Abstract
Purpose
To explore the prevalence of anhedonia (ANH) in major depressive disorder (MDD) and treatment expectation and satisfaction among patients with MDD and physicians in the Asia-Pacific region.
Methods
This cross-sectional web-based survey was conducted in April-May 2023 among physicians and individuals aged ≥18 years with self-reported physician diagnosis of MDD (9-item Patient Health Questionnaire [PHQ-9] score ≥ 10) further stratified by anhedonia as measured by the Snaith-Hamilton Pleasure Scale (SHAPS): MDD-ANH (SHAPS score > 2) and MDD non-ANH (SHAPS score ≤ 2). The study assessed the prevalence of anhedonia in MDD as well as the perspectives on the treatment of anhedonia in MDD in terms of expectations and satisfaction among patients and physicians.
Results
The regional estimated prevalence of MDD was 16.1% where 52.5% of MDD respondents had ANH (SHAPS score ≥2). Depressed mood, mental changes, and changes in sleeping patterns prompted MDD-ANH (n = 1448) or MDD non-ANH (n = 836) respondents to seek medical consultation. Respondents with MDD-ANH (vs MDD non-ANH) reported significantly higher levels of depression and anhedonia, longer treatment duration, and preferred switching their existing medications over adding additional medications (all, p < 0.001). Over half of physicians (55.0%) were not treating anhedonia separately. Anhedonia-specific treatment goals seemed important to all respondents, while avoiding suicidal ideation was significantly important to physicians. MDD-ANH respondents reported in general the lowest level of satisfaction with treatment goals than MDD non-ANH and physician, with “improvements in sexual satisfaction” being the treatment goal with the lowest level of satisfaction.
Conclusion
This first large-scale study conducted across the Asia-Pacific region provides a recent update on the prevalence of MDD and anhedonia in MDD and highlights unmet needs in the current therapeutic landscape for anhedonia in MDD, emphasizing the need for novel treatment.
Keywords: anhedonia, apathy, depression, emotional blunting, epidemiology, prevalence, treatment goals
Introduction
Major depressive disorder (MDD) is estimated to affect 185 million individuals globally and accounts for 37.2 million disability-adjusted life years.1 In Asia, the point aggregate prevalence of depression (1994–2014) (16.7%) is the second-highest among continents.2 Country-wise epidemiological studies conducted across China,3 Taiwan,4 and other countries5 in Asia-Pacific have reported a lifetime prevalence of MDD of 1.2–5.8%; albeit using different diagnostic criteria.
Individuals with MDD commonly exhibit symptoms of anhedonia, altered behavioral and emotional processing, and lowered mood.6 Anhedonia (impaired capacity to experience pleasure and interest) is one of the core criteria for depression diagnosis using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).
Patients with depressive disorders and anhedonia demonstrate higher suicidal ideation,7 MDD severity,8,9 cognitive deficits,10 prolonged persistence of depression,11 and poor psychological functioning.12 Moreover, treatments, such as specific antidepressants and psychotherapy, may have a greater impact on depression-specific attributes than anhedonia-related symptoms13,14 resulting in poor prognosis in patients with anhedonia in MDD.15
Emotional blunting is defined as the incapacity to experience both pleasant and negative emotions.16 The phenotypical presentation of emotional blunting and anhedonia may be similar in a clinical setting; however, the neuromodulations underlying each symptom are not the same.17
To date, data on the epidemiology of MDD,3–5 treatment patterns,18 and patient expectations and preferences19,20 for MDD treatment have been reported for individual countries from the Asia-Pacific region but not adequately explored on a regional level. Hence, the present study aimed to estimate the prevalence and current treatment practices of anhedonia in MDD patients; to understand physicians’ awareness towards emotional blunting; and to elucidate patients’ and physicians’ perspectives towards anhedonia in MDD in terms of treatment satisfaction and expectations in the Asia-Pacific region. The insights of this study could provide a more comprehensive understanding of the burden of anhedonia in MDD and its therapeutic perspectives as well as inform the development of more effective treatment strategies to optimize treatment outcomes and shared decision-making to manage anhedonia in MDD patients in Asia-Pacific.
Materials and Methods
Study Design and Data Source
This observational, cross-sectional, web-based self-reported survey was conducted in April-May 2023 across Australia, China, Japan, Malaysia, South Korea, and Taiwan (Figure S1).
The survey was administered to eligible respondents in the general population (non-physicians) aged ≥18 years who had met the inclusion and exclusion criteria, provided informed consent, and were subsequently screened for MDD and anhedonia. The 20–30-minute survey questionnaire contained items on functioning, treatment satisfaction, and treatment expectations and was administered to MDD respondents with or without anhedonia. The non-physician respondents were recruited via opt-in databases with a similar gender and age group breakdown as the most recent nationwide census of each country.
Eligible physicians were recruited via a local database of physicians/panels consolidated from various sources through purposive sampling – Australian Health Practitioner Regulation Agency (AHPRA), JKT and hospital website in China, Medical Database Provision Business (MDB) in Japan, National Specialist Register (NSR) in Malaysia, Health Insurance Review & Assessment (HIRA) in South Korea, and hospital websites and public healthcare provider registries in Taiwan. Physicians who completed the screener and provided consent were invited to complete a 15-minute questionnaire on treatment satisfaction, treatment expectations, and emotional blunting.
The research was conducted according to Good Epidemiological Practices (GEP) and complied with the Declaration of Helsinki. The study protocol received approval from Toukeikai Kitamachi Clinic ERB in Japan (approval number: EJP09413) and exemption from the Pearl Institutional Review Boards of the remaining countries/territories (Australia, China, Malaysia, South Korea, and Taiwan) according to 45 CFR 46.104(d)(2) Tests, Surveys, Interviews (IRB number: 023–0025).
Eligibility Criteria
Non-physician respondents aged ≥18 years without diagnosis of bipolar disorder or schizophrenia were further stratified into MDD-ANH or MDD non-ANH groups: (i) MDD-ANH: respondents with a self-reported physician diagnosis of MDD and self-reported experiencing MDD in the past 2 weeks based on 9-item Patient Health Questionnaire (PHQ-9) score ≥1021 and Snaith-Hamilton Pleasure Scale (SHAPS) score >2;22,23 (ii) MDD non-ANH: respondents with self-reported physician diagnosis of MDD and self-reported experiencing MDD in the past 2 weeks based on PHQ-9 ≥10 and SHAPS score ≤2 (Table S1).
The SHAPS score of >2 for determining anhedonia in MDD patients is a valid and reliable scale.22–24 Translated and validated versions of SHAPS were administered in respective participating countries.25–28 For Taiwan, no validated version of SHAPS was available at the start of study, therefore SHAPS was translated by a certified native speaker subject specialist of the local language (Traditional Chinese) and verified by a subject specialist. The translated SHAPS was back translated to English to cross-check with the original validated English SHAPS version.
Physicians practicing as a psychiatrist with ≥3 years of clinical experience; spending ≥30% of the time in direct patient care and treated ≥20 MDD patients in the past month were recruited for the study.
Study measures
Prevalence and severity of MDD and MDD-ANH
The prevalence of MDD and MDD-ANH was calculated based on PHQ-9 and SHAPS scores as follows:
Sociodemographic and Health Characteristics
Sociodemographic and health characteristics, and severity of depression of MDD respondents with or without ANH were included in the analysis. Physicians’ characteristics included country of residence, number of years of clinical experience, percentage of time in providing direct patient care, patient caseload in the past month, and severity of anhedonia among MDD patients.
Treatment journey
Depression-specific characteristics, time since depression diagnosis, treatment details and duration, number of days using a prescription in the past month, and reasons for switching medications were included (Table S2).
Physicians’ Perspectives Towards Anhedonia
The physicians’ perspectives towards anhedonia were queried through the exploratory statements related to anhedonia. The responses were collected on the 1-9-point Likert scale (1 = strongly disagree to 9 = strongly agree) (Table S3).
Physicians’ awareness and familiarity with emotional blunting were explored by collecting responses for “Whether know the concept of emotional blunting in MDD (Yes/No)” and “Level of familiarity with the definition of emotional blunting in MDD” (Not at all familiar; slightly familiar; somewhat familiar; moderately familiar; extremely familiar). Current therapeutics used to manage anhedonia were also collected (Table S3).
Importance of Treatment Goals and Treatment Satisfaction
The perceived importance of treatment goals was assessed on a 1-5-point Likert scale (1-not at all important, 5-extremely important) and the satisfaction with current treatments in achieving their treatment goals was measured on a 1-9-point Likert scale (1-extremely dissatisfied, 9-extremely satisfied) among MDD respondents and physicians. Higher scores indicate higher treatment importance or satisfaction (Table S4).
Statistical analyses
All study outcomes were analyzed descriptively. Continuous or discrete variables were reported as mean with standard deviation (SD) and categorical variables as frequencies and percentages. Chi-square and independent-sample t-tests were used to determine significant differences across subgroups (MDD-ANH vs MDD non-ANH) for categorical and continuous variables, respectively.
Age-, gender- and country-weighting factor was applied to all the responses to ensure regional representativeness. Statistical analyses were conducted using SPSS version 29 (IBM) and/or R 4.2.2 and/or SAS 9.4.
Results
Prevalence of MDD with anhedonia
The regional estimated age-gender-country weighted prevalence of MDD was 16.1% (N = 6226). Anhedonia was reported by 52.5% of MDD respondents (Table 1).
Table 1.
Country, Age, and Gender Weighted Prevalence of Respondents with Major Depressive Disorder (MDD) Measured Using 9-Item Patient Health Questionnaire (PHQ-9) and Anhedonia as Measured by 14-Item Snaith-Hamilton Pleasure Scale (SHAPS)
| Variables | Total Survey Sample | Total MDD Sample | Total Anhedonia Sample |
|---|---|---|---|
| N=38623 | n=6226 | n=3271 | |
| n (%) | n (%) | n (%) | |
| PHQ-9 | |||
| No Depression (0–4) | 24,403 (63.2) | 0 (0.0) | 0 (0.0) |
| Mild (5–9) | 5417 (14.0) | 0 (0.0) | 0 (0.0) |
| Moderate (10–14) | 3864 (10.0) | 2278 (36.6) | 919 (28.1) |
| Moderate-Severe (15–19) | 2697 (7.0) | 2104 (33.8) | 1076 (32.9) |
| Severe (20–27) | 2242 (5.8) | 1844 (29.6) | 1277 (39.0) |
| Presence of MDDa | 6226 (16.1) | 6226 (100.0) | 3271 (100.0) |
| SHAPSb | |||
| 0 | 1290 (3.3) | 1290 (20.7) | 0 (0.0) |
| 1–2 | 653 (1.7) | 653 (10.5) | 0 (0.0) |
| Presence of Anhedonia (≥3) | 3271 (8.5) | 3271 (52.5) | 3271 (100.0) |
Notes: aRespondents were classified under MDD if they reported Moderate Depression or greater (PHQ-9 ≥ 10) and a self-reported physician diagnosis of depression. bSHAPS was surveyed among respondents with Moderate Depression or greater and a self-reported physician diagnosis of depression. Prevalence weights were produced using UN population estimates for Australia, China, Japan, Malaysia, South Korea, and Taiwan.
Abbreviations: ANH, anhedonia; PHQ-9, 9-item Patient Health Questionnaire; MDD, major depressive disorder; SHAPS, 14-item Snaith-Hamilton Pleasure Scale.
Respondent Characteristics
Respondents with MDD
A total of 2284 MDD respondents (MDD-ANH: n = 1448; MDD non-ANH: n = 836) completed the survey (Figure S1 and Table 2).
Table 2.
Demographic Characteristics of Respondents with Major Depressive Disorder (MDD) with or without Anhedonia (ANH)
| Variables | MDD-ANH | MDD non-ANH |
|---|---|---|
| n=1448 | n=836 | |
| Country, n (%) | ||
| Australia | 241 (16.6) | 156 (18.7) |
| China | 234 (16.2) | 163 (19.5) |
| Japan | 307 (21.2) | 54 (6.5) |
| Malaysia | 214 (14.8) | 186 (22.3) |
| South Korea | 260 (18.0) | 119 (14.2) |
| Taiwan | 192 (13.3) | 158 (18.9) |
| Sex, n (%) | ||
| Male | 691 (47.7) | 329 (39.4) |
| Female | 757 (52.3) | 507 (60.7) |
| Education, n (%) | ||
| Primary school | 5 (0.4) | 5 (0.6) |
| Secondary school | 94 (6.5) | 61 (7.3) |
| Senior secondary school | 272 (18.8) | 130 (15.6) |
| Vocational Education and Training | 191 (13.2) | 83 (9.9) |
| University | 739 (51.0) | 463 (55.4) |
| Graduate school or above | 130 (9.0) | 85 (10.2) |
| No school | 1 (0.1) | 1 (0.1) |
| Others | 13 (0.9) | 6 (0.7) |
| Decline to answer | 3 (0.2) | 2 (0.2) |
| Age Category, n (%) | ||
| 18 to <25 | 148 (10.2) | 113 (13.5) |
| 25 to <35 | 458 (31.6) | 287 (34.3) |
| 35 to <45 | 396 (27.4) | 253 (30.3) |
| 45 to <55 | 264 (18.2) | 107 (12.8) |
| 55 to <65 | 125 (8.6) | 52 (6.2) |
| 65 and older | 57 (3.9) | 24 (2.9) |
| CCI Score Categories, n (%) | ||
| 0 | 803 (55.5) | 442 (52.9) |
| 1 | 272 (18.8) | 111 (13.3) |
| 2 | 143 (9.88) | 83 (9.9) |
| 3+ | 230 (15.9) | 200 (23.9) |
| Depression Severity, mean (SD) | ||
| PHQ-9 scorea,* | 16.61 (4.5) | 15.08 (4.1) |
| SHAPS scoreb,* | 7.23 (3.3) | 0.73 (0.8) |
| Time Since Depression diagnosis (years), mean (SD)* | 7.98 (8.4) | 4.00 (7.0) |
Note: *P<0.001.
Abbreviations: ANH, anhedonia; MDD, major depressive disorder; CCI, Charlson comorbidity index; PHQ-9, 9-item Patient Health Questionnaire; SD, standard deviation; SHAPS, Snaith-Hamilton Pleasure Scale. aPHQ-9 (range 0 to 27); a higher score indicates more severe depression. Scores of 5, 10, 15, and 20 represent cutoffs for mild, moderate, moderately severe, and severe depression, respectively. bSHAPS (range 0 to 14); a higher score indicates higher levels of anhedonia.
Respondents with MDD-ANH had a significantly higher level of depression (mean [SD] PHQ-9 score: 16.61 [4.5] vs 15.08 [4.1]; p < 0.001) and anhedonia (mean [SD] SHAPS score: 7.23 [3.3] vs 0.73 [0.8]; p < 0.001) compared to MDD non-ANH respondents. Mean (SD) time since depression diagnosis was significantly longer for MDD-ANH than MDD non-ANH (7.98 [8.4] vs 4.00 [7.0] years; p < 0.001) (Table 2).
Physicians’ Characteristics
The characteristics of the 340 physicians who completed the survey are described in Table S5. Physicians had an average of 16.77 (8.5) years of clinical experience and had seen an average of 129.22 patients with MDD in the past month. Physicians reported that 49.5% of their MDD patients had anhedonia.
Treatment Journal of Respondents with MDD
The top three symptoms prompting medical consultation were the same for MDD-ANH and MDD non-ANH, but at significantly different proportions: depressed mood or other emotional problems (79.3% vs 68.6%), mental changes (55.7% vs 44.3%), and changes in sleeping pattern (47.0% vs 56.2%) (p < 0.05 for all) (Table 3).
Table 3.
Country, Age, and Gender Weighted Depression-Specific Characteristics and Treatment Patterns of Respondents with Major Depressive Disorder (MDD) with or Without Anhedonia (ANH)
| Variable | MDD-ANH n=1193 | MDD non-ANH n=743 | p-value |
|---|---|---|---|
| Depression Characteristics | |||
| Depression Symptoms Prompting Diagnosisa, n (%) | |||
| Depressed mood and other emotional problems | 946 (79.3) | 510 (68.6) | 0.0048 |
| Mental changes | 665 (55.7) | 329 (44.3) | 0.0081 |
| Sleep pattern changes | 561 (47.0) | 418 (56.2) | 0.0329 |
| Social problems | 377 (31.6) | 161 (21.7) | 0.0119 |
| Eating pattern changes | 331 (27.8) | 217 (29.3) | 0.6970 |
| Physical problems | 301 (25.2) | 206 (27.7) | 0.5101 |
| None of these | 10 (0.9) | 9 (1.3) | 0.5719 |
| Diagnosing HCPa, n (%) | |||
| Psychiatrist | 560 (46.9) | 226 (30.5) | <0.0001 |
| Psychologist | 476 (39.9) | 301 (40.6) | |
| General Practitioner / Family Practitioner | 95 (8.0) | 160 (21.5) | |
| Neurologist [No Japan] | 34 (2.8) | 34 (4.5) | |
| General Internist / Internal medicine physician [China, Japan only] | 16 (1.3) | 18 (2.5) | |
| Other | 13 (1.1) | 4 (0.5) | |
| Duration of Treatment in Months, mean (SD) | 36.76 (44.7) | 25.22 (28.2) | 0.0002 |
| Prescription Use | |||
| Current Prescription Use for Depression, n (%) | |||
| Yes | 735 (61.6) | 498 (67.1) | 0.1844 |
| No | 458 (38.4) | 245 (32.9) | |
| Current Use of Multiple Prescriptions for Depressionb, n (%) | n=735 | n=498 | |
| Yes | 303 (41.3) | 276 (55.4) | 0.0087 |
| No | 432 (58.8) | 222 (44.6) | |
| Prescribing multiple prescriptions for depressionc, n (%) | |||
| Psychiatrist | 616 (83.9) | 303 (60.9) | |
| General Practitioner / Family Practitioner | 97 (13.2) | 173 (34.8) | <0.0001 |
| General Internist / Internal medicine physician [CHINA, JAPAN ONLY] | 17 (2.4) | 21 (4.2) | |
| Other | 4 (0.6) | 1 (0.2) | |
| Prior Depression Medication Useb, n (%) | |||
| Yes | 232 (31.7) | 317 (63.6) | <0.0001 |
| No | 503 (68.4) | 181 (36.4) | |
| Current Medication Replaced or Added to Previous Medication, n (%) | |||
| Replaced my existing medication, treatment or therapy | 129 (55.8) | 117 (36.9) | 0.0005 |
| Added on to my existing medication, treatment or therapy | 95 (41.0) | 198 (62.6) | |
| Not sure | 7 (3.1) | 2 (0.5) | |
| Reasons for Switching, n (%) | n=129 | n=117 | |
| Doctor’s recommendation | 86 (66.7) | 99 (85.0) | 0.0552 |
| To reduce side effects | 57 (44.1) | 53 (45.3) | 0.9146 |
| I was not responding to the previous treatment | 55 (42.4) | 19 (16.2) | 0.0116 |
| The dosing of the current treatment is more convenient | 38 (29.4) | 18 (15.7) | 0.1702 |
| The form or mode of administration is more convenient | 27 (20.6) | 1 (0.8) | <0.0001 |
| Lower cost | 21 (15.9) | 21 (17.6) | 0.8428 |
| Others | 2 (1.7) | 0 (0.1) | 0.0004 |
Notes: aAsked of those reporting a depression diagnosis who answered all questions in the depression module (MDD-ANH, MDD non-ANH). bAsked of those reporting having experienced depression in the past 12 months who are taking a prescription medication (MDD-ANH, MDD non-ANH). cIn cases where there were different providers prescribing the medications within a group, the following hierarchy was used: Psychiatrist > GP > General Internist/Internal Medicine Physician > other.
Abbreviations: ANH, anhedonia; HCP, healthcare provider; MDD, major depressive disorder; SD, standard deviation.
Similar proportions of respondents with MDD-ANH (61.6%) and MDD non-ANH (67.1%) had a current prescription for depression. The mean duration of treatment was longer for MDD-ANH than MDD non-ANH (36.76 vs 25.22 months; p = 0.0002). Compared to MDD-ANH respondents, a significantly higher proportion of MDD non-ANH respondents were on multiple prescriptions for depression (41.3% vs 55.4%; p = 0.0087); primarily, prescribed by psychiatrists for MDD-ANH (83.9%) and MDD non-ANH (60.9%) (Table 3). A significantly lower proportion of respondents with MDD-ANH (vs MDD non-ANH) had prior depression medication use (31.7% vs 63.6%; p < 0.0001).
More MDD-ANH respondents than MDD non-ANH switched their existing medications (55.8% vs 36.9%), instead of adding on additional medications (41.0% vs 62.6%) (p = 0.0005) (Table 3). There is a significantly higher proportion of MDD-ANH respondents who switched (42.4% vs 16.2%) because they were not responding to the previous treatment.
Physician’s Perspectives Towards Anhedonia in MDD
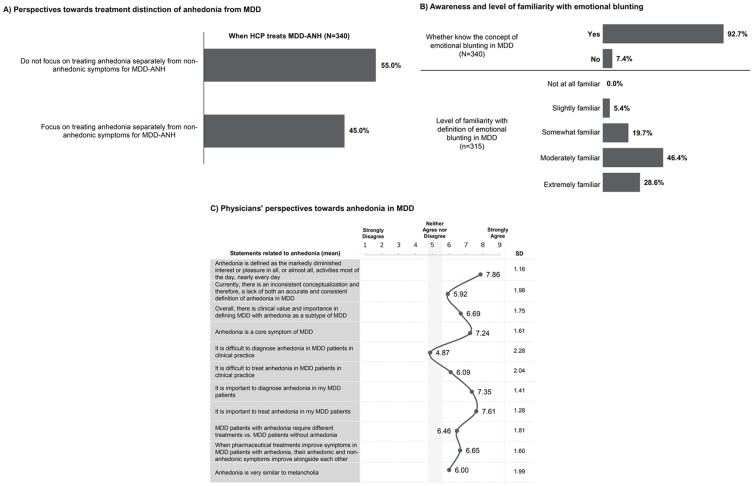
Over half of the physicians (55.0%) stated that they did not focus on treating anhedonia separately from MDD (Figure 1A). The majority of physicians (92.7%) were aware of the concept of emotional blunting in MDD, whereas three-quarters were extremely (28.6%) or moderately (46.4%) familiar with the definition of emotional blunting (Figure 1B). Physicians moderately-strongly agreed (mean [SD] score: 7.86 [1.16]) with the definition of anhedonia and moderately agreed with nearly all statements on anhedonia (Figure 1C). Physicians moderately-strongly agreed that it is important to diagnose (mean [SD] score: 7.35 [1.41]) and treat anhedonia (mean [SD] score: 7.61 [1.28]) in MDD patients. However, physicians were neutral on the statement that anhedonia is difficult to diagnose in MDD patients (Figure 1C).
Figure 1.
Physicians’ perspectives towards anhedonia in major depressive disorder (MDD) and emotional blunting. (A) Physician’s perspectives towards treatment distinction of anhedonia from MDD. (B) Physicians’ awareness and level of familiarity with emotional blunting. (C) Physicians’ perspectives towards anhedonia in MDD.
Abbreviations: ANH, anhedonia; HCP, healthcare provider; MDD, major depressive disorder; SD, standard deviation.
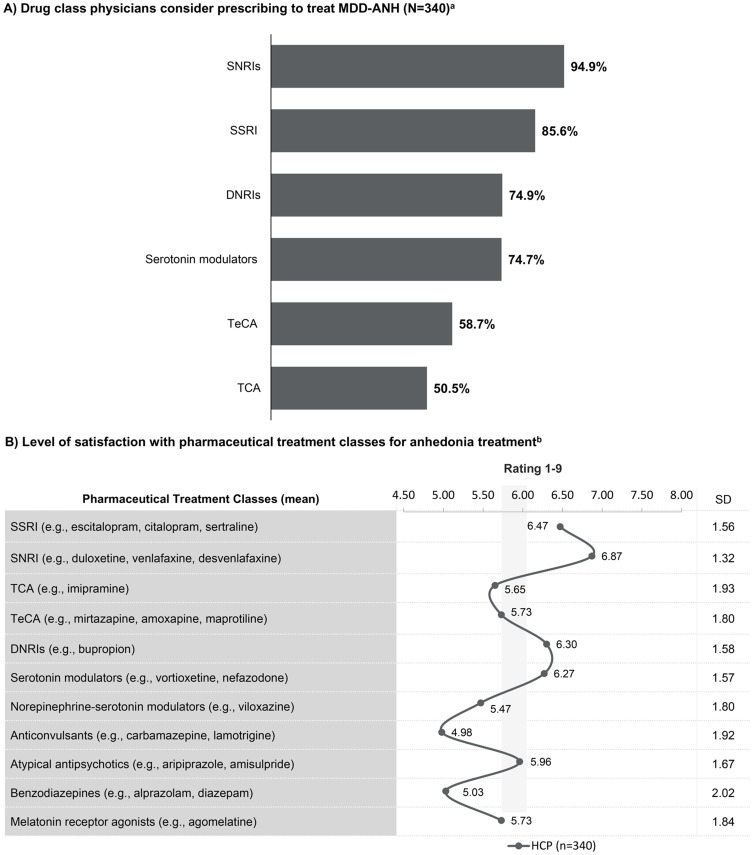
The three most commonly prescribed classes of therapeutics included serotonin-norepinephrine reuptake inhibitors (SNRIs) (94.9%) followed by selective serotonin reuptake inhibitors (SSRIs) (85.6%), and dopamine norepinephrine reuptake inhibitors (DNRIs) (74.9%) to treat MDD-ANH (Figure 2A). Physicians were moderately satisfied (>6 on the 1-9-point satisfaction scale) with commonly recommended classes of therapeutics, whereas 7 out of 11 classes received a below-moderate level of satisfaction (<6 on the satisfaction scale) (Figure 2B).
Figure 2.
Therapeutics considered for the treatment of anhedonia by physicians. (A) Drug classes that physicians considered prescribing to treat anhedonia in MDD patients. (B) Physicians’ perceived level of satisfaction towards the different pharmaceutical treatment classes for anhedonia treatment on a scale of 1–9, where 1=least satisfied and 9=most satisfied. aDrug class HCP considers prescribing to treat MDD-ANH. bLevel of satisfaction with pharmaceutical treatment classes (scale 1–9, 1-extremely dissatisfied, 9-extremely satisfied).
Abbreviations: ANH, anhedonia; MDD, major depressive disorder; SD, standard deviation; DNRIs, Dopamine norepinephrine reuptake inhibitors; SNRIs, Serotonin-norepinephrine reuptake inhibitors; SSRI, Selective serotonin reuptake inhibitors; TCA, Tricyclic antidepressants; TeCA, Tetracyclic antidepressants.
Treatment Goals and Satisfaction for MDD-ANH: MDD and Physician Respondents
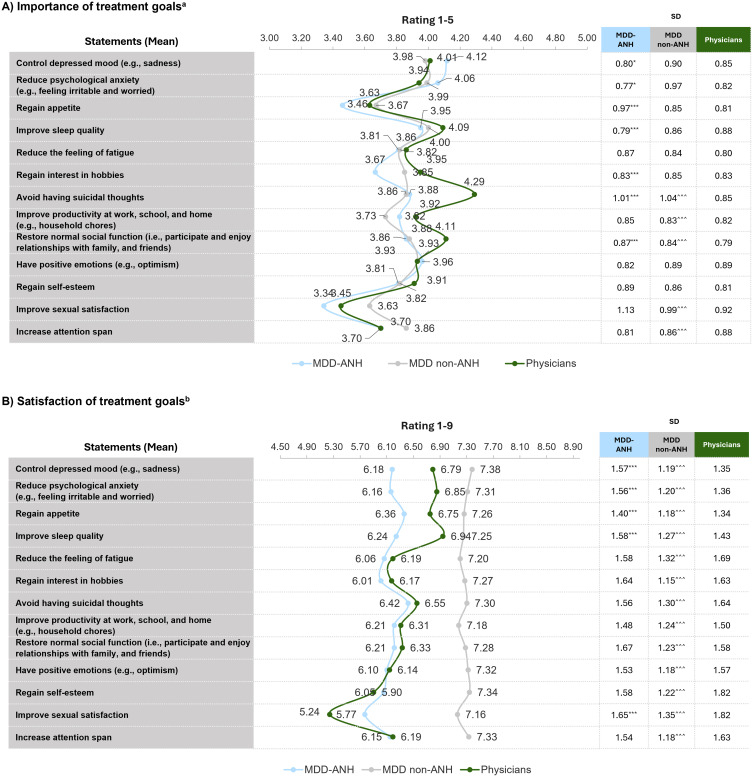
Improved sleep quality (mean [SD] scores: 3.95 [0.79], 4.00 [0.86], 4.09 [0.88]), control depressed mood (mean [SD] scores: 4.12 [0.80], 3.98 [0.90], 4.01 [0.85]) and reduced psychological anxiety (mean [SD] scores: 4.06 [0.77], 3.99 [0.87], 3.94 [0.82]) were equally important to respondents with MDD-ANH, MDD non-ANH, and physicians, respectively. Avoiding suicidal ideation (4.29 [0.85]) was a significantly important goal for physicians compared to respondents with MDD-ANH (3.88 [1.01]) and MDD non-ANH (3.86 [1.04]); (p < 0.0001 for both) (Figure 3A).
Figure 3.
Importance and satisfaction of treatment goals among major depressive disorder patients with anhedonia (MDD-ANH), major depressive disorder patients without anhedonia (MDD non-ANH), and physicians. (A) Perceived importance of treatment goals among MDD-ANH, MDD non-ANH and physicians based on a scale of 1–5 where 1=least important and 5=most important. (B) Perceived satisfaction on treatment goals towards current therapeutics among MDD-ANH, MDD non-ANH, and physicians on a scale of 1–9 where 1=least satisfied and 9=most satisfied. aLevel of importance of treatment goals (scale 1–5, 1-not at all important, 5-extremely important). bLevel of satisfaction with treatment goals (scale 1–9, 1-extremely dissatisfied, 9-extremely satisfied). T-test: MDD-ANH vs physicians – *p<0.05, ***p<0.001; MDD non-ANH vs HCP –^^^p<0.001.
Abbreviations: ANH, anhedonia; MDD, major depressive disorder; SD, standard deviation.
Respondents with MDD-ANH (range of mean scores: 5.77–6.42) reported the lowest satisfaction on the majority of treatment goals followed by physicians (range of mean scores: 5.24–6.94), whereas respondents with MDD non-ANH reported moderate level of satisfaction on all treatment goals (range of mean scores: 7.16–7.38) on the 1–9 satisfaction scale (Figure 3B). Respondents with MDD-ANH reported lower level of satisfaction for control depressed mood (6.18 [1.57] vs 6.79 [1.35]), reduced psychological anxiety (6.16 [1.56] vs 6.85 [1.36]), regained appetite (6.36 [1.40] vs 6.75 [1.34]), and improved sleep quality (6.24 [1.58] vs 6.94 [1.43]) compared to physicians (p < 0.0001, for all). Both respondents with MDD-ANH (5.77 [1.65]) and physicians reported the least level of satisfaction with the improvement in sexual satisfaction (5.24 [1.82]) among others.
Discussion
To the best of our knowledge, this is the first large-scale study to report the epidemiology of anhedonia in MDD in the Asia-Pacific region. This cross-sectional, web-based study estimated the prevalence of anhedonia in MDD and evaluated treatment patterns and perspectives in terms of treatment goals and satisfaction among MDD respondents with or without anhedonia and physicians. Consistent with previous studies, MDD-ANH respondents had significantly higher PHQ-9 scores than MDD non-ANH, indicating greater severity of depression.8,9 Physicians agreed of the need to diagnose and treat anhedonia distinctly from MDD even though 55.0% of the physicians did not focus on treating anhedonia separately from MDD in our study. The transdiagnostic trait of anhedonia (as a symptom of MDD and schizophrenia),29 and lack of approved antidepressants30 and distinct treatment guidelines for anhedonia in MDD make the diagnosis and treatment further challenging.
The majority of physicians reported moderate level of satisfaction with commonly prescribed classes and a below-moderate level of satisfaction for the majority of current treatment classes. Treatment goals specific to anhedonia seemed (very) important to all respondents, whereas avoiding suicidal ideation was more important to physicians than MDD-ANH respondents.
Prevalence of anhedonia among respondents with MDD (52.5%) estimated in this study is within the range reported previously across the US,31 China,32 and Canada.33 A study that assessed the possibility of different ways of meeting DSM-5 criteria reported that about 82% of the patients with MDD had “loss of interest or pleasure”.31 Another study reported that 43% of MDD patients in China were anhedonic as per SHAPS cut-off score >2.32 Overall, the prevalence estimated in this study reflects real-world observations of physician diagnosis of anhedonia among MDD patients.
Respondents from both groups (MDD-ANH and MDD non-ANH) cited the same symptoms which prompted them to seek medical consultation. These findings are in tandem with prior studies reporting these as bothersome symptoms by MDD patients.20,34 Additionally, MDD-ANH respondents had longer mean time since diagnosis of depression compared to MDD non-ANH respondents, which could hint at chronic depression or a greater disease severity8,9 or that anhedonia persisted despite the treatment35 among MDD-ANH respondents. Additionally, anhedonia has been reported as a prognostic indicator of a longer recovery time and few depression-free days.15 These findings potentially suggest inadequate management of their conditions within this study population.
Literature suggests that a combination of antidepressants for severe cases of depression or non-responders results in clinically superior outcomes.36 In this study, a significantly lower proportion of MDD-ANH respondents were receiving multiple prescriptions. This could be attributed to the higher proportion of MDD-ANH respondents (vs MDD non-ANH) switching their existing medication compared to those adding other medications which have been observed in this study. Notably, a higher proportion of MDD-ANH respondents cited non-responsiveness to previous treatment than MDD non-ANH respondents for changing their medication regimen. Collectively, the findings support the hypothesis that patients with depressive disorders exhibiting anhedonia are not responsive to conventional treatments, thus prompting treatment switches instead of add-ons.37 This indicates a need for targeted treatments to manage anhedonia in MDD patients.
Notably, at least one-quarter of physicians were unfamiliar with the distinctive concept of emotional blunting. Although emotional blunting is commonly confused with anhedonia due to overlapping clinical presentations, the symptoms are not identical.38 A survey among depressed patients and physicians reported that the physicians tend to underestimate the impact of emotional blunting on treatment discontinuation and daily functioning of patients compared to patients perspectives.38, Therefore, it is important that physicians diagnose and manage emotional blunting distinctly from anhedonia.
In this study, a large proportion of physicians (85.6%) had prescribed SSRIs (a first-line antidepressant) to manage anhedonia in MDD. This contrasted with existing literature suggesting that first-line antidepressants might not be effective for anhedonia treatment, and rather a variety of different novel antidepressants (agomelatine, ketamine, and vortioxetine) could be considered.30,37,39 Due to the lack of specific recommendations or approved antidepressants30 for anhedonia management within MDD, physicians in this survey might have prescribed these antidepressants as treatments for MDD. As conventional antidepressants and psychotherapy have demonstrated higher efficacy for treating depression-specific symptoms than anhedonia-specific symptoms,13,14 recent interventions should focus on targeting anhedonia-specific symptoms which could prompt personalized treatment.39
The treatment goal considered most important differed between MDD-ANH (control depressed mood), MDD non-ANH (improve sleep quality), and physicians (avoid suicidal ideation). A qualitative study reported that for both, patients and physicians, goal-setting was very important to boost treatment outcomes.40
The lowest satisfaction for the majority of treatment goals was reported by MDD-ANH respondents; likely owing to the association of anhedonia with poor prognosis15 and non-response and non-remission among individuals with MDD.41 Respondents with MDD-ANH perceived lower level of satisfaction for controlling depressed mood, reducing psychological anxiety, regaining appetite, and improving sleep quality than physicians. Conversely, physicians were least satisfied with improvement in the sexual satisfaction and regained self-esteem. Sexual satisfaction was commonly perceived to the lowest by both MDD-ANH and physicians. The prominent feature of anhedonia “lack pleasure/satisfaction after receiving rewards” and higher usage of SSRIs that ebb the neural processing of rewarding stimuli could be related to inadequate treatment response35,42 resulting in lower levels of satisfaction. Therefore, shared decision-making incorporating patients’ perspectives,19 careful patient monitoring, and assessing potential predictors (sociodemographic and clinical variables, genetic factors) to propose the personalized antidepressant treatment for MDD patients is necessary.30
The study has some limitations. We may have included a subset of patients with atypical depression since it also includes overlapping symptoms of increased appetite, hypersomnia, and rejection sensitivity. As this is a web-based survey study, respondents without internet access or comfort with the online administration, institutionalized patients, elderly people, and those with severe comorbidities and disabilities may have been under-represented. This study was conducted among individuals with a self-reported physician diagnosis of MDD and self-reported responses to PHQ-9 and SHAPS, verification could not be performed, and the findings may not be generalizable to individuals with specific subtypes of MDD or other mental health disorders with anhedonia. A causal inference cannot be elucidated owing to recollection bias created by the self-reported nature of the study; however, necessary measures were taken to minimize intentional false reporting.
Conclusion
Overall, about half of patients with MDD in Asia-Pacific were found to have anhedonia, indicating a high prevalence of anhedonia in MDD. The study provided insights into the characteristics of anhedonia and highlighted the key unmet needs, specifically, in terms of the current therapeutic landscape for anhedonia from the perspectives of both patients and physicians.
The findings implied that the presence of anhedonia in MDD may lead to poorer outcomes seen from higher levels of depression and anhedonia, and longer treatment duration among MDD individuals with anhedonia than MDD individuals without anhedonia. While physicians had agreed that it was important to treat anhedonia in MDD patients, about half of physicians did not focus on treating anhedonia separately from MDD. Furthermore, MDD individuals with anhedonia had reported the lowest satisfaction for the majority of treatment goals, especially with sexual satisfaction improvement being the lowest for both MDD individuals with anhedonia and physicians. Collectively, the findings underscored the need for shared decision-making and novel specific treatments.
Acknowledgments
Medical writing support was provided by Vaishnavi Punja, M.Pharm and Ashwini Atre, PhD of Indegene Pvt. Ltd., Bangalore, India, and funded by Janssen Asia Pacific, a division of Johnson & Johnson Pte Ltd. Editorial support was provided by Amanda Woo, PhD, of Oracle Life Sciences, Singapore, funded by Janssen Asia Pacific, a division of Johnson & Johnson Pte Ltd., Singapore.
Funding Statement
This study was funded by Janssen Asia Pacific, a division of Johnson & Johnson Pte Ltd.
Abbreviations
AHPRA, Australian Health Practitioner Regulation Agency; ANH, anhedonia; DNRI, dopamine norepinephrine reuptake inhibitor; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; GEP, Good Epidemiological Practices; HIRA, Health Insurance Review & Assessment; MDB, Medical Database Provision Business; MDD, major depressive disorder; MDD-ANH, major depressive disorder patients with anhedonia; MDD non-ANH, major depressive disorder patients without anhedonia; NSR, National Specialist Register; PHQ-9, 9-item Patient Health Questionnaire; SD, standard deviation; SHAPS, Snaith-Hamilton Pleasure Scale; SNRI, serotonin-norepinephrine reuptake inhibitors; SSRI, selective serotonin intake inhibitors.
Data Sharing Statement
The authors confirm that all relevant data are within the manuscript and its supporting information files.
Ethics approval and informed consent
The study protocol received approval from Toukeikai Kitamachi Clinic ERB in Japan (approval number: EJP09413) and exemption from the Pearl Institutional Review Boards of the remaining countries/territories (Australia, China, Malaysia, South Korea, and Taiwan) according to 45 CFR 46.104(d)(2) Tests, Surveys, Interviews (IRB number: 023-0025).
Each respondent provided electronic consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Michael Berk is supported by a NHMRC Senior Principal Research Fellowship and Leadership 3 Investigator grant (1156072 and 2017131); reports grant funding from Wellcome Trust, MRFF, Victorian Government Department of Jobs, Precincts and Regions, Cooper University USA, Janssen Lundbeckfonden Copenhagen, St. Biopharma, Psychscene.com, WFSBP, NeuroSAS, CINP, Shanghai Mental Health Center, Penn State College of Medicine, Precision Psych Fondamental, ISBD, Milken Baszucki Brain Research Fund, Stanley Medical Research Institute, Danmarks Frie Forskningsfond Psykiatrisk Center Kobenhavn, Patient-Centered Outcomes Research Institute (PCORI), Australian Eating Disorders Research and Translation Centre AEDRTC, USA Department of Defense Office of the Congressionally Directed Medical Research Programs (CDMRP), Equity Trustees Limited. Lectures: Global Congress of Biological Psychiatry India, Otsuka CNS, RANZCP New Zealand, Eisai Australia, Sandoz, Allori, Lundbeck, World Congress of Psychiatry, African College of Neuropsychopharmacology, SVI Inaugural Health Matters Webinar Series, Argentine Association of Psychiatrists Congress of Psychiatry and Mental Health, Global Bipolar Cohort (GBC). In addition, he reports patents “Modulation of Physiological processes and agents useful for same” pending to Bush, Copolov, Berk. 60/325061, “Modulation of diseases of the central nervous system and related disorders” licensed to Berk, Laupu. 2014900627, “Xanthone-rich plant extracts or compounds therefrom for modulating diseases of the central nervous system and related disorders” licensed to Berk, Laupu, Deakin University2015222697. Wei-Lieh Huang has received consultation fees from Janssen, Servier, and Boehringer Ingelheim. He has given lectures with personal honoraria for Janssen, Servier, Pfizer/Viatris, Sumitomo, Otsuka, and Boehringer Ingelheim. He also reports grants from the National Health Research Institutes, Taiwan, National Science and Technology Council, Taiwan, and the National Taiwan University Hospital Yunlin Branch, Taiwan. Tadafumi Kato reports personal fees from Janssen Asia Pacific/Vista Health related to this work, reports grants from Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Teijin Pharma, Daiichi Sankyo Co., Ltd., EA Pharma Co., Ltd., and Eisai Co., Ltd., personal fees from Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eisai Co., Ltd., Meiji Seika Pharma Co., Ltd., Shionogi & Co., Ltd., Mochida Pharmaceutical Co., Janssen Pharmaceutical K.K., Janssen Asia Pacific, Vista Health, Yoshitomiyakuhin, MSD K.K., Japan Boehringer Ingelheim, Kyowa Pharmaceutical Industry Co., Ltd., Viatris, Mylan EPD, H.U. Frontier, Lundbeck Japan K.K., Nihon Medi-physics Co., Ltd., Glaxo-SmithKline, Novartis Pharma, EA Pharma Co., and Ono Pharmaceutical Co., Ltd., outside the submitted work. Lawrence Philip Vandervoort is an employee of Oracle Life Sciences, Singapore. Keira Herr and Thomas David Webb are employees of Janssen Asia Pacific, a division of Johnson & Johnson Pte Ltd., Singapore. Mami Kasahara-Kiritani is an employee of Janssen Pharmaceutical K.K., Tokyo, Japan. Jung Goo Lee, Chong Guan Ng, and Zhen Wang declare no conflicts of interest in this work.
References
- 1.Global Health metrics. Major depressive disorder — level 4 cause. The institute for health metrics and evaluation. 2019. Available from: https://www.healthdata.org/results/gbd_summaries/2019/major-depressive-disorder-level-4-cause,https://www.healthdata.org/results/gbd_summaries/2019/major-depressive-disorder-level-4-cause. Accessed November 15, 2023.
- 2.Lim GY, Tam WW, Lu Y, et al. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. 2018;8:2861. doi: 10.1038/s41598-018-21243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu L, Xie J, Long J, et al. Epidemiology of major depressive disorder in mainland China: a systematic review. PLoS One. 2013;8:e65356. doi: 10.1371/journal.pone.0065356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao S-C, Chen WJ, Lee M-B, et al. Low prevalence of major depressive disorder in Taiwanese adults: possible explanations and implications. Psychol Med. 2012;42:1227–1237. doi: 10.1017/S0033291711002364 [DOI] [PubMed] [Google Scholar]
- 5.Chong SA, Vaingankar J, Abdin E, et al. The prevalence and impact of major depressive disorder among Chinese, Malays and Indians in an Asian multi-racial population. J Affective Disorders. 2012;138:128–136. doi: 10.1016/j.jad.2011.11.038 [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2013. Available from: 10.1176/appi.books.9780890425596. Accessed November 29, 2023. [DOI]
- 7.Ducasse D, Loas G, Dassa D, et al. Anhedonia is associated with suicidal ideation independently of depression: a meta-analysis. Depress Anxiety. 2018;35:382–392. doi: 10.1002/da.22709 [DOI] [PubMed] [Google Scholar]
- 8.Gabbay V, Johnson AR, Alonso CM, et al. Anhedonia, but not irritability, is associated with illness severity outcomes in adolescent major depression. J Child Adolesc Psychopharmacol. 2015;25:194–200. doi: 10.1089/cap.2014.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8:22. doi: 10.1186/1744-859X-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong L, Yin Y, He C, et al. Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J Psychiatr Res. 2017;84:9–17. doi: 10.1016/j.jpsychires.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 11.Spijker J, Bijl RV, de Graaf R, et al. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands mental health survey and incidence study (NEMESIS). Acta Psychiatr Scand. 2001;103(2):122–130. doi: 10.1034/j.1600-0447.2001.103002122.x [DOI] [PubMed] [Google Scholar]
- 12.Vinckier F, Gourion D, Mouchabac S. Anhedonia predicts poor psychosocial functioning: results from a large cohort of patients treated for major depressive disorder by general practitioners. Eur Psychiatry. 2017;44:1–8. doi: 10.1016/j.eurpsy.2017.02.485 [DOI] [PubMed] [Google Scholar]
- 13.Dunn BD, German RE, Khazanov G, et al. Changes in positive and negative affect during pharmacological treatment and cognitive therapy for major depressive disorder: a secondary analysis of two randomized controlled trials. Clin Psychol Sci. 2020;8(1):36–51. doi: 10.1177/2167702619863427 [DOI] [Google Scholar]
- 14.Craske MG, Meuret AE, Ritz T, et al. Positive affect treatment for depression and anxiety: a randomized clinical trial for a core feature of anhedonia. J Consult Clin Psychol. 2019;87:457–471. doi: 10.1037/ccp0000396 [DOI] [PubMed] [Google Scholar]
- 15.McMakin DL, Olino TM, Porta G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51:404–411. doi: 10.1016/j.jaac.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen MC, Ren H, Fagiolini A. Emotional blunting in patients with depression. Part I: clinical characteristics. Ann Gen Psychiatry. 2022;21:10. doi: 10.1186/s12991-022-00387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma H, Cai M, Wang H. Emotional blunting in patients with major depressive disorder: a brief non-systematic review of current research. Frontiers in Psychiatry. 2021;12:792960. doi: 10.3389/fpsyt.2021.792960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu T, Dong S, Yang L, et al. Investigation of the pharmacological treatment patterns of Chinese patients with major depressive disorder under real-world settings using multi-channel sequence analysis. Front Psychiatry. 2023;14:1089504. doi: 10.3389/fpsyt.2023.1089504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang C-M. What do patients want in the treatment of major depressive disorder? Taiwan’s TAILOR survey. Neurol Ther. 2023;12:21–29. doi: 10.1007/s40120-023-00471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baune BT, Florea I, Ebert B, et al. Patient Expectations and experiences of antidepressant therapy for major depressive disorder: a qualitative study. Neuropsychiatr Dis Treat. 2021;17:2995–3006. doi: 10.2147/NDT.S325954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snaith RP, Hamilton M, Morley S, et al. A scale for the assessment of hedonic tone the Snaith-Hamilton pleasure scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- 23.Trøstheim M, Eikemo M, Meir R, et al. Assessment of anhedonia in adults with and without mental illness: a systematic review and meta-analysis. JAMA Network Open. 2020;3:e2013233. doi: 10.1001/jamanetworkopen.2020.13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Wang L, Zhu Y, et al. Clinical utility of the Snaith-Hamilton-pleasure scale in the Chinese settings. BMC Psychiatry. 2012;12(1):184. doi: 10.1186/1471-244X-12-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagayama H, Kubo S, Hatano T, et al. Validity and reliability assessment of a Japanese version of the Snaith-Hamilton pleasure scale. Intern Med. 2012;51:865–869. doi: 10.2169/internalmedicine.51.6718 [DOI] [PubMed] [Google Scholar]
- 26.Yee A, Loh HS, Ng CG. Factorial validity and reliability of the simplified-Chinese version of Snaith- Hamilton pleasure scale: a study among depressed patients at an out-patient clinic in Malaysia. ASEAN J Psychiatry. 2013;15:66–71. [Google Scholar]
- 27.Ng CG, Chin SC, Yee AHA, et al. Validation of Malay Version of Snaith-Hamilton Pleasure Scale: comparison between depressed patients and healthy subjects at an out-patient clinic in Malaysia. Malays J Med Sci. 2014;21:62–70. [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S-M, Han C-S, Lee S-J, et al. The Korean version of the Snaith-Hamilton pleasure scale: a preliminary study. Mood Emotion. 2011;9:202–205. [Google Scholar]
- 29.Su Y-A, Si T. Progress and challenges in research of the mechanisms of anhedonia in major depressive disorder. Gen Psychiatr. 2022;35:e100724. doi: 10.1136/gpsych-2021-100724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perna G, Alciati A, Daccò S, et al. Personalized psychiatry and depression: the role of sociodemographic and clinical variables. Psychiatry Invest. 2020;17:193–206. doi: 10.30773/pi.2019.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmerman M, Ellison W, Young D, et al. How many different ways do patients meet the diagnostic criteria for major depressive disorder? Compr Psychiatry. 2015;56:29–34. doi: 10.1016/j.comppsych.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 32.Tang W, Liu H, Chen L, et al. Inflammatory cytokines, complement factor H and anhedonia in drug-naïve major depressive disorder. Brain Behav Immun. 2021;95:238–244. doi: 10.1016/j.bbi.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 33.Cao B, Park C, Subramaniapillai M, et al. The efficacy of vortioxetine on anhedonia in patients with major depressive disorder. Front Psychiatry. 2019;10:17. doi: 10.3389/fpsyt.2019.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujii RK, Goren A, Annunziata K, et al. Prevalence, awareness, treatment, and burden of major depressive disorder: estimates from the national health and wellness survey in Brazil. Value Health Reg Issues. 2012;1:235–243. doi: 10.1016/j.vhri.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 35.Cao B, Zhu J, Zuckerman H, et al. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:109–117. doi: 10.1016/j.pnpbp.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 36.Henssler J, Alexander D, Schwarzer G, et al. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(4):300–312. doi: 10.1001/jamapsychiatry.2021.4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serretti A. Anhedonia and Depressive Disorders. Clin Psychopharmacol Neurosci. 2023;21:401–409. doi: 10.9758/cpn.23.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen MC, Ren H, Fagiolini A. Emotional blunting in patients with depression. Part IV: differences between patient and physician perceptions. Ann Gen Psychiatry. 2022; 21: 22. doi: 10.1186/s12991-022-00391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winer ES, Jordan DG, Collins AC. Conceptualizing anhedonias and implications for depression treatments. Psychol Res Behav Manag. 2019;12:325–335. doi: 10.2147/PRBM.S159260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kan K, Jörg F, Buskens E, et al. Patients’ and clinicians’ perspectives on relevant treatment outcomes in depression: qualitative study. BJPsych Open. 2020;6:e44. doi: 10.1192/bjo.2020.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vrieze E, Demyttenaere K, Bruffaerts R, et al. Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disord. 2014;155:35–41. doi: 10.1016/j.jad.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCabe C, Mishor Z, Cowen PJ, et al. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry. 2010;67:439–445. doi: 10.1016/j.biopsych.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all relevant data are within the manuscript and its supporting information files.