Abstract
Purpose
Diabetes mellitus (DM) negatively impacts chronic hepatitis B patients, but its role in those with HBV-related hepatocellular carcinoma (HCC) undergoing ablation remains unclear. This study aims to evaluate the influence of DM on recurrence patterns and overall survival (OS) among patients with HBV-related HCC undergoing ablation.
Patients and Methods
We retrospectively enrolled 372 patients receiving thermal ablation for HBV-related HCC, including 96 (25.8%) patients with DM. Factors associated with local tumor progression (LTP), distant recurrence, and OS were analyzed. The prognostic value of DM in IMbrave050-defined high-risk population was validated.
Results
DM did not correlate with LTP, whereas patients with DM had significantly higher risk of distant recurrence (median time to recurrence 23.7 versus 46.2 months, p=0.032), poorer OS (median OS 75.6 versus 106 months, p=0.011), and poorer post-recurrence survival (70.7 versus 106 months, p=0.009). In multivariate analysis, DM (hazard ratio (HR)=1.466, p=0.012), FIB-4 score, multiple tumors, and AFP level were independent predictors of distant recurrence, while DM (HR=1.424, p=0.028), ALBI score, tumor size, AFP and creatinine levels were significantly associated with OS. A DM-based risk score effectively discriminated the risk of distant recurrence. The IMbrave050 criteria could stratify the risk of LTP but not distant recurrence. DM status further discriminated the risk of distant recurrence and mortality in the IMbrave050-defined high-risk population.
Conclusion
Patients with DM had an increased risk of distant recurrence and mortality after thermal ablation for HBV-related HCC, highlighting the importance of increasing awareness of DM and implementing rigorous post-ablation monitoring for diabetic HCC patients.
Keywords: hepatitis B virus, hepatocellular carcinoma, diabetes mellitus, ablation, recurrence, survival
Introduction
Thermal ablation therapies, such as radiofrequency ablation (RFA) and microwave ablation (MWA), are among the first-line loco-regional treatments for early-stage hepatocellular carcinoma (HCC).1 These therapies provide a significant survival advantage, achieving over a 90% complete response rate and approximately a 70% 5-year survival rate, even among patients unsuitable for resection.1,2 Nevertheless, HCC recurrence following thermal ablation treatment arises in up to 80% of cases within five years.3–5
Notably, the patterns of recurrence differ between ablation and resection, particularly in terms of local tumor progression (LTP) and distant recurrence. LTP, occurring near the ablation site, often results from insufficient ablation or aggressive tumor characteristics. Conversely, distant recurrence is generally associated with metastatic activity, indicative of the tumor’s inherent properties or new carcinogenesis, especially in patients with cirrhosis, active viral hepatitis, or metabolic risk factors.2,6 Identifying predictive factors for these distinct HCC recurrence patterns after thermal ablation remains a critical challenge in clinical practice.
Chronic hepatitis B virus (HBV) infection is the major risk factor for HCC and contributes to majority of HCC cases in the highly endemic Asia-Pacific region. Additionally, the prevalence of type 2 diabetes mellitus (DM) has been steadily increasing in the general population as well as in patients with chronic hepatitis B (CHB).7 Both viral and metabolic factors may contribute to disease progression in CHB patients.8 Epidemiologic studies have revealed that patients with DM have an elevated risk of developing HCC, including those with CHB.9 Recent studies have demonstrated that the presence of DM is associated with an increased risk of HCC recurrence after surgical resection.10,11 Growing evidence indicates that diabetes-related risk factors, including hyperglycemia, hyperinsulinemia, obesity, metabolic dysfunction-associated steatotic liver disease, alterations in gut microbiota, and immune modulation, may contribute to hepatocarcinogenesis through multiple mechanisms.12 Additionally, increased viscoelasticity may serve as a predictive risk factor for more invasive characteristics of HCC in patients with DM.13 However, the impact of DM on the recurrence pattern and long-term outcomes after ablation for HBV-related HCC remains unclear. This study aims to evaluate the influence of DM on recurrence patterns and overall survival among patients with HBV-related HCC undergoing thermal ablation therapy.
Materials and Methods
Patients
This retrospective study enrolled patients diagnosed with HBV-related HCC who underwent curative RFA or MWA at Taipei Veterans General Hospital from October 1, 2007, to January 31, 2023 (Supplementary Figure 1). Inclusion criteria included individuals aged 20 years or older, testing positive for HBsAg, and receiving RFA or MWA as the primary treatment for HCC. Exclusion criteria consisted of patients who did not achieve complete tumor ablation in a single session, had mixed HCC-cholangiocarcinoma, underwent concurrent treatments for HCC, were classified as BCLC stage C, or died or were lost to follow-up within 3 months post-ablation. HCC diagnosis was established before ablation using contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) following the diagnostic guidelines of the American Association for the Study of Liver Diseases (AASLD).1 Alternatively, pathological confirmation through percutaneous liver biopsy was conducted if the imaging criteria were not met. Complete ablation, defined as the absence of residual viable tumor, was confirmed by CECT or MRI one month post-ablation. After achieving complete ablation, patients underwent follow-up assessments every 3 months during the initial 2 years, and subsequently every 4–6 months, including routine evaluations of hepatic functions, alpha-fetoprotein (AFP) levels, ultrasound scans, and CECT and/or MRI imaging. Tumor recurrence was confirmed through CECT or MRI scans.
This study adhered to the ethical guidelines and principles of the Helsinki Declaration and received approval from the Institutional Review Board of Taipei Veterans General Hospital (IRB number: 2023–03-004CC). Due to the retrospective nature of the study, the Institutional Review Board waived the need for written informed consent.
Outcome Assessment
The primary endpoints of this study included local tumor progression (LTP) and distant recurrence. LTP was defined as the presence of tumor foci at the margin of the ablation zone, whereas distant recurrence was defined as the presence of tumor foci outside the ablation zone or the presence of extrahepatic metastasis, as confirmed by follow-up CT or MRI scans following complete ablation.14 The dates of first LTP and distant recurrence were documented as separate endpoints. Secondary endpoints included overall survival (OS), defined as the duration from initial complete ablation to death, and post-recurrence survival, defined as the duration from the time of recurrence to death.
Clinical Information
Baseline clinical parameters were collected before the initial ablation session, including age, gender, DM status, medications for DM, nucleos(t)ide analogue (NUC) treatment, body mass index (BMI), Barcelona Clinic Liver Cancer (BCLC) stage, Child-Pugh score, serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, creatinine, total bilirubin, platelet counts, hemoglobin A1c (HbA1c) and alpha-fetoprotein (AFP) levels. Serum AFP levels were quantified using a chemiluminescent microparticle immunoassay (ARCHITECT AFP assay, Abbott Ireland Diagnostics Division, Sligo, Ireland). Biochemical analyses were conducted using a systemic multi-autoanalyzer (Technicon SMAC, Technicon Instruments Corp., Tarrytown, NY). Quantitative HBsAg level was measured by the Elecsys HBsAg II assay (Roche Diagnostics, Mannheim, Germany) or Abbott Architect HBsAg assay (Abbott Diagnostics, Abbott Park, IL) with a detection limit of 0.05 IU/mL.15 Serum HBV DNA level was measured by Roche Cobas Taqman HBV DNA assay (Roche Diagnostics, Switzerland) with a detection limit of 20 IU/mL. The Fibrosis-4 index (FIB-4) and Albumin-Bilirubin (ALBI) grade were computed as previously described.16,17 Type 2 DM was diagnosed based on plasma glucose criteria following the American Diabetes Association guidelines.18
All tumors were assessed based on pre-ablation CT, MRI, or ultrasound images to determine their proximity to the liver capsule or major blood vessels. A subcapsular tumor was defined as a tumor with its nearest margin less than 5mm from the liver capsule.19,20 A perivascular tumor was characterized as a tumor located within 5mm of a blood vessel with a diameter of at least 3mm.20,21
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics V26 (IBM, Armonk, NY). Descriptive statistics are presented as median (interquartile range, IQR) or mean ± standard deviation, as appropriate. Continuous variables were compared using the Mann–Whitney U-test, while categorical variables were compared using Pearson’s chi-square analysis or Fisher’s exact test, as appropriate. Missing data were handled using complete case analysis. Recurrence and survival rates were estimated using the Kaplan-Meier method, and survival curves were compared using the Log rank test. Prognostic factors for local tumor progression, distant recurrence, and overall survival were analyzed using Cox proportional-hazards models. Variables with p<0.1 in univariate analysis were included in multivariate analysis using a forward stepwise Cox proportional-hazards model. A two-tailed p<0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 461 consecutive patients diagnosed with HBV-related HCC undergoing RFA or MWA were initially screened. After excluding 89 patients based on predefined criteria, a total of 372 patients were considered eligible for analysis, including 96 (25.8%) patients with a diagnosis of DM (Supplementary Figure 1). Table 1 provides an overview of their baseline characteristics. Patients with DM exhibited significantly higher BMI and creatinine levels, whereas the quantitative HBsAg levels were significantly lower. The majority of patients in the cohort presented with a single tumor, Child-Pugh class A, and received NUC antiviral therapy for CHB after ablation. The distribution of Child-Pugh class, ALBI grades, and BCLC stages between the two groups was similar.
Table 1.
Baseline Characteristics of the 372 Patients with HBV-Related HCC Undergoing Thermal Ablation
| Characteristics | Overall (n = 372) | DM (n = 96; 25.8%) | No DM (n = 276; 74.2%) | P |
|---|---|---|---|---|
| Age (years) | 66.4 ± 12.2 | 66.7 ± 10.9 | 66.4 ± 12.7 | 0.936 |
| Sex (male), n (%) | 266 (71.5) | 71 (74.0) | 195 (70.7) | 0.536 |
| BMI (kg/m2) | 25.2 ± 4.5 | 26.7 ± 4.2 | 24.6 ± 4.5 | <0.001 |
| BMI >25 | 178 (48.5) | 60 (63.8) | 118 (43.2) | 0.001 |
| HbA1c (%) | – | 7.5 ± 1.9 | – | – |
| Metformin use (%) | – | 44 (45.8) | – | – |
| Child-Pugh class A/B, n (%) | 342/29 (92.2/7.8) | 87/8 (91.6/8.4) | 255/21 (92.4/7.6) | 0.799 |
| HBsAg (log IU/mL)a | 2.18 ± 1.28 | 1.86 ± 1.62 | 2.30 ± 1.12 | 0.036 |
| HBsAg <100 IU/mL, n (%) | 89 (32.0) | 32 (42.1) | 57 (28.8) | 0.039 |
| HBV DNA (log IU/mL)a | 4.77 ± 1.82 | 4.57 ± 1.88 | 4.83 ± 1.81 | 0.498 |
| Undetectable HBV DNA, n (%) | 122 (41.2) | 35 (46.1) | 87 (39.5) | 0.320 |
| HBV DNA >2000 IU/mL | 133 (44.9) | 28 (36.8) | 105 (47.7) | 0.100 |
| HBeAg-positive, n (%) | 26 (7.0) | 7 (7.3) | 19 (6.9) | 0.893 |
| Nucleos(t)ide analog treatment, n (%) | 286 (76.9) | 72 (75.0) | 214 (77.5) | 0.612 |
| BCLC stage 0/A/B, n (%) | 179/187/6 (48.1/50.3/1.6) | 40/54/2 (41.7/56.3/2.1) | 139/133/4 (50.4/48.2/1.4) | 0.330 |
| Tumor size (cm) | 2.15 ± 0.81 | 2.20 ± 0.76 | 2.13 ± 0.83 | 0.390 |
| Tumor size >2 cm, n (%) | 170 (45.7) | 49 (51.0) | 121 (43.8) | 0.222 |
| Tumor numbers 1/2/3, n (%) | 328/35/9 (88.2/9.4/2.4) | 83/8/5 (86.5/8.3/5.2) | 245/27/4 (88.8/9.8/1.4) | 0.113 |
| AFP (ng/mL) | 9.8 (4.3–46.7) | 8.3 (4.1–42.2) | 10.2 (4.4–51.3) | 0.363 |
| Albumin (g/dL) | 3.9 ± 0.6 | 3.9 ± 0.6 | 3.9 ± 0.6 | 0.321 |
| Total bilirubin (mg/dL) | 0.88 ± 0.68 | 0.81 ± 0.58 | 0.90 ± 0.71 | 0.264 |
| ALBI score | −2.602 ± 0.570 | −2.569 ± 0.554 | −2.614 ± 0.576 | 0.507 |
| ALBI grade 1/2/3, n (%) | 216/140/15 (58.2/37.7/4.0) | 49/42/4 (51.6/44.2/4.2) | 167/98/11 (60.5/35.5/4.0) | 0.302 |
| Platelet count (109/L) | 135 ± 61 | 125 ± 48 | 138 ± 65 | 0.099 |
| ALT (U/L) | 42.6 ± 35.4 | 41 ± 29 | 43 ± 38 | 0.566 |
| AST (U/L) | 44.9 ± 32.3 | 41 ± 21 | 46 ± 35 | 0.721 |
| FIB-4 score | 4.70 ± 4.25 | 4.22 ± 2.34 | 4.86 ± 4.72 | 0.409 |
| Creatinine (mg/dL) | 1.10 ± 1.17 | 1.32 ± 1.77 | 1.04 ± 0.86 | 0.039 |
| RFA/MWA, n (%) | 364/8 (97.8/2.2%) | 93/3 (96.9%/3.1%) | 271/5 (98.2%/1.8%) | 0.430 |
| Difficult tumor location, n (%) | 273 (73.4) | 69 (71.9) | 204 (73.9) | 0.697 |
| Subcapsular, n (%) | 148 (39.8) | 36 (37.5) | 122 (40.6) | 0.595 |
| Near gallbladder or hilum, n (%) | 26 (7.0) | 6 (6.3) | 20 (7.2) | 0.742 |
| Near bowel, n (%) | 18 (4.8) | 8 (8.3) | 10 (3.6) | 0.064 |
| Near major vessel, n (%) | 96 (25.8) | 23 (24.0) | 73 (26.4) | 0.631 |
| Median follow-up, months | 68.8 (30.4–100.4) | 50.1 (25.1–102.1) | 63.2 (32.8–99.1) | 0.148 |
| HCC recurrence, n (%) | 257 (69.1) | 72 (75.0) | 185 (67.0) | 0.145 |
| Local tumor progression, n (%) | 141 (37.9) | 35 (36.5) | 106 (38.4) | 0.735 |
| Distant recurrence, n (%) | 222 (59.7) | 63 (65.6) | 159 (57.6) | 0.168 |
| Death, n (%) | 192 (51.6) | 59 (61.5) | 133 (48.2) | 0.025 |
Notes: aAvailable baseline quantitative HBsAg data, n=278; available HBV DNA data, n=296; available HbA1c data, n=58.
Abbreviations: BMI, body mass index; RFA, radiofrequency ablation; MWA, microwave ablation; ALBI, Albumin-Bilirubin.
Impact of DM on HCC Recurrence Pattern
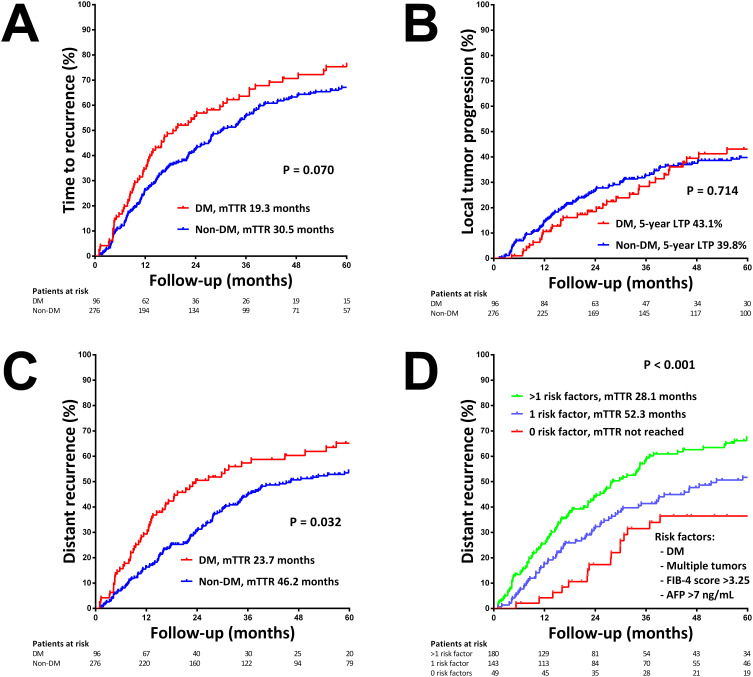
During a median follow-up period of 68.8 months, tumor recurrence was observed in 257 (68.1%) patients following ablation, consisting of 141 patients with local tumor progression (LTP) and 222 patients with distant recurrence. The 5-year cumulative recurrence rate was 75.3% for patients with DM and 67.1% for patients without DM (p=0.070, Figure 1A).
Figure 1.
Kaplan-Meier curves for HCC recurrence following thermal ablation. (A) Cumulative incidence of HCC recurrence in patients with and without diabetes mellitus (DM). (B) Cumulative incidence of local tumor progression (LTP) in patients with and without DM. (C) Cumulative incidence of distant recurrence in patients with and without DM. (D) Cumulative incidence of distant recurrence stratified by the number of risk factors present, including DM, multiple tumors, FIB-4 score >3.25, and AFP >7 ng/mL.
Abbreviation: mTTR, median time to recurrence.
The rates of LTP were similar between patients with and without DM, with 5-year LTP rates of 43.1% and 39.8%, respectively (p=0.714, Figure 1B). Multivariate analysis identified tumor size (hazard ratio (HR)=1.444, p<0.001) and AFP (log10 transformed, HR=1.277, p=0.020) as independent predictors of LTP (Supplementary Table 1).
Notably, diabetic patients exhibited a significantly higher risk of distant recurrence compared to non-diabetic patients, with a median time to distant recurrence of 23.7 months versus 46.2 months (p=0.032, Figure 1C). In multivariate analysis, DM (HR=1.466, p=0.012), FIB-4 score (HR=1.035, p=0.009), multiple tumors (HR=1.680, p=0.012) and AFP (log10 transformed, HR=1.206, p=0.025) were identified as independent predictors of distant recurrence (Table 2).
Table 2.
Univariate and Multivariate Analyses of Factors Associated with Distant Recurrence
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.001 | 0.990–1.012 | 0.849 | |||
| Gender | ||||||
| Male vs Female | 1.150 | 0.850–1.555 | 0.365 | |||
| Diabetes mellitus | ||||||
| Yes vs no | 1.375 | 1.027–1.842 | 0.033 | 1.466 | 1.087–1.978 | 0.012 |
| BMI | 0.997 | 0.967–1.029 | 0.868 | |||
| FIB-4 score | 1.034 | 1.009–1.061 | 0.008 | 1.035 | 1.008–1.062 | 0.009 |
| ALBI score | 1.364 | 1.078–1.725 | 0.010 | NS | ||
| HBV DNA | ||||||
| Detectable vs undetectable | 1.060 | 0.788–1.425 | 0.701 | |||
| qHBsAg (IU/mL) | 0.979 | 0.872–1.099 | 0.719 | |||
| HBeAg | ||||||
| Positive vs negative | 1.032 | 0.628–1.695 | 0.902 | |||
| NUC therapy | ||||||
| Yes vs No | 1.045 | 0.751–1.454 | 0.795 | |||
| Tumor size | 1.143 | 0.980–1.332 | 0.088 | NS | ||
| Tumor number | ||||||
| Multiple vs single | 1.554 | 1.043–2.314 | 0.030 | 1.680 | 1.119–2.524 | 0.012 |
| AFP (log ng/mL) | 1.204 | 1.028–1.410 | 0.021 | 1.206 | 1.023–1.422 | 0.025 |
| Creatinine (mg/dL) | 0.909 | 0.710–1.163 | 0.448 | |||
| ALT | 1.005 | 1.000–1.011 | 0.040 | NS | ||
| AST | 1.009 | 1.003–1.014 | 0.003 | NS | ||
| Tumor location | ||||||
| Subcapsular | 1.230 | 0.942–1.607 | 0.128 | |||
| Near gallbladder or hilum | 1.228 | 0.748–2.016 | 0.417 | |||
| Near bowel | 1.233 | 0.703–2.162 | 0.465 | |||
| Near major vessel | 1.055 | 0.782–1.424 | 0.727 | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; NS, not significant; ALBI, Albumin-Bilirubin.
A simple risk model was proposed based on four independent predictors of distant recurrence. The cutoff levels were determined using usual clinical thresholds: FIB-4 score >3.25, indicating advanced fibrosis, and AFP >20 ng/mL, the upper limit of normal in clinical practice. One point was assigned for each of the following factors: presence of DM, multiple tumors, FIB-4 score >3.25, and AFP >20 ng/mL, given the similar hazard ratio for each factor (Supplementary Table 2). Patients were categorized as low- (score 0), intermediate- (score 1), and high-risk (score 2–4) for distant recurrence. This simple DM-based risk model effectively discriminated the risk of distant recurrence in HCC patients, with 5-year cumulative distant recurrence rates of 36.5%, 51.7%, and 67.1% in the low-, intermediate-, and high-risk groups, respectively (p<0.001, Figure 1D).
Impact of DM on Overall Survival
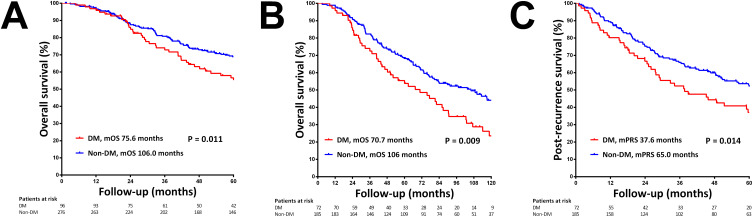
During the follow-up period, 192 (51.6%) patients died. Patients with DM exhibited significantly poorer survival compared to those without DM, with median OS of 75.6 and 106 months, respectively (p=0.011, Figure 2A). Multivariate analysis indicated that DM (HR=1.424, p=0.028), ALBI score (HR=2.078, p<0.001), tumor size (HR=1.203, p=0.019), AFP (log10 transformed, HR=1.404, p<0.001), and creatinine level (HR=1.234, p<0.001) were significantly associated with OS (Table 3).
Figure 2.
Kaplan-Meier curves for overall survival (OS) following thermal ablation. (A) OS in patients with and without diabetes mellitus (DM). (B) OS in patients with HCC recurrence stratified by DM status. (C) Post-recurrence survival in patients with and without DM.
Abbreviations: mOS, median OS; mPRS, median post-recurrence survival.
Table 3.
Univariate and Multivariate Analyses of Factors Associated with Overall Survival
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.035 | 1.023–1.048 | <0.001 | 1.041 | 1.027–1.055 | <0.001 |
| Gender | ||||||
| Male vs Female | 1.010 | 0.733–1.393 | 0.950 | |||
| Diabetes mellitus | ||||||
| Yes vs no | 1.484 | 1.091–2.019 | 0.012 | 1.424 | 1.039–1.950 | 0.028 |
| BMI | 0.998 | 0.947–1.051 | 0.940 | |||
| FIB-4 score | 1.037 | 1.015–1.060 | 0.001 | NS | ||
| ALBI score | 2.092 | 1.645–2.659 | <0.001 | 2.078 | 1.600–2.698 | <0.001 |
| HBV DNA | ||||||
| Detectable vs undetectable | 0.935 | 0.688–1.308 | 0.694 | |||
| qHBsAg (IU/mL) | 0.910 | 0.804–1.031 | 0.138 | |||
| HBeAg | ||||||
| Positive vs negative | 0.997 | 0.588–1.692 | 0.993 | |||
| NUC therapy | ||||||
| Yes vs No | 0.633 | 0.464–0.864 | 0.004 | NS | ||
| Tumor size | 1.381 | 1.187–1.606 | <0.001 | 1.203 | 1.030–1.404 | 0.019 |
| Tumor number | ||||||
| Multiple vs single | 1.318 | 0.852–2.041 | 0.215 | |||
| AFP (log ng/mL) | 1.254 | 1.048–1.550 | 0.013 | 1.404 | 1.161–1.698 | <0.001 |
| Creatinine (mg/dL) | 1.156 | 1.073–1.246 | <0.001 | 1.234 | 1.141–1.336 | <0.001 |
| ALT | 1.001 | 0.993–1.008 | 0.826 | |||
| AST | 1.006 | 0.998–1.014 | 0.116 | |||
| Tumor location | ||||||
| Subcapsular | 1.033 | 0.774–1.380 | 0.825 | |||
| Near gallbladder or hilum | 1.870 | 1.175–2.977 | 0.008 | NS | ||
| Near bowel | 1.152 | 0.626–2.119 | 0.650 | |||
| Near major vessel | 0.883 | 0.630–1.237 | 0.469 | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; NS, not significant; ALBI, Albumin-Bilirubin.
Among patients experiencing HCC recurrence, OS was significantly worse in those with DM (70.7 versus 106 months, p=0.009, Figure 2B). Further analysis revealed that patients with DM exhibited significantly poorer post-recurrence survival compared to those without DM (37.6 versus 65 months, p=0.014, Figure 2C).
Impact of DM on IMbrave050-Defined High-Risk Patients
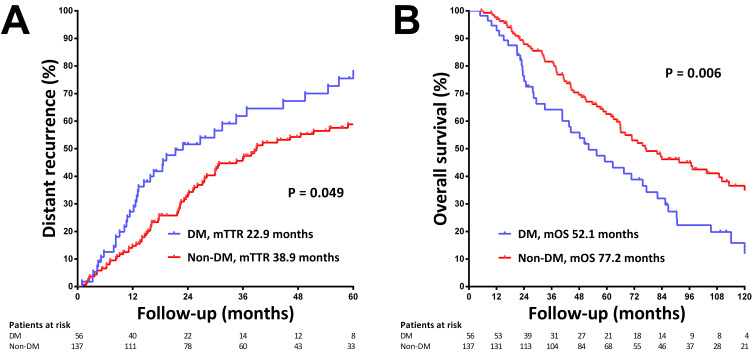
We validated the IMbrave050 high-risk criteria concerning HCC recurrence pattern and survival following thermal ablation. The IMbrave050-defined high-risk patients exhibited a significantly higher risk of LTP (5-year LTP rate 48.3% versus 31.9%, p=0.001, Supplementary Figure 2A). However, the IMbrave050 criteria were less effective in discriminating the risk of distant recurrence, particularly within the first two years (5-year distant recurrence rate 63.7% versus 49.9%, p=0.056, Supplementary Figure 2B). Within the IMbrave050-defined high-risk group, the presence of DM further stratified the risk of distant recurrence (median time to distant recurrence 22.9 versus 38.9 months, p=0.049, Figure 3A).
Figure 3.
The risk of distant recurrence and survival stratified by diabetes mellitus (DM) status among IMbrave050-defined high-risk patients. (A) Cumulative incidence of distant recurrence in patients with and without DM. (B) Overall survival in patients with and without DM.
Abbreviations: mTTR, median time to recurrence; mOS, median OS.
The IMbrave050 criteria significantly stratified overall survival (median OS 70.9 versus 128.7 months, p<0.001, Supplementary Figure 2C). Moreover, within the IMbrave050-defined high-risk group, the presence of DM further stratified OS (median OS 52.1 versus 77.2 months, p=0.006, Figure 3B).
Impact of Metformin Use and Glycemic Control in Diabetic Patients
Among patients with DM, 45.8% received metformin treatment, and 53.4% achieved adequate DM control with HbA1c <7%. We did not observe a significant correlation between metformin use and the risk of distant recurrence or OS in diabetic patients (Supplementary Figure 3A and B). Similarly, there was no correlation between glycemic control, as presented by HbA1c level, and the risk of distant recurrence or OS (Supplementary Figure 3C and D).
Discussion
The full impact of DM on recurrence pattern and long-term survival outcomes in HBV-related HCC patients undergoing thermal ablation remains incompletely understood. In this study, we demonstrated that DM is associated with significantly higher risks of distant recurrence and mortality. Notably, DM further stratifies the risk of distant recurrence and mortality in the IMbrave050-defined high-risk population and leads to poor survival outcomes after recurrence. Identifying significant predictors of recurrence and survival could optimize monitoring strategies post-ablation for HBV-related HCC and potentially facilitate the selection of ideal candidates for adjuvant immunotherapy.
In this study, the prevalence of DM was around 25% among patients with HBV-related HCC, indicating that DM is a growing metabolic disorder not only among CHB patients but also among those with HBV-related HCC.22 Patients with DM exhibited poorer renal function, a known predictor of poor survival, underscoring the additional adverse impacts of DM on both hepatic and extrahepatic systems.22 Interestingly, diabetic patients had significantly lower HBsAg levels. While metabolic factors such as obesity and hepatic steatosis have been shown to correlate with HBsAg seroclearance,23 the impact of diabetes on HBsAg seroclearance remains unclear. Nevertheless, a high glycemic burden may contribute to HCC development and fibrosis progression among CHB patients with DM.24 In this study, 46.6% of HCC patients with diabetes had a baseline HbA1c >7%, highlighting the importance of glycemic control awareness in patients with CHB and HCC.
Although some studies have shown that DM is associated with a higher recurrence rate in HCC patients undergoing resection,10,11 limited data exist regarding the impact of DM on recurrence patterns in patients with HBV-related HCC undergoing thermal ablation. Notably, there are distinct recurrence patterns between surgical resection and thermal ablation. In this study, we demonstrated that DM significantly correlates with distant recurrence, while its impact on LTP is neutral. Our study is the first to delineate the differential effects of DM on HCC recurrence patterns following ablation for HBV-related HCC. The increased risk of distant recurrence in diabetic patients may reflect a higher rate of de novo HCC occurrence post-ablation. Insulin resistance and hyperinsulinemia, hallmarks of DM, can lead to increased expression of pro-inflammatory cytokines such as interleukin-6 and tumor necrosis factor-α, as well as elevated levels of reactive oxygen species.6 These factors collectively contribute to hepatic inflammation, oxidative stress and the subsequent development of HCC. The upregulation of insulin-like growth factor 1 associated with insulin resistance has also been shown to altered cell cycle regulation, promoting autophagy and thereby contributing to the carcinogenesis and invasiveness of HCC.12 DM may also promote HCC progression through enhanced extracellular matrix viscoelasticity independent of liver fibrosis.13
The negative impact of DM on overall survival among patients with CHB and HCC patients has been documented.25 However, few studies have focused on patients undergoing thermal ablation. Our study fills this gap by demonstrating a comparable survival disadvantage of DM in patients with HBV-related HCC undergoing thermal ablation. We observed the negative impact of DM, especially in patients who developed HCC recurrence. DM contributes to a higher risk of distant recurrence and worse post-recurrence survival.
Adjuvant therapies have become available for patients at high risk of recurrence after ablation.26 In the IMbrave050 trial, the criteria for selecting candidates for immunotherapy based on recurrence risk were defined solely by tumor size and number in patients undergoing thermal ablation.27 However, multiple factors contribute to different recurrence patterns after ablation, and the optimal criteria for selecting high-risk candidates for adjuvant therapy remain unclear. In this study, we validated the IMbrave050 criteria in patients with HBV-related HCC undergoing thermal ablation and showed that while IMbrave050 effectively discriminates the risk of LTP, its predictive ability for distant recurrence is unsatisfactory. In addition to tumor number, DM status, FIB-4 score, and AFP levels are important predictors of distant recurrence, but these factors are neglected in the IMbrave050 criteria. Integrating DM and other independent risk factors can effectively discriminate the risk of distant recurrence after thermal ablation. Moreover, DM status can further stratify the risk of distant recurrence and survival in the IMbrave050-defined high-risk population.
The use of antidiabetic drugs may act as confounding factors in assessing HCC treatment outcomes in patients with DM. Antidiabetic medications might interfere the outcomes of HCC, either through direct effects on the disease process or by influencing metabolic parameters. In the last decade, increasing evidence has suggested that metformin may reduce the risk of HCC.28,29 However, we did not observe a correlation between metformin use and the risk of recurrence and survival in HCC patients with DM. Glucagon-like peptide-1 receptor agonists have recently been shown to be associated with a reduced risk of incident HCC and hepatic decompensation compared to other anti-diabetes medications in patients with DM.30 The association between newer antidiabetic agents and the risk of HCC recurrence warrants further study. Although glycemic control is associated with adverse hepatic outcomes in CHB patients with DM,24 we did not show a significant correlation between baseline HbA1c levels and outcomes in patients with HBV-related HCC. The prognostic value and strategy of glycemic control in patients with HBV-related HCC warrant future research.
HBV viral load and antiviral therapy have been shown to correlate with HCC recurrence after curative treatment.31,32 However, we did not observe a correlation between the HBV viral factors and outcomes of HCC after ablation. Since NUC therapy is now widely applied to the majority of patients with HBV-related HCC after ablation, the prognostic value of these viral factors might be mitigated.16
This study is subject to some limitations. First, the single-center nature of the study may limit the generalizability of the findings and introduce selection bias. Second, the sample size in the DM group is relatively small. Future multicenter studies with larger cohorts, diverse races and long-term follow-up are necessary to confirm our findings and to explore the impact of different geographic, ethnic, and treatment modality factors on the association between DM and HCC outcomes. Third, the retrospective study design may be subject to information bias, as complete data may not always be available. Finally, although we introduced a straightforward DM-based risk score to assess the risk of distant recurrence, this model requires further external validation to assess its predictive performance and generalizability.
Conclusion
In summary, DM significantly correlates with an increased risk of distant recurrence and mortality in patients with HBV-related HCC undergoing thermal ablation. DM status may further discriminate the risk of distant recurrence and mortality in the IMbrave050-defined high-risk population. With the rising prevalence of DM among patients with CHB and HCC, it is essential to increase awareness of DM and implement rigorous post-ablation monitoring and treatment approaches for diabetic HCC patients to enhance survival outcomes.
Acknowledgments
The authors thank the Clinical Research Core Laboratory, Taipei Veterans General Hospital for providing their facilities to conduct this study.
Funding Statement
The study was supported by grants from Taipei Veterans General Hospital, Taipei, Taiwan (V113C-157), and Ministry of Science and Technology, Taiwan (NSTC 112-2628-B-A49-016-MY3).
Abbreviations
DM, diabetes mellitus; HCC, hepatocellular carcinoma; RFS, recurrence-free survival; OS, overall survival; LTP, local tumor progression; HR, hazard ratio; RFA, radiofrequency ablation; WMA, microwave ablation; HBV, hepatitis B virus; CHB, chronic hepatitis B; BCLC, Barcelona Clinic Liver Cancer; CECT, contrast-enhanced computed tomography; MRI, magnetic resonance image; AASLD, American Association for the Study of Liver Disease; AFP, alpha-fetoprotein; NUC, nucleos(t)ide analogue; BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; ALBI, albumin-bilirubin; TTR, time to recurrence.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval Statement
Our study adhered to the prevailing ethical guidelines and principles of the Helsinki Declaration and received approval from the Institutional Review Board of Taipei Veterans General Hospital (IRB number: 2023-03-004CC).
Patient Consent Statement
The Institutional Review Board waived the need for written informed consent due to the retrospective nature of the study. All patient data were handled in accordance with institutional guidelines and relevant privacy regulations, and patient privacy was protected by anonymizing all identifiable information.
Disclosure
ICL has received honoraria from Gilead Sciences, Bristol-Meyers Squibb, Abbvie, Merck Sharp & Dohme, Bayer, Eisai, Eli Lilly, Ipsen, AstraZeneca and Roche, and has served in an advisory role for Gilead Sciences and AstraZeneca. YHH has received research grants from Gilead Sciences and Bristol-Meyers Squibb, and honoraria from Abbvie, Gilead Sciences, Bristol-Meyers Squibb, Ono Pharmaceutical, Merck Sharp & Dohme, Eisai, Eli Lilly, Ipsen, AstraZeneca and Roche, and has served in an advisory role for Abbvie, Gilead Sciences, Bristol-Meyers Squibb, Ono Pharmaceuticals, Eisai, Eli Lilly, Ipsen, Merck Sharp & Dohme, AstraZeneca and Roche. Other authors declare no conflicts of interest.
References
- 1.Singal AG, Llovet JM, Yarchoan M, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78(6):1922–1965. doi: 10.1097/HEP.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nault JC, Sutter O, Nahon P, Ganne-Carrie N, Seror O. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68(4):783–797. doi: 10.1016/j.jhep.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 3.Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107(4):569–577;quiz578. doi: 10.1038/ajg.2011.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YS, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58(1):89–97. doi: 10.1016/j.jhep.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 5.Lee IC, Huang YH, Chau GY, et al. Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int J Cancer. 2013;133(12):2895–2902. doi: 10.1002/ijc.28311 [DOI] [PubMed] [Google Scholar]
- 6.Talamantes S, Lisjak M, Gilglioni EH, Llamoza-Torres CJ, Ramos-Molina B, Gurzov EN. Non-alcoholic fatty liver disease and diabetes mellitus as growing aetiologies of hepatocellular carcinoma. JHEP Rep. 2023;5(9):100811. doi: 10.1016/j.jhepr.2023.100811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spradling PR, Simons B, Narayanan M, et al. Incidence of diabetes mellitus in a population-based cohort of persons with chronic hepatitis B virus infection. J Viral Hepat. 2013;20(7):510–513. doi: 10.1111/jvh.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee IC, Huang YH, Chan CC, et al. Impact of body mass index and viral load on liver histology in hepatitis B e antigen-negative chronic hepatitis B. Clin Nutr. 2011;30(5):647–652. doi: 10.1016/j.clnu.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 9.Nakatsuka T, Tateishi R. Development and prognosis of hepatocellular carcinoma in patients with diabetes. Clin Mol Hepatol. 2023;29(1):51–64. doi: 10.3350/cmh.2022.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen GL, Lu Y, Liang L, et al. Impact of diabetes mellitus on the long-term prognosis of patients with hepatocellular carcinoma after hepatectomy. Expert Rev Gastroenterol Hepatol. 2022;16(5):473–478. doi: 10.1080/17474124.2022.2063837 [DOI] [PubMed] [Google Scholar]
- 11.Huo TI, Wu JC, Lui WY, et al. Diabetes mellitus is a recurrence-independent risk factor in patients with hepatitis B virus-related hepatocellular carcinoma undergoing resection. Eur J Gastroenterol Hepatol. 2003;15(11):1203–1208. doi: 10.1097/00042737-200311000-00009 [DOI] [PubMed] [Google Scholar]
- 12.Mai Y, Meng L, Deng G, Qin Y. The role of type 2 diabetes mellitus-related risk factors and drugs in hepatocellular carcinoma. J Hepatocell Carcinoma. 2024;11:159–171. doi: 10.2147/JHC.S441672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan W, Adebowale K, Vancza L, et al. Matrix viscoelasticity promotes liver cancer progression in the pre-cirrhotic liver. Nature. 2024; 626(7999):635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273(1):241–260. doi: 10.1148/radiol.14132958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee IC, Lei HJ, Chau GY, et al. Predictors of long-term recurrence and survival after resection of HBV-related hepatocellular carcinoma: the role of HBsAg. Am J Cancer Res. 2021;11(7):3711–3725. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee IC, Chau GY, Yeh YC, et al. Risk of recurrence in chronic hepatitis B patients developing hepatocellular carcinoma with antiviral secondary prevention failure. PLoS One. 2017;12(11):e0188552. doi: 10.1371/journal.pone.0188552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee IC, Hung YW, Liu CA, et al. A new ALBI-based model to predict survival after transarterial chemoembolization for BCLC stage B hepatocellular carcinoma. Liver Int. 2019;39(9):1704–1712. doi: 10.1111/liv.14194 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Professional Practice C. 2. classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. doi: 10.2337/dc22-S002 [DOI] [PubMed] [Google Scholar]
- 19.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242(2):158–171. doi: 10.1097/01.sla.0000171032.99149.fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu KC, Lee IC, Chi CT, et al. Impact of HCV eradication on recurrence pattern and long-term outcomes in patients with HCV-related hepatocellular carcinoma undergoing radiofrequency ablation. Aliment Pharmacol Ther. 2024;60(7):940–952. doi: 10.1111/apt.18199 [DOI] [PubMed] [Google Scholar]
- 21.DSK L, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234(3):954–960. doi: 10.1148/radiol.2343040153 [DOI] [PubMed] [Google Scholar]
- 22.Cai C, Zeng J, Wu H, et al. Association between hepatitis B virus infection and diabetes mellitus: a meta-analysis. Exp Ther Med. 2015;10(2):693–698. doi: 10.3892/etm.2015.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao X, Cheung KS, Peng C, et al. Steatosis, HBV-related HCC, cirrhosis, and HBsAg seroclearance: a systematic review and meta-analysis. Hepatology. 2023;77(5):1735–1745. doi: 10.1002/hep.32792 [DOI] [PubMed] [Google Scholar]
- 24.Mak LY, Hui RW, Lee CH, et al. Glycemic burden and the risk of adverse hepatic outcomes in patients with chronic hepatitis B with type 2 diabetes. Hepatology. 2023;77(2):606–618. doi: 10.1002/hep.32716 [DOI] [PubMed] [Google Scholar]
- 25.Lee YB, Moon H, Lee JH, et al. Association of metabolic risk factors with risks of cancer and all-cause mortality in patients with chronic hepatitis B. Hepatology. 2021;73(6):2266–2277. doi: 10.1002/hep.31612 [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Pinyol R, Yarchoan M, et al. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat Rev Clin Oncol. 2024;21(4):294–311. doi: 10.1038/s41571-024-00868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin S, Chen M, Cheng AL, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, Phase 3 trial. Lancet. 2023;402(10415):1835–1847. doi: 10.1016/S0140-6736(23)01796-8 [DOI] [PubMed] [Google Scholar]
- 28.Plaz Torres MC, Jaffe A, Perry R, Marabotto E, Strazzabosco M, Giannini EG. Diabetes medications and risk of HCC. Hepatology. 2022;76(6):1880–1897. doi: 10.1002/hep.32439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wabitsch S, McCallen JD, Kamenyeva O, et al. Metformin treatment rescues CD8(+) T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol. 2022;77(3):748–760. doi: 10.1016/j.jhep.2022.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Berger NA, Kaelber DC, Xu R. Association of GLP-1 Receptor Agonists and Hepatocellular Carcinoma Incidence and Hepatic Decompensation in Patients With Type 2 Diabetes. Gastroenterology. 2024;2024:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51(5):890–897. doi: 10.1016/j.jhep.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 32.Lee TY, Lin JT, Zeng YS, Chen YJ, Wu MS, Wu CY. Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Hepatology. 2016;63(5):1517–1527. doi: 10.1002/hep.28266 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.