ABSTRACT
Several molecular mismatch assessment approaches exist, but data on their combined use are limited. In this study, we aimed to define distinct risk groups for rejection based on the combination of three molecular mismatch assessment approaches (i.e., eplet mismatch count, the number of highly immunogenic eplets and PIRCHE‐II score) in 439 consecutive immunological standard risk transplantations. For each molecular mismatch assessment approach, ROC analyses were used to define cut‐offs for prediction of (sub) clinical rejection according to Banff 2019 classification within the first year post‐transplant as a reference. If all three scores were below the cut‐off, the patient was assigned to the low‐risk group (19% of patients); if all three scores were above the cut‐off, the patient was assigned to the high‐risk group (21% of patients). The one‐year incidence of (sub) clinical rejection was 12% in the low‐risk group and 33% in the high‐risk group (p = 0.003). Internal validation of the assigned risk groups for prediction of other outcomes revealed a high consistency: clinical rejection (6% vs. 24%; p = 0.004), ATG‐treated rejection (1% vs. 16%; p < 0.001) and development of de novo HLA‐DSA at 5 years post‐transplant (6% vs. 25%; p = 0.003). The molecular mismatch risk group was an independent predictor for (sub) clinical rejection (high‐risk vs. low‐risk: hazard ratio 3.11 [95%‐CI 1.50–6.45]; p = 0.002). We conclude that combining molecular mismatch approaches allows us to distinguish low‐ and high‐risk groups among standard renal allograft recipients. Independent validation in other patient populations and different ethnicities is required.
Keywords: HLA eplets, immunological risk stratification, Molecular mismatch assessment, PIRCHE II, renal allograft rejection
1. Introduction
Differences among the HLA molecules between the donor and recipient are the major driving force for allograft rejection. Patients having a kidney transplantation with a presumed HLA‐directed memory response inferred by the presence of donor‐specific HLA antibodies (HLA‐DSA) are widely accepted as an immunological high‐risk group [1, 2, 3]. On the other end of the immunological risk spectrum, HLA identical transplants clearly have the lowest risk of rejection and are considered as low‐risk transplants [4]. Between these two distinct groups, patients without preformed HLA‐directed memory, but with at least one HLA mismatch account for most of the transplantations and can be regarded as immunological standard risk situations [3, 5, 6]. However, this group is still very heterogeneous. Further stratification has been attempted by traditional HLA matching methods. However, this approach has a major limitation because it does not assess differences of molecular structures on the HLA molecules (i.e., epitopes), which are the true targets of the alloimmune response [7, 8, 9].
Two main molecular mismatch assessment approaches have been developed during the last two decades (i.e., the eplet mismatch analysis and the predicted indirectly recognisable HLA epitopes [PIRCHE] II algorithm) [10, 11]. Both approaches were investigated in many studies, showing a clear and independent association with the development of de novo HLA‐DSA, occurrence of rejection and graft loss [12, 13, 14, 15, 16, 17]. A combination of both approaches might improve risk prediction because the eplet mismatch analysis and the PIRCHE II algorithm try to simulate the B cell‐ and T cell alloimmune response, respectively. Indeed, two groups performed such analyses and described some advantages when using two approaches together [18, 19, 20]. Theoretically, the eplet mismatch analysis could be further enhanced by adding information on the immunogenicity of individual eplets. Recently, we and others provided first data on the immunogenicity of eplets with an emphasis on the highly immunogenic ones [21, 22, 23, 24, 25, 26].
The aim of this study was to explore three molecular mismatch tools (eplet mismatch count, highly immunogenic eplet count, PIRCHE II score) for prediction of rejection within the first year post‐transplant in a well‐characterised standard risk cohort having contemporary immunosuppression with tacrolimus‐mycophenolate‐prednisone and basiliximab induction.
2. Methods and Materials
2.1. Patient Population
The retrospective observational cohort study was approved by the local ethics committee (EKNZ 2023–01992). The patient flow is summarised in Figure 1. All patients who had a kidney transplantation between January 2014 and August 2022 at the University Hospital in Basel were eligible for this study (n = 666). We excluded 227 patients for the following reasons: (i) high immunological risk (n = 152) such as pre‐transplant HLA‐DSA determined by virtual crossmatching (n = 79), husband‐to‐wife transplantations with mutual children (n = 27) and ABO‐incompatible transplants (n = 46) [27, 28]; (ii) no HLA‐associated immunological risk (n = 22); (iii) no tacrolimus/mycophenolate‐based maintenance immunosuppression or no induction with basiliximab (n = 23); (iv) no high‐resolution HLA typing of the donor and recipient (n = 21) and (v) death within the first year post‐transplant (n = 9). Thus, the final population consisted of 439 patients considered as immunological standard risk having an identical immunosuppression consisting of tacrolimus (Tac), mycophenolate (MPA) and prednisone with basiliximab induction, as well as high‐resolution HLA typing of the donors/recipients and a minimal follow‐up of 1 year.
FIGURE 1.
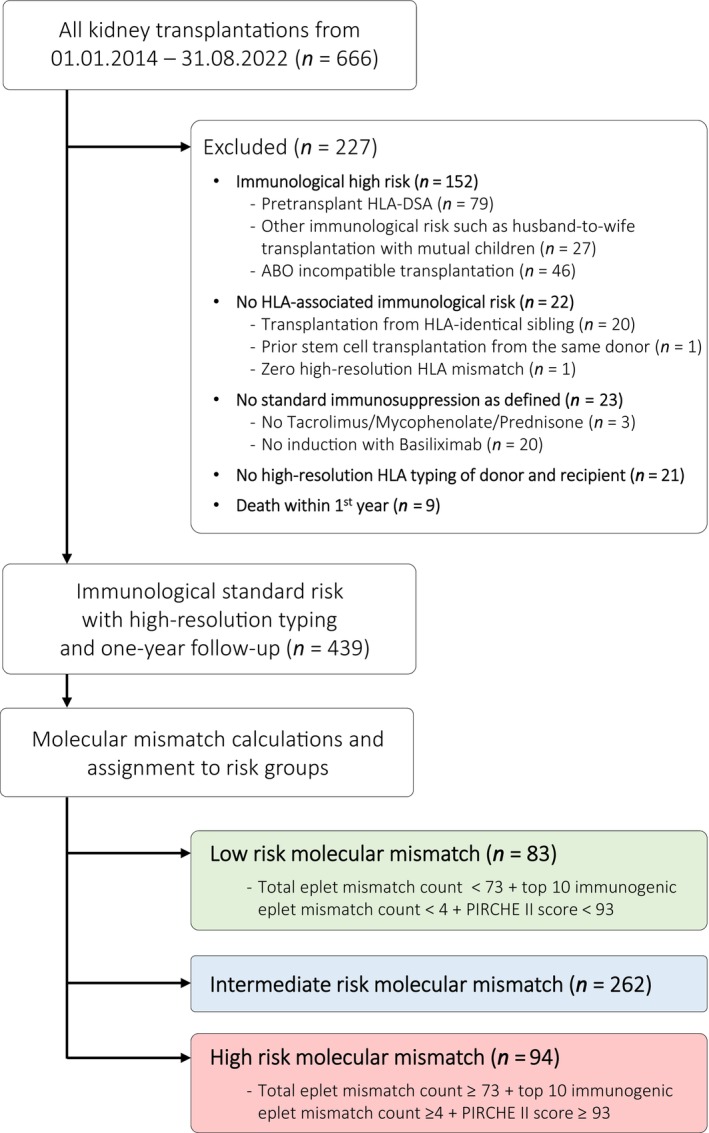
Patient flow.
2.2. HLA Typing and Molecular Mismatch Analyses
Two‐field HLA typing of 11 loci was performed by next‐generation sequencing with the NGSgoMX11‐3 kits from GenDx (gendx.com). The eplet mismatch count was calculated using the definition of HLA Eplet Registry (epregistry.com.br; accessed in December 2023) as well as the definition of the HLAMatchmaker Software Version 2.2. The definition of the top 10 immunogenic eplets for each class/locus is based on an own study in a human pregnancy model [21, 22], which was expanded for this study to all loci and both eplet definitions (i.e., HLA Eplet Registry and HLAMatchmaker Software Version 2.2). PIRCHE II scores for all HLA loci were calculated using the software version 4.1 (Frost 1.1 binding prediction, 200% binding rank cut‐off; pirche.com).
The target outcome to be predicted by the molecular mismatch analyses was the occurrence of clinical or subclinical rejection within the first year post‐transplant defined by the Banff 2019 classification based on the individual Banff scores [29]. We calculated the area‐under‐the‐curve (AUC) regarding this binary outcome for all individual molecular mismatch scores. The best AUC for each molecular mismatch score (i.e., eplet mismatch count, Top 10 immunogenic eplet count, PIRCHE II score) was determined, and its cut‐off and sensitivity and specificity were calculated. All possible combinations of individual molecular mismatch scores were investigated as well.
2.3. Investigated Outcomes
Besides the occurrence of clinical or subclinical rejection within the first year post‐transplant defined by the Banff 2019 classification to calibrate the molecular mismatch analyses, various other outcomes were investigated as follows: (i) the incidence of early clinical rejection within the first 30 days post‐transplant as well as clinical rejection within the first year post‐transplant, defined by either the Banff 2015 or the 2019 classification; (ii) the incidence of rejection requiring treatment with anti T‐lymphocyte globulin (ATG; either Thymoglobulin or Grafalon); (iii) incidence of de novo HLA‐DSA; (iv) estimated GFR at one‐year post‐transplant calculated by the CKD‐EPI 2021 equation; (v) incidence of graft loss within the first year post‐transplant and (vi) incidence of triple immunosuppression at 1‐year post‐transplant.
Overall, 269 clinical biopsies and 284 surveillance biopsies were obtained within the first year post‐transplant. One‐hundred and twenty‐one of 439 patients (28%) did not have any allograft biopsies within the first year post‐transplant. As mentioned previously, the individual Banff scores were used to define rejection according to the Banff 2015 or 2019 classification, respectively. Notably, the Banff 2015 and Banff 2019 classifications mainly differ in the definition of borderline T cell‐mediated rejection (TCMR), which is the most common rejection phenotype in immunological standard risk patients [30, 31]. De novo HLA‐DSA were assessed by single‐antigen beads (OneLambda; ThermoFisher) at one‐year post‐transplant in 388/439 patients (88%) and every 2 years thereafter. All 11 HLA loci were included for the analysis and an MFI > 500 was considered as a positive result.
2.4. Immunosuppressive Drug Exposure
Target Tac trough levels were 10–12 μg/L in the first‐month post‐transplant, then 8–10 μg/L until month 3 and 6–8 μg/L thereafter. We aimed for MPA trough levels > 2 mg/L unless clinical side effects required a dose reduction. Prednisone was reduced to 0.1 mg/kg body weight by month 3 post‐transplant and withdrawn, if no prior clinical or subclinical rejection occurred. To assess the exposure to Tac and MPA, all trough levels measured within the first year post‐transplant were retrieved from the laboratory information system. Five of 439 patient (1%) experiencing primary non‐function had to be excluded from this analysis. We calculated the frequency of trough levels below a certain threshold by dividing the number of values below the threshold by the total number of measurements.
Therapeutic interventions were based on the histological diagnosis according to the Banff 2013/2015 classification. Most rejection episodes were treated with steroid pulses plus optimisation of maintenance immunosuppression.
2.5. Statistics
Categorical data are presented as count (percentage) and were analysed by chi‐square test or Fishers exact test as appropriate. Continuous data are shown as median (interquartile range) and compared by the Wilcoxon rank sum test unless stated otherwise. For all tests, a two‐tailed p value < 0.05 was considered to indicate statistical significance. Time‐to‐event analyses were performed by the Kaplan–Meier method and compared by the log‐rank test. Multivariate analysis to assess independent predictors for (sub) clinical rejection within the first year post‐transplant was performed by the Cox proportional hazard method, including covariates associated with rejection (e.g., recipient age, re‐transplant, delayed graft function). We used JMP Pro 16 for statistical analyses.
3. Results
3.1. Classification of the Molecular Mismatch Risk Groups
The top 10 immunogenic eplets using the HLA Eplet Registry definition are summarised in Figure 2A. First, we calculated the AUC's for all different molecular mismatch scores regarding the target outcome in all 439 patients (i.e., occurrence of clinical or subclinical rejection within the first year post‐transplant defined by the Banff 2019 classification) without correction for other covariates. Among the eplet mismatch analyses using the HLA Eplet Registry definition, the total count had the highest AUC with 0.60. Among the top 10 immunogenic eplet analyses using the HLA Eplet Registry definition, the total count had the highest AUC with 0.56. Among the PIRCHE II analyses, the total score had the highest AUC with 0.60. When the calculation was restricted to only those 318 patients having at least one allograft biopsy within the first year post‐transplant, the AUC and cut‐off were very similar (Figure 2B). The eplet mismatch and top 10 immunogenic eplet analyses using HLAMatchmaker Software Version 2.2 revealed similar AUC of 0.60 and 0.56, respectively.
FIGURE 2.
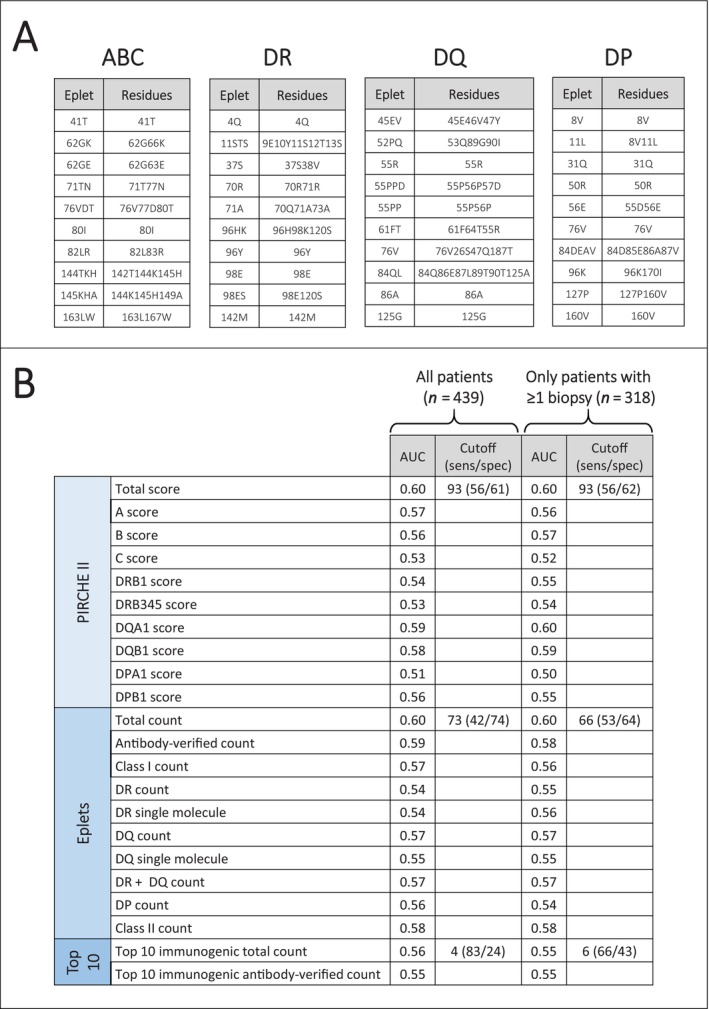
Molecular mismatch analyses. (A) Top 10 immunogenic eplets for each class/locus. (B) Receiver‐operating characteristic analyses of individual molecular mismatch approaches.
Next, we used the best cut‐off of the three scores to classify the cohort into low‐/high‐risk groups, as well as tertiles to classify the cohort into low/intermediate/high‐risk groups. A statistically significant distinction could be seen for some individual scores. Combinations of two of the three scores also revealed statistically significant results, but either the intermediate‐risk group aligned with the low‐ or the high‐risk group (Figure S1). Finally, we combined all three scores together. Patients were assigned to the low‐risk group, if all three individual scores were below the cut‐off (total eplet mismatch count < 73, top 10 immunogenic eplet mismatch count < 4, PIRCHE II score < 93) and to the high‐risk group, if all three individual scores were above the cut‐off (total eplet mismatch count ≥ 73, top 10 immunogenic eplet mismatch count ≥ 4, PIRCHE II score ≥ 93). The intermediate‐risk group consisted of all remaining patients, who did neither qualify for the low‐ nor the high‐risk group. This led to a better separation of the risk groups regarding the target outcome (Figure 3A).
FIGURE 3.
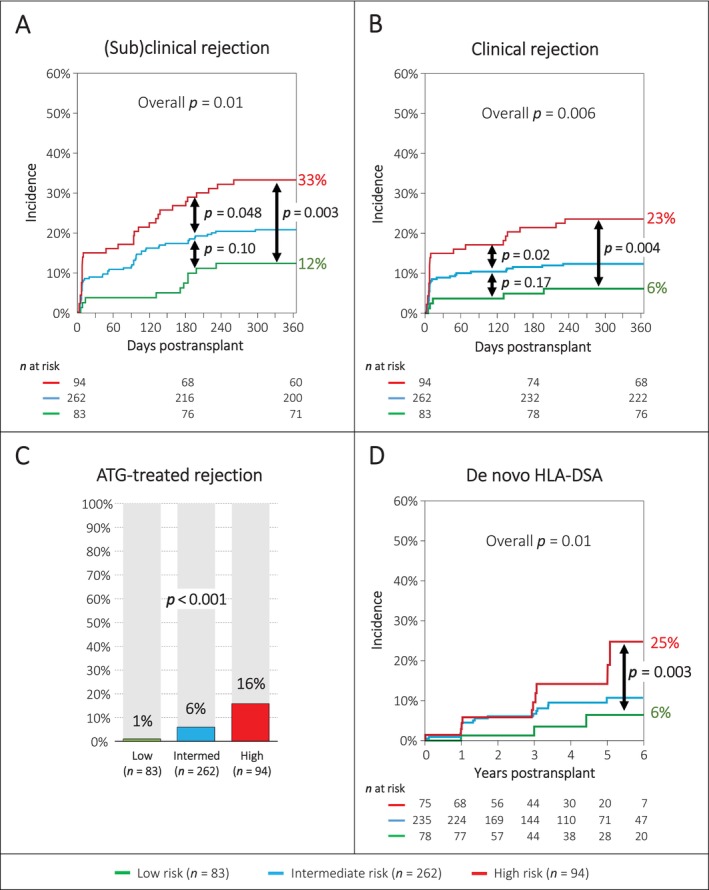
Pertinent outcomes among the three molecular mismatch risk groups. (A) Incidence of clinical or subclinical rejection within the first year post‐transplant defined by the Banff 2019 classification. (B) Incidence of clinical rejection within the first year post‐transplant defined by the Banff 2019 classification. (C) Frequency of ATG‐treated rejection (thymoglobulin or grafalon). (D) Incidence of de novo HLA‐DSA up to 5 years post‐transplant.
3.2. Baseline Characteristics
Based on the combined molecular mismatch score, we assigned the 439 patients into a low‐risk group (n = 83; 19%), an intermediate‐risk group (n = 262; 60%) and a high‐risk group (n = 94; 21%). The baseline characteristics among these groups are detailed in Table 1. We observed no statistically significant differences regarding major donor and recipient variables, except a higher frequency of prior kidney transplantation in the low‐risk group (percentage of re‐transplants: 13.3% vs. 7.3% vs. 3.2%; p = 0.04). As expected, the number of high‐resolution HLA mismatches, the eplet mismatch count, the top 10 immunogenic eplet mismatch count and the PIRCHE II scores were significantly different among the three groups.
TABLE 1.
Baseline characteristics.
| Parameter | Low risk (n = 83) | Intermediate risk (n = 262) | High risk (n = 94) | p across all 3 groups | p low vs high risk |
|---|---|---|---|---|---|
| Recipient sex female | 23 (28%) | 78 (30%) | 27 (29%) | 0.93 | 1.00 |
| Recipient age | 56 (44–63) | 55 (43–64) | 56 (47–64) | 0.79 | 0.57 |
| Deceased donor | 50 (60%) | 174 (66%) | 70 (74%) | 0.12 | 0.05 |
| Cold ischemia time [h] | 6.3 (2–9.1) | 7.8 (2.1–10.6) | 8 (2.6–10.3) | 0.05 | 0.02 |
| Donor age | 57 (43–67) | 58 (47–64) | 56 (47–64) | 0.87 | 0.74 |
| Renal disease | |||||
| Polycystic kidney disease | 14 (16.9%) | 43 (16.4%) | 23 (24.5%) | 0.62 | 0.42 |
| Diabetic nephropathy | 4 (4.8%) | 26 (9.9%) | 9 (9.6%) | ||
| Glomerulonephritis | 30 (36.1%) | 93 (35.5%) | 27 (28.7%) | ||
| Interstitial nephropathy | 5 (6%) | 14 (5.3%) | 7 (7.5%) | ||
| Vascular nephropathy | 12 (14.5%) | 26 (9.9%) | 10 (10.6%) | ||
| Other nephropathies | 13 (15.7%) | 33 (12.6%) | 9 (9.6%) | ||
| Unknown nephropathy | 5 (6%) | 27 (10.3%) | 9 (9.6%) | ||
| Renal replacement therapy | |||||
| Preemptive transplantation | 20 (24.1%) | 39 (14.9%) | 13 (13.8%) | 0.27 | 0.19 |
| Haemodialysis | 55 (66.3%) | 175 (66.8%) | 65 (69.2%) | ||
| Home haemodialysis | — | 2 (0.8%) | 1 (1.1%) | ||
| Peritoneal dialysis | 8 (9.6%) | 46 (17.6%) | 15 (16%) | ||
| Sensitising events | |||||
| any | 35 (42.2%) | 99 (37.8%) | 42 (44.7%) | 0.46 | 0.74 |
| prior kidney transplantation | 11 (13.3%) | 19 (7.3%) | 3 (3.2%) | 0.04 | 0.01 |
| pregnancies | 13 (15.9%) | 51 (19.7%) | 20 (21.3%) | 0.63 | 0.36 |
| blood transfusions | 23 (29.1%) | 54 (20.9%) | 22 (23.9%) | 0.32 | 0.44 |
| Current cPRA (11 loci) [%] | 5.4 (0–46.4) | 1.6 (0–30.4) | 1.5 (0–28.8) | 0.57 | 0.29 |
| High‐resolution HLA typing mismatches | 7 (5–9) | 10 (8–13) | 14 (12–15) | < 0.001 | < 0.001 |
| Total eplet mismatch count | 35 (26–44) | 58 (46–69) | 84 (77–91) | < 0.001 | < 0.001 |
| Top 10 immunogenic eplet mismatch count | 2 (0–3) | 7 (5–10) | 11 (8–14) | < 0.001 | < 0.001 |
| PIRCHE II score | 45 (25–67) | 80 (56–110) | 127 (113–150) | < 0.001 | < 0.001 |
Abbreviation: cPRA, calculated population‐reactive antibodies.
High‐resolution HLA typing mismatches correlated with the total eplet mismatch count (r 2 = 0.62; p < 0.001), the top 10 immunogenic eplet mismatch count (r 2 = 0.26; p < 0.001) and the PIRCHE II score (r 2 = 0.44; p < 0.001). The three molecular mismatch scores also correlated strongly with each other (r 2 between 0.21 and 0.42; all p < 0.001). The relationship among the three molecular mismatch scores, the high‐resolution HLA typing mismatches and molecular mismatch risk groups are detailed in two correlation plots (Figure S2).
3.3. Outcomes
The target outcome (i.e., incidence of subclinical or clinical rejection within the first year after transplantation using the Banff classification 2019), which was used to classify the patients based on the molecular mismatch scores, occurred in 10/83 (12.0%) patients of the low‐risk group, in 54/262 (20.6%) patients of the intermediate‐risk group and in 31/94 (33.0%) patients of the high‐risk group (p = 0.01) (Table 2 and Figure 3A). The rejection phenotypes were mainly TCMR (84/95; 88.4%), while antibody‐mediated rejection (AMR) was rarely observed (11/95; 11.6%). The incidence of (sub) clinical TCMR was significantly different among the three groups (12.0% vs. 17.2% vs. 30.9%; p = 0.008), but the incidence of (sub) clinical AMR was similar (0% vs. 3.4% vs. 2.1%; p = 0.26). The incidence of clinical rejection among the three groups was significantly different (6% vs. 12% vs. 23%; p = 0.006) (Figure 3B). When the analysis was restricted to only those 318 patients having at least one allograft biopsy within the first year post‐transplant, the incidence of rejection was higher as expected, but the separation among the three groups was identical (Figure S3).
TABLE 2.
One‐year outcomes.
| Outcome | Low risk (n = 83) | Intermediate risk (n = 262) | High risk (n = 94) | p across all 3 groups | p low vs high risk |
|---|---|---|---|---|---|
| Rejection according to Banff 2019 classification | |||||
| clinical rejection ≤ 30 days post‐transplant | 3 (3.6%) | 23 (8.8%) | 14 (14.9%) | 0.03 | 0.01 |
| clinical rejection within 1st year | 5 (6.0%) | 32 (12.2%) | 22 (23.4%) | 0.003 | 0.001 |
| clinical or subclinical rejection within 1st year | 10 (12.1%) | 54 (20.6%) | 31 (33.0%) | 0.003 | 0.001 |
| Other one‐year outcomes | |||||
| rejection treated with ATG | 1 (1.2%) | 16 (6.1%) | 15 (16.0%) | < 0.001 | < 0.001 |
| graft loss within 1st year | 2 (2.4%) | 4 (1.5%) | 6 (6.4%) | 0.05 | 0.20 |
| triple immunosuppression at 1 year (n = 427) | 28/81 (34.6%) | 91/258 (35.3%) | 46/88 (52.3%) | 0.01 | 0.02 |
| eGFR (n = 427) | 53 (39–72) | 54 (41–68) | 54 (41–71) | 0.82 | 0.67 |
| eGFR < 25 mL/min at 1 year (n = 427) | 2/81 (2.5%) | 7/258 (2.7%) | 4/88 (4.6%) | 0.68 | 0.47 |
| Urine protein/creat ratio [mg/mmol] (n = 417) | 12 (8–20) | 12 (7–20) | 14 (7–24) | 0.65 | 0.77 |
| de novo HLA‐DSA at 1 year (n = 388) | 1/77 (1.3%) | 10/236 (4.2%) | 4/75 (5.3%) | 0.32 | 0.16 |
| Rejection according to Banff 2015 classification | |||||
| clinical rejection ≤ 30 days post‐transplant | 6 (7.2%) | 48 (18.3%) | 21 (22.3%) | 0.01 | 0.005 |
| clinical rejection within 1st year | 13 (15.7%) | 66 (25.2%) | 29 (30.9%) | 0.05 | 0.02 |
| clinical or subclinical rejection within 1st year | 24 (28.9%) | 121 (46.2%) | 52 (55.3%) | 0.001 | < 0.001 |
Abbreviations: ATG, anti T‐lymphocyte globulin (thymoglobulin or grafalon); eGFR, estimated glomerular filtration rate; HLA‐DSA, donor‐specific HLA‐antibodies.
As an internal validation, the three risk groups were also assessed regarding other pertinent outcomes. We found significant differences among the three groups regarding the incidence of early clinical rejection within 30 days post‐transplant and the incidence of clinical rejection within the first year post‐transplant, for both Banff 2015 and 2019 classifications (Table 2). Furthermore, the incidence of ATG‐treated rejection was significantly different among the three groups (1.2% vs. 6.1% vs. 16.0%; p < 0.001) (Table 2 and Figure 3C).
The incidence of de novo HLA‐DSA at one‐year post‐transplant was not statistically different among the three groups (1.3% vs. 4.2% vs. 5.3%; p = 0.32). Six of 15 (40%) de novo DSA were against class I, 8/15 (53%) against class II and 1/15 (7%) against class I + II. The median MFI was 1264, and 12/15 de novo DSA were detected at the one‐year routine screening. At 5 years post‐transplant, we noticed a significantly different incidence of de novo DSA among the three groups (6% vs. 11% vs. 25%; overall p = 0.01; low‐ vs. high‐risk group p = 0.003) (Figure 3D).
We observed no differences among the three groups regarding graft loss, eGFR and proteinuria at one‐year post‐transplant. Interestingly, patients in the high‐risk group were more often on triple immunosuppression (52.3% vs. 35.3% and 34.6%, respectively; p = 0.01) (Table 2).
3.4. Exposure to Immunosuppression
The overall Tac and MPA exposure in the whole cohort reflected the intended protocol and is shown in Figure S4. We observed no differences among the three risk groups regarding the number of Tac and MPA measurements, as well as the frequency of measurement below specific trough levels (Table S1). Considering intra‐patient Tac level variability as a potential proxy for adherence did not correlate with de novo HLA‐DSA (data not shown).
3.5. Multivariable Analysis
In the multivariable cox regression analysis, the molecular mismatch risk group was an independent risk factor (high‐risk vs. low‐risk hazard ratio 3.11 [95%‐CI 1.50–6.45]; p = 0.002) for clinical or subclinical rejection within the first year post‐transplant. In addition, the percentage of Tac levels below 3 μg/L increased the risk of rejection, while older age was associated with a lower risk of rejection. The donor source (deceased vs. living donor), repeated transplantation or delayed graft function were not independent risk factors (Table 3). Due to a significant collinearity of the total eplet mismatch count, the top 10 immunogenic eplet mismatch count, the PIRCHE II score and the molecular mismatch risk group, these four parameters could not be evaluated in a combined model. The corrected Akaike's Information Criterion and the Bayesian Information Criterion assessed in the Cox model for all individual parameters are similar, and all are independently associated with clinical or subclinical rejection within the first year post‐transplant (data not shown).
TABLE 3.
Multivariable cox regression analysis for clinical or subclinical rejection (n = 95) within the first year post‐transplant according to the Banff 2019 classification.
| Parameter | Hazard ratio (95% CI) | p |
|---|---|---|
| Molecular mismatch risk group | ||
| Low risk (reference) | — | — |
| Intermediate risk | 1.73 (0.88–3.43) | 0.11 |
| High risk | 3.11 (1.50–6.45) | 0.002 |
| Recipient age per decade older | 0.82 (0.70–0.96) | 0.01 |
| Recipient female sex | 1.27 (0.82–1.96) | 0.28 |
| Deceased donor source | 1.09 (0.65–1.83) | 0.75 |
| Donor age per decade older | 1.05 (0.94–1.19) | 0.38 |
| Re‐transplant | 1.23 (0.56–2.72) | 0.60 |
| Delayed graft function | 1.30 (0.78–2.15) | 0.31 |
| % of tacrolimus trough levels below 3 μg/L | 1.13 (1.00–1.26) | 0.03 |
| % of mycophenolate trough level below 1 mg/L | 1.01 (0.99–1.02) | 0.27 |
4. Discussion
To our knowledge, this is the first study exploring an enhanced risk stratification based on the combination of three individual molecular mismatch approaches. It highlights that this approach can distinguish low‐ and high‐risk patients among immunological standard‐risk transplants. Indeed, patients having scores below the threshold for all three molecular mismatch algorithms are at very low risk to experience an immunological event within the first year post‐transplant. In contrast, patients demonstrating elevated scores in all three molecular mismatch assessment approaches have a three times increased risk to develop rejection, and about half of them require treatment with ATG. In addition, these patients have a four‐fold higher incidence of de novo HLA‐DSA at 5 years post‐transplant.
The number of top 10 immunogenic eplets alone was not significantly associated with the development of rejection. However, the cut‐off of 4 had a rather high sensitivity of 83%, which helped in combination with the total eplet mismatch count and the PIRCHE II score to better delineate low‐risk patients (Figure S1G,I). This observation also indicates that the definition of the immunogenicity of eplet is still incomplete and needs further refinements [25, 26, 32, 33]. Theoretically, one highly immunogenic HLA epitope could induce a strong alloimmune response, leading to allograft rejection [32, 33].
In our study, the total eplet mismatch count and the total PIRCHE II scores had higher AUC's compared to the analyses of individual classes or loci (e.g., class II or DR/DQ). At first sight, this is surprising and contradictory to many pertinent studies reporting a strong association with DR/DQ eplet mismatch counts or DR/DQ PIRCHE II scores and the development of de novo DR/DQ‐DSA as well as TCMR and AMR [13, 15]. Our data do not argue against this well‐established observation, but it suggests that the alloimmune response within the first year post‐transplant, which is very often detectable as a TCMR phenotype, can be induced by all HLA molecules, either alone or in combination.
Wiebe et al. clearly demonstrated in a very well characterised cohort that the development of de novo DR/DQ‐DSA is driven by a high DR/DQ eplet mismatch count, low Tac levels, younger age and poor adherence [34, 35]. Our multivariable Cox regression model is fully in line with the first three parameters described by Wiebe et al., but unfortunately, we had no reliable data on the adherence of the patients in our study.
How can we use the molecular mismatch risk grouping clinically? Patients in the low‐risk group could be ideal candidates for immunosuppression minimisation protocols or tolerance induction studies performed in HLA mismatched situations. Patients in the high‐risk group might benefit from an intensified induction therapy (i.e., ATG) and an increased maintenance immunosuppression. In addition, such high‐risk patients can enrich studies aiming to explore novel treatments against allograft rejection. The clinical application of the molecular mismatch risk grouping requires (i) high‐resolution HLA typing of the donor and recipient and (ii) the availability of the molecular mismatch assessment tools. For living donor kidney transplantation, both requirements can easily be fulfilled. For deceased donor transplantations, high‐resolution HLA typing of the donor is currently almost never available before transplantation and would have to be imputed from intermediate‐resolution data. This will lead to some errors in the calculations of the molecular mismatch scores [36, 37]. Notably, recent advances in the nanopore sequencing method will likely resolve this problem very soon because it is possible to generate high‐resolution HLA typing data within 4–6 h [38].
Our study has some advantages compared to other study assessing molecular mismatch assessment approaches [18, 19, 20]. The population consists only of patients having an HLA‐associated risk but no preformed HLA‐DSA. In addition, the cohort has a standardised immunosuppression and a reasonable frequency of the target outcome (n = 95) to facilitate the generation of risk groups. Furthermore, the target outcome to develop the risk grouping (i.e., clinical or subclinical rejection within the first year post‐transplant) is clinically important and precedes the occurrence of de novo HLA‐DSA. In fact, unresolved and persisting rejection starting within the first year post‐transplant is strongly associated with later development of de novo DSA [39].
We acknowledge several limitations of this study. First, it is a single‐centre study with almost exclusively Caucasian patients. Therefore, it requires validation in other cohorts with more diverse ethnicities. As an alternative to external validation, we investigated the robustness of the grouping by assessment of various other outcomes, which were not used for the development of the risk grouping, such as the incidence of clinical rejection according to different Banff classification schemas and the incidence of ATG‐treated rejection. The validity of the risk grouping is also supported by the clear association with the development of de novo HLA‐DSA, as has been shown by many large studies [13, 14, 15]. Second, the study population is not large, but it was still sufficient to detect statistically significant results. Third, the definitions of eplets and the PIRCHE II algorithms are evolving and still have significant limitations [40]. In addition, the determination of the immunogenicity of individual eplets depends on the characteristics and the HLA background of the investigated cohort. Interestingly, both investigated eplet definitions (version 2.2 and the most recent from the HLA Eplet Registry) revealed very similar results, suggesting that the total eplet mismatch count is a quite robust parameter despite significant changes between the two versions. Forth, we cannot demonstrate the statistical superiority of the combined molecular mismatch risk group compared to the individual scores. However, the molecular mismatch risk groups showed a more distinct internal separation, and we assume that categorical risk groups will have a better clinical applicability.
In conclusion, a combination of molecular mismatch approaches allows to distinguish low‐ and high‐risk groups among immunological standard‐risk renal allograft recipients. This might help to better tailor immunosuppression to the individual needs of patients. However, it requires validation in independent cohorts and ultimately a prospective randomised trial to investigate whether such an enhanced risk stratification improves clinical outcomes.
Author Contributions
C.J. and S.S. designed the study. G.H., C.W., H.H., T.M., P.A., and M.D. provided data and important knowledge. C.J., M.N., and S.S. performed the statistical analyses and wrote the first draft of the manuscript. All authors revised the manuscript.
Disclosure
Matthias Niemann is an employee of PIRCHE AG.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Appendix S1.
Acknowledgements
The authors would like to thank the teams of the renal transplant outpatient clinic and the laboratory for HLA diagnostics.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Tambur A. R., Campbell P., Chong A. S., et al., “Sensitization in Transplantation: Assessment of Risk (STAR) 2019 Working Group Meeting Report,” American Journal of Transplantation 20, no. 10 (2020): 2652–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bestard O., Couzi L., Crespo M., Kessaris N., and Thaunat O., “Stratifying the Humoral Risk of Candidates to a Solid Organ Transplantation: A Proposal of the ENGAGE Working Group,” Transplant International 34, no. 6 (2021): 1005–1018. [DOI] [PubMed] [Google Scholar]
- 3. Wiebe C., Ho J., Gibson I. W., Rush D. N., and Nickerson P. W., “Carpe Diem‐Time to Transition From Empiric to Precision Medicine in Kidney Transplantation,” American Journal of Transplantation 18, no. 7 (2018): 1615–1625. [DOI] [PubMed] [Google Scholar]
- 4. Yacoub R., Nadkarni G. N., Cravedi P., et al., “Analysis of OPTN/UNOS Registry Suggests the Number of HLA Matches and Not Mismatches Is a Stronger Independent Predictor of Kidney Transplant Survival,” Kidney International 93, no. 2 (2018): 482–490. [DOI] [PubMed] [Google Scholar]
- 5. Wehmeier C., Amico P., Sidler D., et al., “Pre‐Transplant Donor‐Specific HLA Antibodies and Risk for Poor First‐Year Renal Transplant Outcomes: Results From the Swiss Transplant Cohort Study,” Transplant International 34, no. 12 (2021): 2755–2768. [DOI] [PubMed] [Google Scholar]
- 6. Wehmeier C., Honger G., and Schaub S., “Caveats of HLA Antibody Detection by Solid‐Phase Assays,” Transplant International 33, no. 1 (2020): 18–29. [DOI] [PubMed] [Google Scholar]
- 7. Tambur A. R., Rosati J., Roitberg S., Glotz D., Friedewald J. J., and Leventhal J. R., “Epitope Analysis of HLA‐DQ Antigens: What Does the Antibody See?,” Transplantation 98, no. 2 (2014): 157–166. [DOI] [PubMed] [Google Scholar]
- 8. Crivello P., Zito L., Sizzano F., et al., “The Impact of Amino Acid Variability on Alloreactivity Defines a Functional Distance Predictive of Permissive HLA‐DPB1 Mismatches in Hematopoietic Stem Cell Transplantation,” Biology of Blood and Marrow Transplantation 21, no. 2 (2015): 233–241. [DOI] [PubMed] [Google Scholar]
- 9. Kramer C. S. M., Bezstarosti S., Franke‐van Dijk M. E. I., et al., “Antibody Verification of HLA Class I and Class II Eplets by Human Monoclonal HLA Antibodies,” HLA 103, no. 1 (2024): e15345. [DOI] [PubMed] [Google Scholar]
- 10. Duquesnoy R. J., “A Structurally Based Approach to Determine HLA Compatibility at the Humoral Immune Level,” Human Immunology 67, no. 11 (2006): 847–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Otten H. G., Calis J. J., Kesmir C., van Zuilen A. D., and Spierings E., “Predicted Indirectly Recognizable HLA Epitopes Presented by HLA‐DR Correlate With the de Novo Development of Donor‐Specific HLA IgG Antibodies After Kidney Transplantation,” Human Immunology 74, no. 3 (2013): 290–296. [DOI] [PubMed] [Google Scholar]
- 12. Wiebe C., Kosmoliaptsis V., Pochinco D., Taylor C. J., and Nickerson P., “A Comparison of HLA Molecular Mismatch Methods to Determine HLA Immunogenicity,” Transplantation 102, no. 8 (2018): 1338–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiebe C., Kosmoliaptsis V., Pochinco D., et al., “HLA‐DR/DQ Molecular Mismatch: A Prognostic Biomarker for Primary Alloimmunity,” American Journal of Transplantation 19, no. 6 (2019): 1708–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lachmann N., Niemann M., Reinke P., et al., “Donor‐Recipient Matching Based on Predicted Indirectly Recognizable HLA Epitopes Independently Predicts the Incidence of De Novo Donor‐Specific HLA Antibodies Following Renal Transplantation,” American Journal of Transplantation 17, no. 12 (2017): 3076–3086. [DOI] [PubMed] [Google Scholar]
- 15. Senev A., Coemans M., Lerut E., et al., “Eplet Mismatch Load and De Novo Occurrence of Donor‐Specific Anti‐HLA Antibodies, Rejection, and Graft Failure After Kidney Transplantation: An Observational Cohort Study,” Journal of the American Society of Nephrology 31, no. 9 (2020): 2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Senev A., Van Loon E., Lerut E., et al., “Association of Predicted HLA T‐Cell Epitope Targets and T‐Cell‐Mediated Rejection After Kidney Transplantation,” American Journal of Kidney Diseases 80, no. 6 (2022): 718–729 e1. [DOI] [PubMed] [Google Scholar]
- 17. Geneugelijk K., Niemann M., Drylewicz J., et al., “PIRCHE‐II Is Related to Graft Failure After Kidney Transplantation,” Frontiers in Immunology 9 (2018): 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakamoto S., Iwasaki K., Tomosugi T., et al., “Analysis of T and B Cell Epitopes to Predict the Risk of de Novo Donor‐Specific Antibody (DSA) Production After Kidney Transplantation: A Two‐Center Retrospective Cohort Study,” Frontiers in Immunology 11 (2020): 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mangiola M., Ellison M. A., Marrari M., et al., “Immunologic Risk Stratification of Pediatric Heart Transplant Patients by Combining HLAMatchmaker and PIRCHE‐II,” Journal of Heart and Lung Transplantation 41, no. 7 (2022): 952–960. [DOI] [PubMed] [Google Scholar]
- 20. Ellison M., Mangiola M., Marrari M., et al., “Immunologic Risk Stratification of Pediatric Heart Transplant Patients by Combining HLA‐EMMA and PIRCHE‐II,” Frontiers in Immunology 14 (2023): 1110292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Honger G., Niemann M., Schawalder L., et al., “Toward Defining the Immunogenicity of HLA Epitopes: Impact of HLA Class I Eplets on Antibody Formation During Pregnancy,” HLA 96, no. 5 (2020): 589–600. [DOI] [PubMed] [Google Scholar]
- 22. Schawalder L., Honger G., Kleiser M., et al., “Development of an Immunogenicity Score for HLA‐DQ Eplets: A Conceptual Study,” HLA 97, no. 1 (2021): 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kardol‐Hoefnagel T., Senejohnny D. M., Kamburova E. G., et al., “Determination of the Clinical Relevance of Donor Epitope‐Specific HLA‐Antibodies in Kidney Transplantation,” HLA 103, no. 1 (2024): e15346. [DOI] [PubMed] [Google Scholar]
- 24. Mohammadhassanzadeh H., Oualkacha K., Zhang W., et al., “On Path to Informing Hierarchy of Eplet Mismatches as Determinants of Kidney Transplant Loss,” Kidney International Reports 6, no. 6 (2021): 1567–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kramer C., Heidt S., and Claas F. H. J., “Towards the Identification of the Relative Immunogenicity of Individual HLA Antibody Epitopes,” Human Immunology 80, no. 4 (2019): 218–220. [DOI] [PubMed] [Google Scholar]
- 26. Tambur A. R., McDowell H., Hod‐Dvorai R., Abundis M. A. C., and Pinelli D. F., “The Quest to Decipher HLA Immunogenicity: Telling Friend From Foe,” American Journal of Transplantation 19, no. 10 (2019): 2910–2925. [DOI] [PubMed] [Google Scholar]
- 27. Bielmann D., Honger G., Lutz D., Mihatsch M. J., Steiger J., and Schaub S., “Pretransplant Risk Assessment in Renal Allograft Recipients Using Virtual Crossmatching,” American Journal of Transplantation 7, no. 3 (2007): 626–632. [DOI] [PubMed] [Google Scholar]
- 28. Senn L., Wehmeier C., Honger G., et al., “Outcome of Husband‐To‐Wife Kidney Transplantation With Mutual Children: Single Center Experience Using T Cell‐Depleting Induction and Review of the Literature,” Frontiers in Medicine 8 (2021): 724851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loupy A., Haas M., Roufosse C., et al., “The Banff 2019 Kidney Meeting Report (I): Updates on and Clarification of Criteria for T Cell‐ and Antibody‐Mediated Rejection,” American Journal of Transplantation 20, no. 9 (2020): 2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wehmeier C., Amico P., Hirt‐Minkowski P., et al., “Acute Rejection Phenotypes in the Current Era of Immunosuppression: A Single‐Center Analysis,” Transplantation Direct 3, no. 3 (2017): e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirt‐Minkowski P., Handschin J., Stampf S., et al., “Randomized Trial to Assess the Clinical Utility of Renal Allograft Monitoring by Urine CXCL10 Chemokine,” Journal of the American Society of Nephrology 34, no. 8 (2023): 1456–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maguire C., Crivello P., Fleischhauer K., et al., “Qualitative, Rather Than Quantitative, Differences Between HLA‐DQ Alleles Affect HLA‐DQ Immunogenicity in Organ Transplantation,” HLA 103, no. 4 (2024): e15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kramer C. S. M., Franke‐van Dijk M. E. I., Bakker K. H., et al., “Generation and Reactivity Analysis of Human Recombinant Monoclonal Antibodies Directed Against Epitopes on HLA‐DR,” American Journal of Transplantation 20 (2020): 3341–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiebe C., Rush D. N., Nevins T. E., et al., “Class II Eplet Mismatch Modulates Tacrolimus Trough Levels Required to Prevent Donor‐Specific Antibody Development,” Journal of the American Society of Nephrology 28, no. 11 (2017): 3353–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wiebe C., Balshaw R., Gibson I. W., et al., “A Rational Approach to Guide Cost‐Effective de Novo Donor‐Specific Antibody Surveillance With Tacrolimus Immunosuppression,” American Journal of Transplantation 23, no. 12 (2023): 1882–1892. [DOI] [PubMed] [Google Scholar]
- 36. Engen R. M., Jedraszko A. M., Conciatori M. A., and Tambur A. R., “Substituting Imputation of HLA Antigens for High Resolution HLA Typing: Evaluation of a Multiethnic Population and Implications for Clinical Decision Making in Transplantation,” American Journal of Transplantation 21, no. 1 (2020): 344–352. [DOI] [PubMed] [Google Scholar]
- 37. Senev A., Emonds M. P., Van Sandt V., et al., “Clinical Importance of Extended Second Field High‐Resolution HLA Genotyping for Kidney Transplantation,” American Journal of Transplantation 20, no. 12 (2020): 3367–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devriese M., Da Silva S., Le Mene M., et al., “Two‐Field Resolution On‐Call HLA Typing for Deceased Donors Using Nanopore Sequencing,” HLA 103, no. 3 (2024): e15441. [DOI] [PubMed] [Google Scholar]
- 39. Rampersad C., Balshaw R., Gibson I. W., et al., “The Negative Impact of T Cell‐Mediated Rejection on Renal Allograft Survival in the Modern Era,” American Journal of Transplantation 22, no. 3 (2022): 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tambur A. R. and Das R., “Can we Use Eplets (Or Molecular) Mismatch Load Analysis to Improve Organ Allocation? The Hope and the Hype,” Transplantation 107, no. 3 (2023): 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


