Abstract
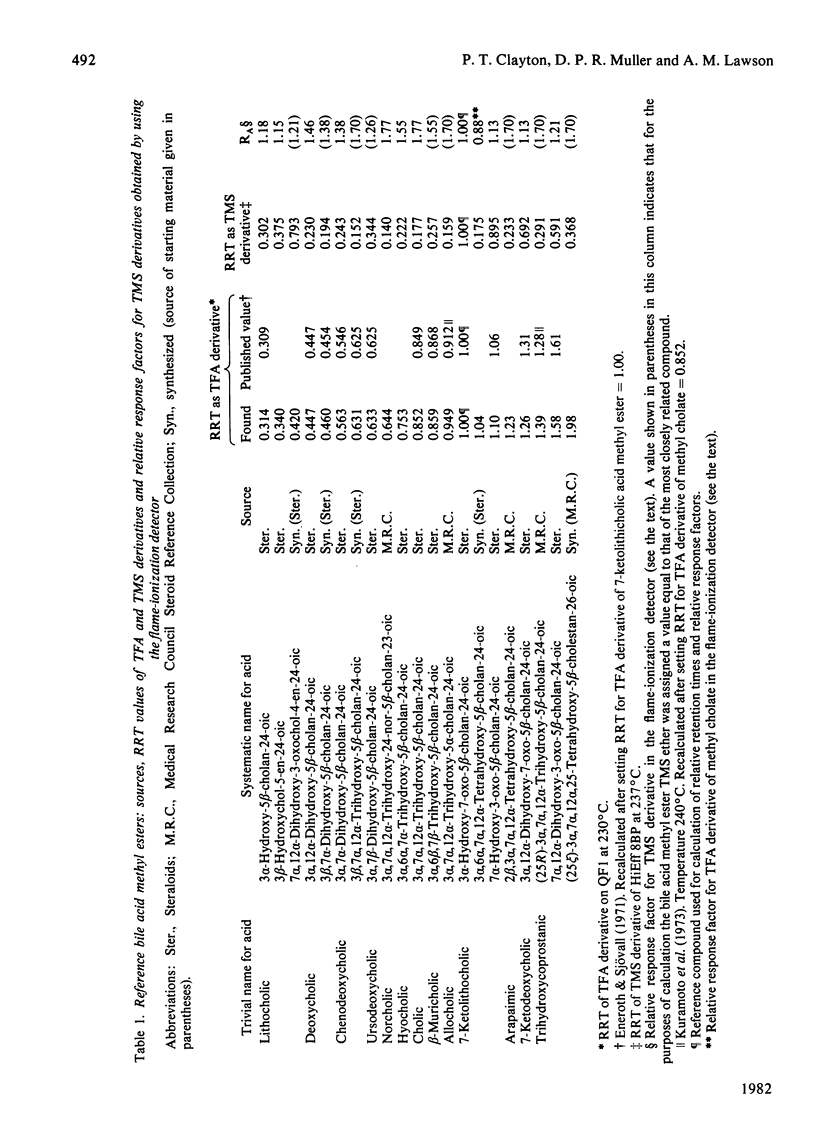
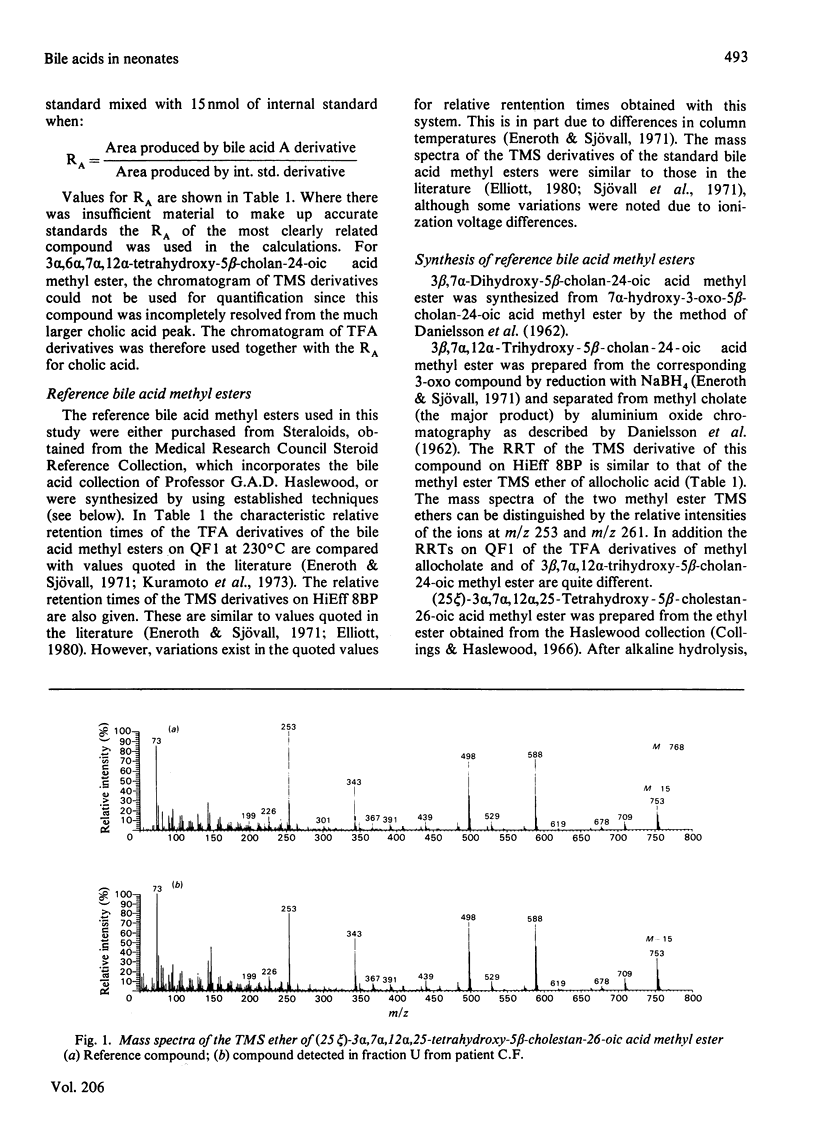
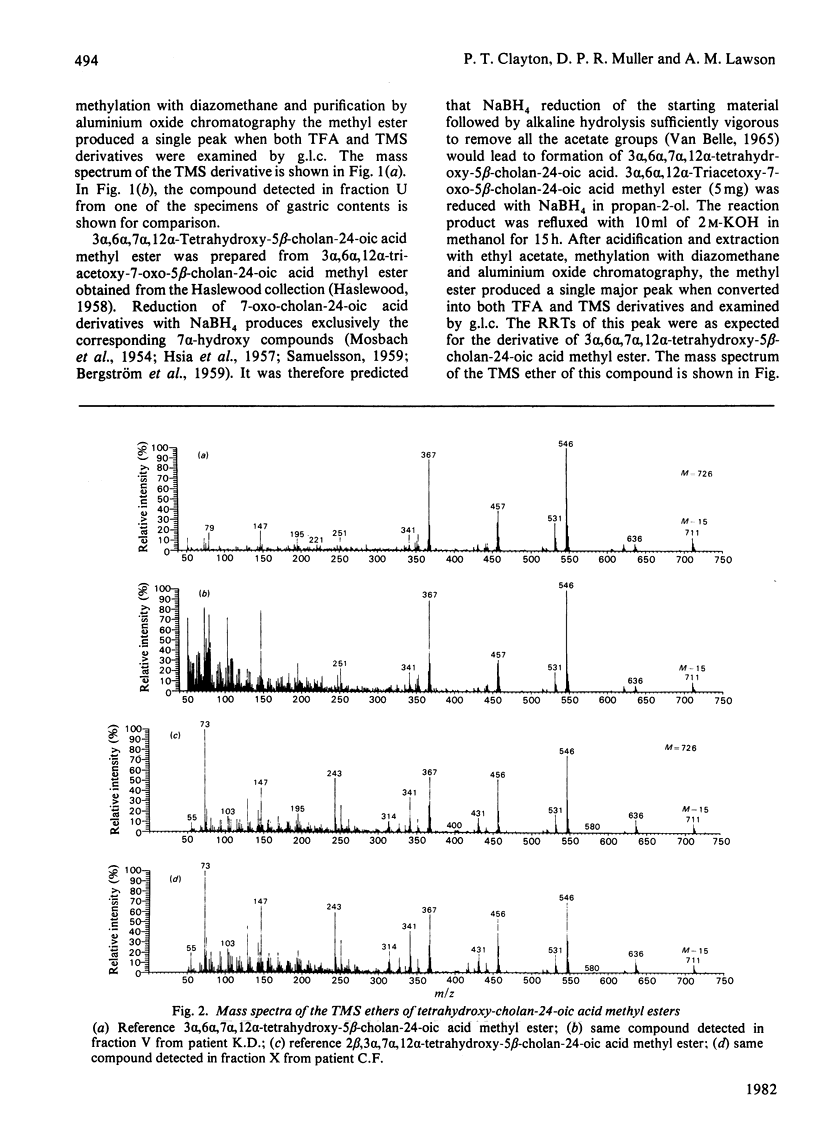
The study was designed to identify 'atypical' bile acids in gastric contents from three neonates with high intestinal obstruction on the basis that this was likely to represent a rich source of primary bile acids. Cholic acid was the major component, and related 'atypical' bile acids included its C-3 and C-7 oxidation products, its 3 beta-epimer and 2 beta- and 6 alpha-hydroxylation products. Allocholic acid was the only 5 alpha-cholanic acid derivative identified. 7 alpha, 12 alpha-Dihydroxy-3-oxochol-4-en-24-oic acid was found in all three specimens and might be an intermediate in a biosynthetic pathway from cholesterol to cholic acid in which side-chain oxidation precedes at least some of the nuclear changes. Side-chain-hydroxylated derivatives of trihydroxycoprostanic acid were also detected and these may represent intermediates in biosynthetic pathways from cholesterol to cholic acid via 5 beta-cholestan-3 alpha, 7 alpha, 12 alpha-triol. The most abundant bile acid of this type was (25 epsilon)-3 alpha, 7 alpha, 12 alpha, 25-tetrahydroxy-5 beta-cholestan-26-oic acid, which suggested that C-25 hydroxylation may be an important step in the shortening of the C8 side chain of the cholestane triol to the C5 side chain of cholic acid in the neonatal period. Bile acids lacking a substituent at C-12 included chenodeoxycholic acid, its C-3 and C-7 oxidation products, its 3 beta-epimer and its 6 alpha-hydroxylation product (hyocholic acid).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almé B., Bremmelgaard A., Sjövall J., Thomassen P. Analysis of metabolic profiles of bile acids in urine using a lipophilic anion exchanger and computerized gas-liquid chromatorgaphy-mass spectrometry. J Lipid Res. 1977 May;18(3):339–362. [PubMed] [Google Scholar]
- Aries V., Hill M. J. Degradation of steroids by intestinal bacteria. II. Enzymes catalysing the oxidoreduction of the 3 alpha-, 7 alpha- and 12 alpha-hydroxyl groups in cholic acid, and the dehydroxylation of the 7-hydroxyl group. Biochim Biophys Acta. 1970 May 5;202(3):535–543. doi: 10.1016/0005-2760(70)90124-4. [DOI] [PubMed] [Google Scholar]
- BERGSTROM S., LINDSTEDT S., SAMUELSSON B. Bile acids and steroids. LXXXII. On the mechanism of deoxycholic acid formation in the rabbit. J Biol Chem. 1959 Aug;234(8):2022–2025. [PubMed] [Google Scholar]
- Back P., Walter K. Developmental pattern of bile acid metabolism as revealed by bile acid analysis of meconium. Gastroenterology. 1980 Apr;78(4):671–676. [PubMed] [Google Scholar]
- Carey J. B., Jr, Wilson I. D., Zaki F. G., Hanson R. F. The metabolism of bile acids with special reference to liver injury. Medicine (Baltimore) 1966 Nov;45(6):461–470. doi: 10.1097/00005792-196645060-00009. [DOI] [PubMed] [Google Scholar]
- Clayton P. T., Muller D. P. A simplified gas-liquid chromatographic methods for the estimation of non-sulphated plasma bile acids. Clin Chim Acta. 1980 Aug 19;105(3):401–405. doi: 10.1016/0009-8981(80)90122-9. [DOI] [PubMed] [Google Scholar]
- DANIELSSON H., ENEROTH P., HELLSTROM K., SJOVALL J. Synthesis of some 3beta-hydroxylated bile acids and the isolation of 3beta, 12alpha-dihydroxy-5beta-cholanic acid from feces. J Biol Chem. 1962 Dec;237:3657–3659. [PubMed] [Google Scholar]
- Eneroth P., Gordon B., Ryhage R., Sjövall J. Identification of mono- and dihydroxy bile acids in human feces by gas-liquid chromatography and mass spectrometry. J Lipid Res. 1966 Jul;7(4):511–523. [PubMed] [Google Scholar]
- Fedorowski T., Salen G., Tint G. S., Mosbach E. Transformation of chenodeoxycholic acid and ursodeoxycholic acid by human intestinal bacteria. Gastroenterology. 1979 Nov;77(5):1068–1073. [PubMed] [Google Scholar]
- HASLEWOOD G. A. Comparative studies of bile salts. 11. 3 alpha:6 alpha:12 alpha-trihydroxycholanic acid and related substances. Biochem J. 1958 Dec;70(4):551–558. doi: 10.1042/bj0700551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIA S. L., MATSCHINER J. T., MAHOWALD T. A., ELLIOTT W. H., DOISY E. A., Jr, THAYER S. A., DOISY E. A. Bile acids. V. Chemical studies on new bile acids from the rat and the hog. J Biol Chem. 1957 Apr;225(2):811–823. [PubMed] [Google Scholar]
- Hanson R. F., Isenberg J. N., Williams G. C., Hachey D., Szczepanik P., Klein P. D., Sharp H. L. The metabolism of 3alpha, 7alpha, 12alpha-trihydorxy-5beta-cholestan-26-oic acid in two siblings with cholestasis due to intrahepatic bile duct anomalies. An apparent inborn error of cholic acid synthesis. J Clin Invest. 1975 Sep;56(3):577–587. doi: 10.1172/JCI108127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslewood G. A., Tökés L. Comparative studies of bile salts. A new type of bile salt from Arapaima gigas (Cuvier) (family Osteoglossidae). Biochem J. 1972 Mar;126(5):1161–1170. doi: 10.1042/bj1261161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepner G. W., Hofmann A. F., Malagelada J. R., Szczepanik P. A., Klein P. D. Increased bacterial degradation of bile acids in cholecystectomized patients. Gastroenterology. 1974 Apr;66(4):556–564. [PubMed] [Google Scholar]
- Hofmann A. F. The enterohepatic circulation of bile acids in man. Clin Gastroenterol. 1977 Jan;6(1):3–24. [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Rane A., Gustafasson J. A. Properties of hydroxylase systems in the human fetal liver active on free and sulfoconjugated steroids. Biochemistry. 1975 Jan 28;14(2):429–437. doi: 10.1021/bi00673a033. [DOI] [PubMed] [Google Scholar]
- Kallner A. A method of synthesis of allocholanoic acids. Bile acids and steroids 182. Acta Chem Scand. 1967;21(2):322–328. doi: 10.3891/acta.chem.scand.21-0322. [DOI] [PubMed] [Google Scholar]
- Kuramoto T., Kikuchi H., Sanemori H., Hoshita T. Bile salts of anura. Chem Pharm Bull (Tokyo) 1973 May;21(5):952–959. doi: 10.1248/cpb.21.952. [DOI] [PubMed] [Google Scholar]
- Lewis R., Gorbach S. Modification of bile acids by intestinal bacteria. Arch Intern Med. 1972 Oct;130(4):545–549. [PubMed] [Google Scholar]
- Mata L. J., Mejicanos M. L., Jiménez F. Studies on the indigenous gastrointestinal flora of Guatemalan children. Am J Clin Nutr. 1972 Dec;25(12):1380–1390. doi: 10.1093/ajcn/25.12.1380. [DOI] [PubMed] [Google Scholar]
- Norman A., Strandvik B. Formation and metabolism of bile acids in extrahepatic biliary atresia. J Lab Clin Med. 1971 Aug;78(2):181–193. [PubMed] [Google Scholar]
- Parmentier G. G., Janssen G. A., Eggermont E. A., Eyssen H. J. C27 bile acids in infants with coprostanic acidemia and occurrence of a 3 alpha,7 alpha,12 alpha-tridhydroxy-5 beta-C29 dicarboxylic bile acid as a major component in their serum. Eur J Biochem. 1979 Dec;102(1):173–183. doi: 10.1111/j.1432-1033.1979.tb06278.x. [DOI] [PubMed] [Google Scholar]
- Ross P. E., Pennington C. R., Bouchier I. A. Gas-liquid chromatographic assay of serum bile acids. Anal Biochem. 1977 Jun;80(2):458–465. doi: 10.1016/0003-2697(77)90668-6. [DOI] [PubMed] [Google Scholar]
- Schoenfield L. J., Sjövall J., Sjövall K. Bile acid composition of gallstones from man. J Lab Clin Med. 1966 Aug;68(2):186–194. [PubMed] [Google Scholar]
- Swell L., Gustafsson J., Danielsson H., Schwartz C. C., Vlahcevic Z. R. Biosynthesis of bile acids in man. An in vivo evaluation of the conversion of R and S 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic and 3 alpha, 7 alpha, 12 alpha-24 xi-tetrahydroxy-5 beta-cholestanoic acids to cholic acid. J Biol Chem. 1981 Jan 25;256(2):912–916. [PubMed] [Google Scholar]
- Une M., Matsumoto N., Kihira K., Yasuhara M., Kuramoto T., Hoshita T. Bile salts of frogs: a new higher bile acid, 3 alpha, 7 alpha, 12 alpha, 26-tetrahydroxy-5 beta-cholestanoic acid from the bile Rana plancyi. J Lipid Res. 1980 Mar;21(3):269–276. [PubMed] [Google Scholar]


