Abstract
Background
Biochemical and histochemical studies have both previously indicated plasma membrane-associated carbonic anhydrase (CA) activity in hepatocytes which has been assumed to be CA IV. However, immunohistochemical data did not support this assignment. Recent northern blotting results indicated the presence of mRNA for the most recently discovered membrane-bound CA isozyme, CA XIV, in the liver. The present study was designed to examine whether CA XIV could contribute to the CA activity described in the hepatocytes.
Methods
Tissue samples from mouse liver were subjected to immunohistochemical staining using the antibodies raised against recombinant mouse CA XIV and CA IV. RT-PCR and western blotting were also performed for CA XIV.
Results
A strong immunofluorescent signal was observed in the plasma membrane of mouse hepatocytes. Although CA XIV was expressed on both the apical and basolateral surfaces, the staining was more prominent at the apical (canalicular) membrane domain. The expression of CA XIV in the liver was confirmed by RT-PCR and western blotting.
Conclusions
The presence of CA XIV in the hepatocyte plasma membrane places this novel enzyme at a strategic site to control pH regulation and ion transport between the hepatocytes, sinusoids and bile canaliculi.
Background
Carbonic anhydrases (CAs) are produced in a variety of tissues where they participate in a broad range of physiological processes such as acid-base homeostasis, carbon dioxide and ion transport, respiration, bone resorption, renal acidification, gluconeogenesis, ureagenesis, and formation of cerebrospinal fluid and gastric acid [1]. The expanding α-CA gene family includes 11 enzymatically active members with different structural and catalytic properties. The cellular distribution and physiological functions of the various CA isozymes have been extensively described in several recent reviews [1-4]. The most recently characterized isozyme is CA XIV, the mRNA of which has been demonstrated in the brain, kidney, liver, skeletal muscle, heart, and lung [5,6]. By immunohistochemistry, CA XIV showed a unique distribution in neurons of mammalian brain, and was expressed particularly strongly in neurons involved in motor function and coordination [7]. These observations made CA XIV a likely candidate for the extracellular CA postulated to have an important role in modulating excitatory synaptic transmission in brain.
In a more recent study, CA XIV was demonstrated in renal tubule cells [8]. Immunofluorescence staining showed strong signal for CA XIV in the apical plasma membrane of the S1 and S2 segments of proximal tubules. The staining was weaker in the basolateral membrane of these tubules. In addition, strong staining was seen in the initial portion of the thin descending limb of Henle. The results suggested that CA XIV probably accounts for a substantial fraction of the bicarbonate reabsorption in the kidney.
The present study was designed to examine the cellular localization of CA XIV in the liver which has previously shown CA XIV mRNA expression in northern blots [5,6]. By histochemical staining, hepatocytes have exhibited plasma membrane-associated CA activity [9]. Moderate membrane-associated staining was reported in the hepatocytes surrounding the portal spaces, and the staining weakened towards the central vein. Prior to discovery of additional membrane-associated CAs, the CA activity in hepatocytes was assumed to be due to CA IV. However, recent immunohistochemical data failed to support this assignment [10]. The present results demonstrate the expression of CA XIV at the hepatocyte plasma membrane, suggesting a key role for this isozyme in the regulation of ion and pH homeostasis in parenchymal cells of the liver.
Materials and Methods
Immunocytochemistry
The rabbit anti-mouse CA XIV antiserum to the secretory form of mouse CA XIV was raised in rabbits as described recently by Parkkila et al. [7]. The rabbit anti-mouse CA IV and rabbit anti-rat CA II antisera have also been described earlier [11,12]. All secondary antibodies used in immunofluorescence were purchased from Molecular Probes (Eugene, OR).
Adult male mice (Balb/c) were sacrificed by decapitation. The abdominal organs were perfused in situ through the abdominal aorta with 3% paraformaldehyde in phosphate-buffered saline (PBS), removed, and cut into slices. The slices were further immersion-fixed in 3% paraformaldehyde for 2 h at room temperature, cryoprotected overnight in 20% sucrose-PBS, and rapidly frozen in liquid nitrogen-cooled isopentane. Sections were cut at 5 μm using a Microm Cryo-Star microtome (Microm, Walldorf, Germany), dried onto Superfrost Plus microscope slides (Menzel, Braunschweig, Germany), and incubated with PBS containing 20% cow colostral whey for 40 min. The sections were then incubated for 2 h with polyclonal anti-CA XIV or preimmune serum, diluted 1:200 in 1% bovine serum albumin (BSA)-PBS, washed three times for 5 min in PBS and incubated for 1 h with Alexa 568-coupled goat anti-rabbit IgG, diluted 1:200 in BSA-PBS. After four 5-min washes in PBS, slides were mounted in Immu-mount (Shandon, Pittsburgh, PA). The sections were examined with a conventional epifluorescence microscope (Nikon Corporation, Tokyo, Japan) or a confocal laser-scanning microscope (Zeiss, Göttingen, Germany).
Western blotting
Mice were sacrificed by decapitation, and the liver and colon were removed. 20 μg of total cell protein per lane from homogenized tissue samples or stably transfected CHO cells expressing wild-type mouse CA XIV [7] was subjected to SDS-PAGE under reducing conditions according to Laemmli [13]. Protein standards for SDS-PAGE were from Bio-Rad Laboratories (Richmond, CA). The electrophoreses were performed in a Novex XCell SureLock electrophoresis unit (Invitrogen Corp/NOVEX, Carlsbad, CA) using a Novex NuPAGE 10% Bis-Tris gels. The proteins were transferred electrophoretically from the gel to a nylon membrane (Millipore; Bedford, MA) in a Novex Blot Module. After the transblotting the membrane was first incubated with TBST buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween-20) containing 10% cow colostral whey (BioTop, Oulu, Finland) for 25 min at 4°C, and then with anti-mouse CA XIV serum or anti-rat CA II serum diluted 1:2000 in TBST buffer for 1 h at room temperature. The sheets were washed four times for 5 min with TBST buffer and incubated for 30 min at room temperature with alkaline phosphatase-conjugated goat anti-rabbit IgG (Bio-Rad Laboratories) diluted 1:3000 in TBST buffer. After washing three times for 5 min in TBST buffer, the polypeptides were visualized by a chemiluminescence substrate (Bio-Rad Laboratories).
Extraction of RNA and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
The liver, colon, jejunum, and kidneys were removed from mice and the samples were used for RT-PCR analysis. Epithelial cells of the intestinal mucosa were obtained by using a scraping technique as described [14]. The mucosa from a fresh tissue sample was gently scraped using the short edge of a microscope slide. The scrapings were quickly transferred to a microtube and frozen in liquid nitrogen. Total cellular RNA was extracted from the cell or tissue samples using TRIzol reagent (Gibco-BRL, Gaithersburg, MD), according to the manufacturer's instructions. The concentration and purity of RNA was determined by spectrophotometer at 260 and 280 nm. Reverse transcription (RT) and polymerase chain reaction (PCR) amplification were performed using a single-step method [15]. Two primers for amplifying CA XIV were chosen based on the published mouse CA XIV sequence [5]; forward 5'-AAGGTGACTTGGATCCTGGCTGCA-3' (nucleotides 290–313) and reverse 5'-TTCTGAGCTGCCTCACTCAAGCTG-3' (nucleotides 700–723), which generated a 434-bp amplification product. Primers for rat glyceraldehyde-6-phosphate (GAPDH) [16] were used to monitor the quality of the RNA samples.
Ten micrograms of total RNA was used as a template for subsequent RT and PCR reactions. Reverse transcription was performed at 42°C for 1 hour followed by denaturation at 95°C for 2 minutes. The PCR cycling protocol consisted of 30 cycles of denaturation at 95°C for 1 minute, annealing at 55°C for 1 minute, and extension at 72°C for 1 minute, followed by final extension at 72°C for 5 minutes. The PCR products were analyzed by electrophoresis on 1.5% agarose gels containing ethidium bromide.
Results
Immunohistochemistry
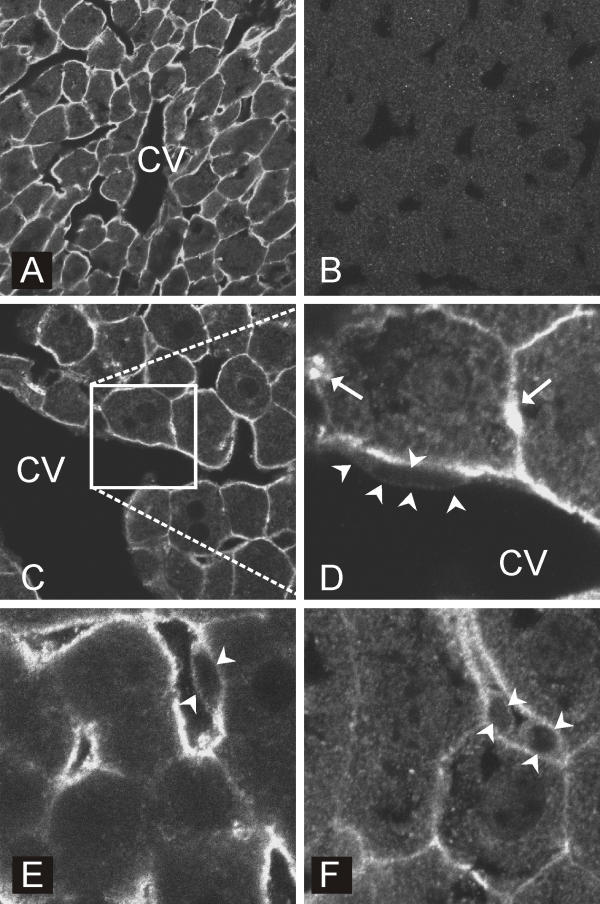
The mouse liver was analyzed for CA XIV protein expression using a specific antibody raised against the recombinant mouse CA XIV. Figure 1A shows that CA XIV was expressed at the plasma membrane of all hepatocytes. The staining intensity showed no apparent zonal variation within a hepatic lobule (data not shown). Both the apical (canalicular) and basolateral (sinusoidal) plasma membrane domains were positive. Arrows in Figure 1D indicate that the area of the canalicular membrane was more strongly stained. Arrowheads in Figure 1E,1F point to the sinusoidal lining cells which were less stained than the hepatocytes. The endothelial cell facing the central vein in panel D was completely devoid of immunostaining.
Figure 1.
Confocal laser scanning images of CA XIV in the mouse liver. CA XIV is expressed at the plasma membrane of all hepatocytes (A). Arrows in panel D indicate the location of the canalicular membrane. The endothelial cell (arrowheads) facing the central vein in panel D does not stain for CA XIV. Arrowheads in panels E and F point to a few sinusoid lining cells. Panel B shows a control section immunostained with preimmune serum. CV = central vein. Original magnifications × 250 (A,B), × 400 (C), × 1000 (D-F).
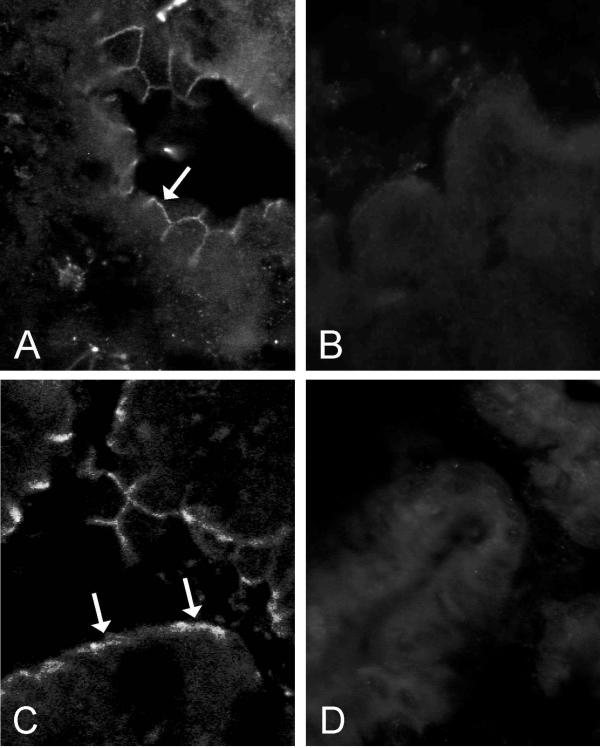
A recent study has indicated that another membrane-bound CA isozyme, CA IV, is coexpressed with CA XIV in some renal tubule cells [8]. Therefore, we set out to study whether similar colocalization can also occur in hepatocytes. Figure 2 shows that CA IV was present in sinusoid lining cells apparently representing endothelial cells, where CA XIV stains weakly (Fig. 1E,1F). Importantly, no staining was observed in the hepatocyte plasma membrane, which can be seen most clearly in the membrane domains located between two adjacent hepatocytes.
Figure 2.
Confocal laser scanning images of CA IV in the mouse liver. The sinusoidal staining pattern indicates that CA IV is most probably expressed in the sinusoid lining cells (arrows). Original magnifications × 250 (A), × 630 (B).
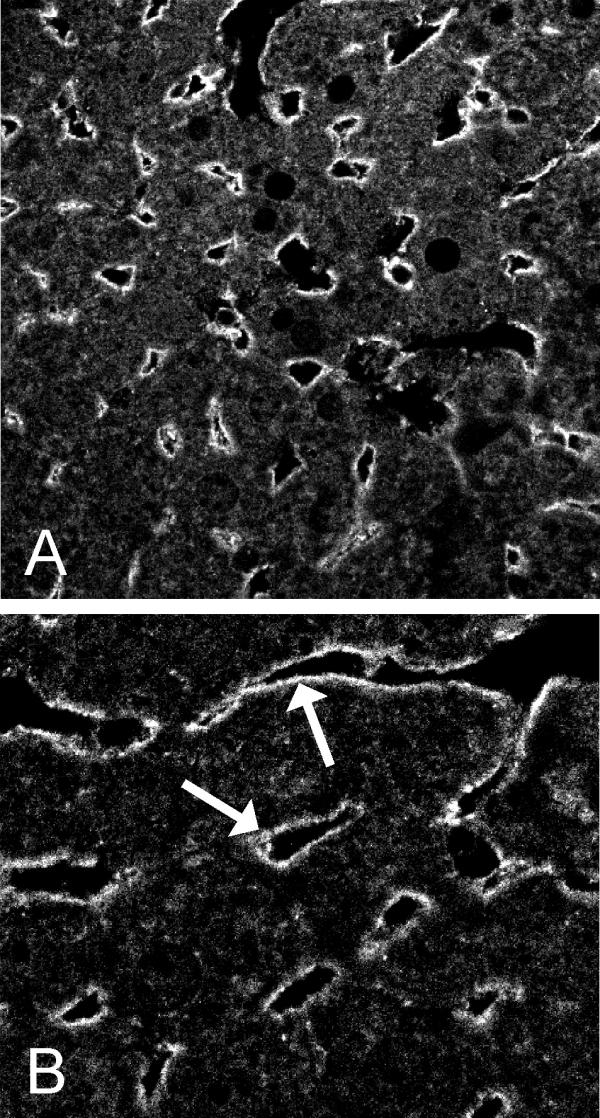
Figure 3 provides examples of CA XIV immunolocalization in some other gastrointestinal tissues of mouse. Panels A and C demonstrate that CA XIV was weakly expressed in the enterocytes of the colon. The positive signal was mainly located in the apical plasma membrane and it was found both in the crypts and the surface epithelial cuff region (data not shown). Immunostaining of mouse jejunum for CA XIV showed no positive reaction (Fig. 3D).
Figure 3.
Conventional epifluorescence (A,D) and confocal laser scanning (C) images of CA XIV in the mouse colon (A,C) and jejunum (D). CA XIV is weakly expressed in the colonic enterocytes (A,C). Immunostaining of mouse jejunum for CA XIV shows no positive reaction (D). Control staining of mouse colon using preimmune serum remained negative (B, conventional epifluorescence image). Original magnifications × 400.
Western blotting and RT-PCR
Figure 4 shows western blots of mouse liver and colon using anti-mouse CA XIV and anti-rat CA II antibodies. CHO cells expressing recombinant CA XIV served as a positive control. Anti-CA XIV serum recognized a 44/46-kDa doublet band in the liver. The results also demonstrate that the overall signal for CA XIV was quite faint in the liver, suggesting that the enzyme may be sensitive to proteolytic degradation in tissue samples. This would explain why preservation of CA XIV antigen for immunohistochemistry requires much faster tissue processing compared to any other CA isozyme. It is notable, however, that the western blotting reaction was stronger in the liver than in the colon, which was also in line with the immunostaining results. The mouse colon, jejunum, and liver were also analyzed for CA XIV mRNA expression. Total RNA isolated from the mouse kidney served as a positive control for the RT-PCR reactions. Figure 5 shows strong 434-kb bands in the liver and kidney, which was consistent with the high expression of CA XIV protein in these tissues. Weak CA XIV mRNA expression was detected in the colon, while the jejunum remained negative.
Figure 4.
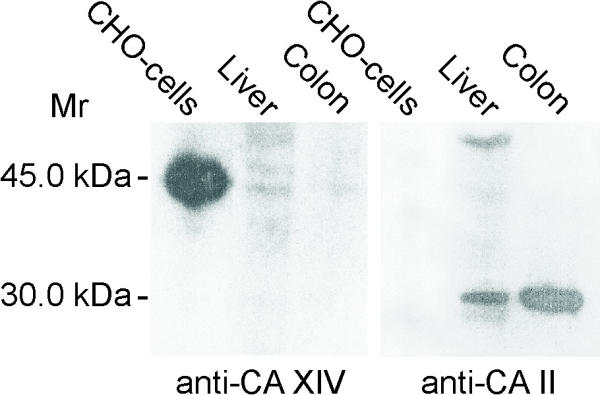
Western blots of mouse liver and colon using anti-mouse CA XIV and anti-rat CA II antibodies. CHO cells expressing recombinant CA XIV served as a positive control. Anti-CA XIV serum recognized a 44/46-kDa doublet band in the liver. The signal was clearly weaker in the colon. Control immunostaining using anti-rat CA II antibody showed strong reactions in both liver and colon.
Figure 5.
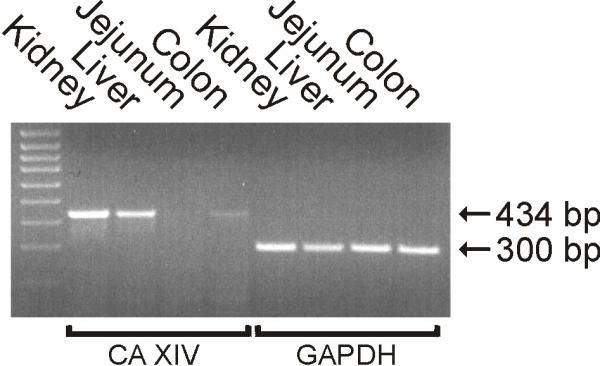
RT-PCR analysis of RNA isolated from mouse colon, jejunum, and liver. 434-bp amplification product of CA XIV mRNA was obtained from liver and kidney. Weak CA XIV mRNA expression was detected in the colon, while the jejunum remained negative. Standards (100-bp ladder) are shown on the left lane.
Discussion
Based on the previous and present data hepatocytes express at least four CA isozymes. Two of them are cytosolic, namely CAs II and III. The function of the high activity isozyme, CA II, has been linked to the intracellular hydration of carbon dioxide and alkalization of hepatic bile [17]. Based on the studies with CA III-overexpressing cells and on the expression profile of CA III in alcoholic liver, it has been proposed to participate in free radical scavenging systems [18,19]. Mitochondrial CA VA plays a key role in gluconeogenesis and ureagenesis in the liver, where bicarbonate is required as a substrate to two enzymes, pyruvate carboxylase and carbamyl phosphate synthetase [20].
In a previous report, we have shown that CA IV, which is anchored to the plasma membrane by glycosyl phosphatidylinositol linkage, is located in the human biliary epithelial cells, but is absent in the human hepatocytes [10]. Here, we demonstrated that CA IV is expressed in the sinusoid lining cells of the mouse liver, while no staining was seen in the hepatocyte plasma membrane. Based on these observations, we conclude that CA XIV is the membrane-associated CA isozyme identified in the hepatocytes by histochemical studies. One discrepancy still remains in that the present results showed no zonal variation in the staining intensity, whereas Ridderstråle et al. [9] reported that the histochemical staining decreased towards the central vein. The exact reason for this difference is not known. It should be noted, however, that Ridderstråle et al. [9] used CA II-deficient mice in the analyses, and the gene defect could potentially change the expression and distribution pattern of other CAs.
The plasma membrane-associated CA activity in hepatocytes was originally demonstrated by Garcia-Marín et al. [21] using a biochemical method and by Ono et al. [22] using a histochemical cobalt precipitation technique. At the same time, information on different ion transport proteins in hepatocyte plasma membrane accumulated that linked the plasma membrane-bound CA activity to ion transport mechanisms. Blitzer and Boyer [23] had earlier demonstrated the localization of Na+,K+-ATPase at the basolateral plasma membrane. Henderson et al. [24] and Renner et al. [25] showed that Na+/H+-exchanger regulates intracellular pH in isolated hepatocytes. Soon after these discoveries, Gleeson et al. [26] and Benedetti et al. [27] demonstrated that a Na+-HCO3- cotransporter and Cl--HCO3- exchanger are important regulators of intracellular pH, in addition to the Na+/H+ exchanger. The Na+/H+ exchanger most probably participates in acid extrusion [28]. The Na+/HCO3- cotransporter has been implicated in Na+ and HCO3- reabsorption [29]. The Cl-/HCO3- exchanger most probably plays a role in recovery from intracellular alkalization by removing HCO3- from the cytoplasm [30]. CA XIV, as a membrane-bound isozyme with its active site on the cell exterior, is a good candidate to participate in regulation of pH homeostasis and ion transport between the hepatocytes, bile canaliculi and hepatic sinusoids.
Although CA IV is not expressed on hepatic plasma membranes, where CA XIV is strongly expressed, CA IV is expressed in the sinusoid lining cells, where CA XIV is weakly expressed. Here both isozymes may participate in ion transport and pH homeostasis across the plasma face of the sinusoidal endothelial cells. CA IV is similarly expressed on the plasma face of pulmonary microcapillaries [31] and cortical capillaries in brain [32], both areas where CO2 flux is high.
It is likely that the membrane-associated CAs in liver appear with differentiation of the hepatocytes. Garcia-Marin et al. [21] observed that membrane-bound CA activity correlates with hepatic regeneration following partial hepatectomy. They measured CA activity in both soluble and plasma membrane-enriched fractions obtained from liver homogenate from rats undergoing hepatectomy. They found no changes in CA activity in the soluble fraction. However, the CA activity in plasma membrane was reduced by 55% soon after hepatectomy and returned to near control value at seven days. It would be of interest to determine the relevant contributions of CA XIV and CA IV to this increase in expression during the regenerative process, and whether the regeneration of the differentiated phenotype is dependent upon expression of the CAs. Testing hepatic regenerative capacity should be included in the physiological studies characterizing CA XIV and CA IV deficient mice when suitable knock-out animal models become available.
Conclusions
The present paper demonstrates for the first time the expression of CA XIV at the hepatocyte plasma membrane. Its localization on both apical and basolateral membrane domains suggests an important role for this isozyme in the regulation of ion and pH homeostasis in the liver.
Competing Interests
None declared.
Authors' contributions
All authors participated in the design of the study. SP, KK and JH collected the tissue samples. SP and AJK drafted the manuscript. SP and AJK performed the western blotting. KK carried out the RT-PCR. SP, AJK, KK, AKP and HR participated in the immunohistochemical staining. AW and WSS provided the antibodies. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgments
The authors thank Ms. Sirpa Kellokumpu and Ms. Lissu Hukkanen for technical assistance. The study has been supported by a grant from Sigrid Juselius Foundation to SP and grants DK40163, GM34182, and GM53405 from the National Institutes of Health to WSS.
Contributor Information
Seppo Parkkila, Email: seppo.parkkila@uta.fi.
Antti J Kivelä, Email: akivela@paju.oulu.fi.
Kari Kaunisto, Email: kari.kaunisto@oulu.fi.
Anna-Kaisa Parkkila, Email: akparkkila@hotmail.com.
Jukka Hakkola, Email: jukka.hakkola@oulu.fi.
Hannu Rajaniemi, Email: hannu.rajaniemi@oulu.fi.
Abdul Waheed, Email: waheeda@slu.edu.
William S Sly, Email: slyws@slu.edu.
References
- Parkkila S. An overview of the distribution and function of carbonic anhydrase in mammals. In: Chegwidden WR, Carter ND, Edwards YH, editor. The carbonic anhydrases. New horizons. Basel, Birkhäuser Verlag; 2000. pp. 79–93. [DOI] [PubMed] [Google Scholar]
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Exp Opin Ther Patents. 2000;10:575–600. [Google Scholar]
- Tashian RE, Hewett-Emmett D, Carter N, Bergenhem NCH. Carbonic anhydrase (CA)-related proteins (CA-RPs), and transmembrane proteins with CA or CA-RP domains. In: Chegwidden WR, Carter ND, Edwards YH, editor. The carbonic anhydrases. New horizons. Basel, Birkhäuser Verlag; 2000. pp. 105–120. [DOI] [PubMed] [Google Scholar]
- Supuran CT, Scozzafava A. Applications of the carbonic anhydrase inhibitors and activators in therapy. Exp Opin Ther Patents. 2002;12:217–242. [Google Scholar]
- Mori K, Ogawa Y, Ebihara K, Tamura N, Tashiro K, Kuwahara T, Mukoyama M, Sugawara A, Ozaki S, Tanaka I, Nakao K. Isolation and characterization of CA XIV, a novel membrane-bound carbonic anhydrase from mouse kidney. J Biol Chem. 1999;274:15701–15705. doi: 10.1074/jbc.274.22.15701. [DOI] [PubMed] [Google Scholar]
- Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S. Human carbonic anhydrase XIV (CA14): cDNA cloning, mRNA expression, and mapping to chromosome 1. Genomics. 1999;61:74–81. doi: 10.1006/geno.1999.5938. [DOI] [PubMed] [Google Scholar]
- Parkkila S, Parkkila AK, Rajaniemi H, Shah GN, Grubb JH, Waheed A, Sly WS. Expression of membrane-associated carbonic anhydrase XIV on neurons and axons in mouse and human brain. Proc Natl Acad Sci USA. 2001;98:1918–1923. doi: 10.1073/pnas.98.4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunisto K, Parkkila S, Rajaniemi H, Waheed A, Grubb J, Sly WS. Carbonic anhydrase XIV: Luminal expression suggests key role in renal acidification. Kidney Int. [DOI] [PubMed]
- Ridderstråle Y, Wistrand PJ, Holm L, Carter ND. Use of carbonic anhydrase II-deficient mice in uncovering the cellular location of membrane-associated isoforms. In: Chegwidden WR, Carter ND, Edwards YH, editor. The carbonic anhydrases. New horizons. Basel, Birkhäuser Verlag; 2000. pp. 143–155. [DOI] [PubMed] [Google Scholar]
- Parkkila S, Parkkila AK, Juvonen T, Waheed A, Sly WS, Saarnio J, Kaunisto K, Kellokumpu S, Rajaniemi H. Membrane-bound carbonic anhydrase IV is expressed in the luminal plasma membrane of the human gallbladder epithelium. Hepatology. 1996;24:1104–1108. doi: 10.1002/hep.510240521. [DOI] [PubMed] [Google Scholar]
- Fleming RE, Moxley MA, Waheed A, Crouch EC, Sly WS, Longmore WJ. Carbonic anhydrase II expression in rat type II pneumocytes. Am J Respir Cell Mol Biol. 1994;10:499–505. doi: 10.1165/ajrcmb.10.5.7514010. [DOI] [PubMed] [Google Scholar]
- Brion LP, Cammer W, Satlin LM, Suarez C, Zavilowitz BJ, Schuster VL. Expression of carbonic anhydrase IV in carbonic anhydrase II-deficient mice. Am J Physiol. 1997;273:F234–F245. doi: 10.1152/ajprenal.1997.273.2.F234. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Waheed A, Parkkila S, Saarnio J, Fleming RE, Zhou XY, Tomatsu S, Britton RS, Bacon BR, Sly WS. Association of HFE protein with transferrin receptor in crypt enterocytes of human duodenum. Proc Natl Acad Sci USA. 1999;96:1579–1584. doi: 10.1073/pnas.96.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aatsinki JT, Lakkakorpi JT, Pietilä EM, Rajaniemi H. A coupled one-step reverse transcription PCR procedure for generation of full-length open reading frames. Biotechniques. 1994;16:282–288. [PubMed] [Google Scholar]
- Holappa K, Mustonen M, Parvinen M, Vihko P, Rajaniemi H, Kellokumpu S. Primary structure of a sperm cell anion exchanger and its messenger ribonucleic acid expression during spermatogenesis. Biol Reprod. 1999;61:981–986. doi: 10.1095/biolreprod61.4.981. [DOI] [PubMed] [Google Scholar]
- Parkkila S, Parkkila AK, Juvonen T, Rajaniemi H. Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut. 1994;35:646–650. doi: 10.1136/gut.35.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkila S, Halsted CH, Väänänen HK, Niemelä O. Expression of testosterone-dependent enzyme, carbonic anhydrase III, and oxidative stress in experimental alcoholic liver disease. Digest Dis Sci. 1999;44:2205–2213. doi: 10.1023/A:1026640317233. [DOI] [PubMed] [Google Scholar]
- Räisänen SR, Lehenkari P, Tasanen M, Rahkila P, Härkönen PL, Väänänen HK. Carbonic anhydrase III protects cells from hydrogen peroxide-induced apoptosis. FASEB J. 1999;13:513–522. doi: 10.1096/fasebj.13.3.513. [DOI] [PubMed] [Google Scholar]
- Dodgson SJ. Liver mitochondrial carbonic anhydrase (CA V), gluconeogenesis, and ureagenesis in the hepatocytes. In: Dodgson SJ, Tashian RE, Gros G, Carter ND, editor. The carbonic anhydrases. Cellular physiology and molecular genetics. New York, Plenum Press; 1991. pp. 297–306. [Google Scholar]
- García Marín JJ, Urbieta AT, Barriocanal FP, Barbero ER, Eleno N. Plasma membrane-bound carbonic anhydrase activity in the regenerating rat liver. Biochim Biophys Acta. 1991;1061:9–14. doi: 10.1016/0005-2736(91)90262-7. [DOI] [PubMed] [Google Scholar]
- Ono Y, Ridderstråle Y, Forster RE, Chu ZG, Dodgson SJ. Carbonic anhydrase in the membrane of the endoplasmic reticulum of male rat liver. Proc Natl Acad Sci USA. 1992;89:11721–11725. doi: 10.1073/pnas.89.24.11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer BL, Boyer JL. Cytochemical localization of Na+, K+-ATPase in the rat hepatocyte. J Clin Invest. 1978;62:1104–1108. doi: 10.1172/JCI109216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RM, Graf J, Boyer JL. Na-H exchange regulates intracellular pH in isolated rat hepatocyte couplets. Am J Physiol. 1987;252:G109–G113. doi: 10.1152/ajpgi.1987.252.1.G109. [DOI] [PubMed] [Google Scholar]
- Renner EL, Lake JR, Persico M, Scharschmidt BF. Na+-H+ exchange activity in rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1989;256:G44–G52. doi: 10.1152/ajpgi.1989.256.1.G44. [DOI] [PubMed] [Google Scholar]
- Gleeson D, Smith ND, Boyer JL. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. Evidence for Na+-HCO3- cotransport. J Clin Invest. 1989;84:312–321. doi: 10.1172/JCI114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A, Strazzabosco M, Corasanti JG, Haddad P, Graf J, Boyer JL. Cl--HCO3- exchanger in isolated rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1991;261:G512–G522. doi: 10.1152/ajpgi.1991.261.3.G512. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+ /H+ exchangers. Physiol Rev. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- Boron WF, Hediger MA, Boulpaep EL, Romero M. The renal electrogenic Na+:HCO3- cotransporter. J Exp Biol. 1997;200:263–268. doi: 10.1242/jeb.200.2.263. [DOI] [PubMed] [Google Scholar]
- Alper SL. The band 3-related AE anion exchanger gene family. Cell Physiol Biochem. 1994;4:265–281. [Google Scholar]
- Fleming RE, Crouch EC, Ruzicka CA, Sly WS. Pulmonary carbonic anhydrase IV: developmental regulation and cell-specific expression in the capillary endothelium. Am J Physiol. 1993;265:L627–L635. doi: 10.1152/ajplung.1993.265.6.L627. [DOI] [PubMed] [Google Scholar]
- Ghandour MS, Langley OK, Zhu XL, Waheed A, Sly WS. Carbonic anhydrase IV on brain capillary endothelial cells: a marker associated with the blood-brain barrier. Proc Natl Acad Sci USA. 1992;89:6823–6827. doi: 10.1073/pnas.89.15.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]