Liu-DeRyke X. Hypocalcemia in trauma patients receiving massive transfusion. J Surg Res. 2016;202(1):182–7. doi: 10.1016/j.jss.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 19.De Robertis E, Kozek-Langenecker SA, Tufano R, Romano GM, Piazza O, Zito Marinosci G. Coagulopathy induced by acidosis, hypothermia and hypocalcaemia in severe bleeding. Minerva Anestesiol. 2015;81(1):65–75. [PubMed] [Google Scholar]
- 20.Hagemo JS, Christiaans SC, Stanworth SJ, Brohi K, Johansson PI, Goslings JC, et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry:an international prospective validation study. Crit Care. 2015;19(1):97. doi: 10.1186/s13054-015-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med. 2016;24(1):114. doi: 10.1186/s13049-016-0308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baksaas-Aasen K, van Dieren S, Balvers K, Juffermans NP, Næss PA, Rourke C, et al. Data-driven development of ROTEM and TEG algorithms for the management of trauma hemorrhage:a prospective observational multicenter study. Ann Surg. 2019;270(6):1178–85. doi: 10.1097/SLA.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 23.Jost D, Lemoine S, Lemoine F, Derkenne C, Beaume S, Lanoë V, et al. Prehospital lyophilized plasma transfusion for trauma-induced coagulopathy in patients at risk for hemorrhagic shock:a randomized clinical trial. JAMA Netw Open. 2022;5(7):e2223619. doi: 10.1001/jamanetworkopen.2022.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clement ND, Tennant C, Muwanga C. Polytrauma in the elderly:predictors of the cause and time of death. Scand J Trauma Resusc Emerg Med. 2010;18:26. doi: 10.1186/1757-7241-18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma:a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65(4):951–60. doi: 10.1097/TA.0b013e318187e15b. [DOI] [PubMed] [Google Scholar]
- 26.Perkins ZB, Yet B, Marsden M, Glasgow S, Marsh W, Davenport R, et al. Early identification of trauma-induced coagulopathy:development and validation of a multivariable risk prediction model. Ann Surg. 2021;274(6):e1119–e1128. doi: 10.1097/SLA.0000000000003771. [DOI] [PubMed] [Google Scholar]
- 27.Cole E, Weaver A, Gall L, West A, Nevin D, Tallach R, et al. A decade of damage control resuscitation:new transfusion practice, new survivors, new directions. Ann Surg. 2021;273(6):1215–20. doi: 10.1097/SLA.0000000000003657. [DOI] [PubMed] [Google Scholar]
- 28.James A, Abback PS, Pasquier P, Ausset S, Duranteau J, Hoffmann C, et al. The conundrum of the definition of haemorrhagic shock:a pragmatic exploration based on a scoping review, experts'survey and a cohort analysis. Eur J Trauma Emerg Surg. 2022;48(6):4639–49. doi: 10.1007/s00068-022-01998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan S, Brohi K, Chana M, Raza I, Stanworth S, Gaarder C, et al. Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J Trauma Acute Care Surg. 2014;76(3):561–7. doi: 10.1097/TA.0000000000000146. discussion567-8. [DOI] [PubMed] [Google Scholar]
- 30.Moore HB, Tessmer MT, Moore EE, Sperry JL, Cohen MJ, Chapman MP, et al. Forgot calcium?Admission ionized-calcium in two civilian randomized controlled trials of prehospital plasma for traumatic hemorrhagic shock. J Trauma Acute Care Surg. 2020;88(5):588–96. doi: 10.1097/TA.0000000000002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byerly S, Inaba K, Biswas S, Wang E, Wong MD, Shulman I, et al. Transfusion-related hypocalcemia after trauma. World J Surg. 2020;44(11):3743–50. doi: 10.1007/s00268-020-05712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKay EJ, Stubna MD, Holena DN, Reilly PM, Seamon MJ, Smith BP, et al. Abnormal calcium levels during trauma resuscitation are associated with increased mortality, increased blood product use, and greater hospital resource consumption:a pilot investigation. Anesth Analg. 2017;125(3):895–901. doi: 10.1213/ANE.0000000000002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wray JP, Bridwell RE, Schauer SG, Shackelford SA, Bebarta VS, Wright FL, et al. The diamond of death:Hypocalcemia in trauma and resuscitation. Am J Emerg Med. 2021;41:104–9. doi: 10.1016/j.ajem.2020.12.065. [DOI] [PubMed] [Google Scholar]
- 34.Magnotti LJ, Bradburn EH, Webb DL, Berry SD, Fischer PE, Zarzaur BL, et al. Admission ionized calcium levels predict the need for multiple transfusions:a prospective study of 591 critically ill trauma patients. J Trauma. 2011;70(2):391–5. doi: 10.1097/TA.0b013e31820b5d98. discussion395-7. [DOI] [PubMed] [Google Scholar]
- 35.Vasudeva M, Mathew JK, Fitzgerald MC, Cheung Z, Mitra B. Hypocalcaemia and traumatic coagulopathy:an observational analysis. Vox Sang. 2020;115(2):189–95. doi: 10.1111/vox.12875. [DOI] [PubMed] [Google Scholar]


