Abstract
Objectives
Carica papaya has been widely used commercially for skin care due to its therapeutic benefits. The potential of its flower to promote hair growth has been traditionally recognized in other countries but not in the Philippines. In this study, we explored the effect of various extracts of C. papaya flower in the biological activities associated with hair loss, including 5α-reductase inhibition and antioxidation, as well as identified the putative compounds present in the most potent extract.
Methods
The flowers of C. papaya were macerated separately with ethanol, ethyl acetate, and hexane to obtain their corresponding crude extracts. These extracts were subjected to antioxidant tests via 2,2′-diphenyl-1-picrylhydrazyl (DPPH), and ferric-reducing antioxidant power (FRAP) assays. The total phenolic and flavonoid contents (TPC and TFC) of the crude extracts were determined, as well as the ability of the extracts to inhibit 5α-reductase. The compounds present in the most potent extract were determined using ultraperformance liquid chromatography quadrupole time of flight mass spectrometer (UPLC/MS-QToF).
Results
Ethyl acetate extract displayed significantly higher DPPH activity (0.001755 ± 0.00092 ascorbic acid equivalent antioxidant capacity) and 5α-reductase inhibitory activity (115.18 ± 11.61 mg dutasteride/g) compared to ethanol (DPPH: p=0.0121; 5α-reductase: p=0.0016) and hexane (DPPH: p=0.0038; 5α-reductase: p<0.0001) extracts. Similarly, ethyl acetate extract gave the highest FRAP (0.4842 ± 0.0936 mg ascorbic acid/g) activity, TFC (0.0403 mg quercetin/g), and TPC (0.0463 mg gallic acid/g) among the extracts. Forty-nine compounds were annotated in the ethyl acetate extract, with seven (7) putatively identified as fatty acids (9-hydroxy-10,12-pentadecadienoic acid, 9,12,15-octadecatrienoic acid), hydroxyflavone (5-methylkaempferol), alkaloid (allomatrine), dipeptide derivative (aurantiamide acetate), bufotalinin, and 6β-acetoxy-5-epilimonin based on the Traditional Chinese Medicine Library.
Conclusion
These results suggest that local C. papaya flowers can be a source of hair growth-promoting agents via their antioxidant and 5α-reductase inhibitory potential.
Keywords: papaya, hair grower, 5α-reductase, antioxidant, phenolics, free fatty acids
INTRODUCTION
Alopecia, often known as hair loss, is a complicated phenomenon that may originate from a congenital or genetic illness or may appear later in life.1 The most common form of alopecia is androgenetic alopecia (AGA), which affects approximately 50% of the global population regardless of gender, age, and ethnicity.2,3 It is a condition marked by an increase in the activity and production of the hormone dihydrotestosterone (DHT),4 facilitated by the enzyme 5α-reductase5. It was found that the conversion of testosterone to its more potent version, DHT, causes hair follicle shrinkage and an increase in hair thinning.6,7 Hair loss has also been linked to oxidative stress, characterized by an increased production of free radicals.8 Oxidative stress causes scalp imbalance leading to a damaged scalp, inhibited hair growth, and weakened hair anchoring.9
Since hair is a key aspect of human appearance, AGA may have negative psychosocial consequences.10 It also alters the patient's psychosocial state, social interaction, and daily activities. It may also cause fear, worry, emotional tension, and occasionally sadness.11,12 As a result, the person experiencing hair loss feels less satisfied with their appearance and loses confidence.13
Hair loss treatment often involves inhibition of 5α-reductase enzyme.7 Two commonly used 5α-reductase inhibitors are finasteride and dutasteride, which encourage hair growth, boost hair thickness, and prevent further hair deterioration.14,15 Among the two, dutasteride has been found to yield better hair counts than finasteride.16 Minoxidil is another hair loss agent that prolongs the hair growth phase regardless of the underlying reason for baldness. It functions as a potassium channel blocker that opens blood vessels, allowing more oxygen, blood, and nutrients to reach follicles, thereby, accelerating the anagen phase.2 Studies on the use of antioxidants as components of shampoo and leave-on treatment also proved their benefits in improving scalp condition and hair shedding.17,18 Despite their potency, these drugs are linked to several adverse events such as skin irritation, pruritus, edema, angina pectoris, weight gain, sexual dysfunction, and hypogonadism in males, as well as teratogenic effects in pregnant women.2,19 Given the potential drawbacks associated with conventional treatments, it is crucial to explore alternative therapies that mitigate side effects while effectively addressing the underlying causes of hair loss.
In the Philippines, there is a rich diversity of plant species with medicinal potential, including Carica papaya, commonly known as papaya. This plant is recognized for its various pharmacological activities attributed to the presence of a wide range of phytochemicals in its different plant parts. Ethnobotanical studies in the Philippines have documented the folkloric use of C. papaya in the treatment of gastrointestinal disorders (fruit, root),18–21 dengue (leaf, flowers), skin problems (leaf ),22,23 wounds,24 rheumatism (leaf ),20,25 dysmenorrhea (flowers),23 hypertension,26 and headaches,27 among others. In India, the flower paste of C. papaya has hair growth promoting activity. Scientific investigation also revealed that the flower extracts of C. papaya contain flavonoids,28,29 triterpenoids, saponins and other phytochemicals30 which have been reported to facilitate hair growth31. Thus, we aim to contribute to the scientific understanding of the potential of C. papaya flower extracts to promote hair growth by inhibiting the 5α-reductase enzyme and scavenging free radicals.
MATERIALS AND METHODS
Plant materials
The male and female flowers of C. papaya were collected in Barangay Villa Victoria, Alabat Island, Quezon, Philippines (14°07′11.6″N 122°01′47.6″E) on the mornings of November 2019. Both young and mature flowers were utilized due to the limited amount of flowers collected (Figure 1). The samples were identified and authenticated by the Bureau of Plant Industry (certification number PLT-ID-CRSPD-2246-19). The collected flowers were washed, air-dried, and milled into powder.
Figure 1.

Carica papaya leaf and flower.
Plant extraction
The powdered sample of C. papaya flowers was macerated separately with ethanol, ethyl acetate, and hexane for 48 h followed by sonication for 30 minutes.32,33 The filtrate was collected, evaporated to dryness in a water bath at 40°C, and stored in amber-colored bottles under 2 – 8°C. The percentage yield of the obtained crude extracts was computed.
Antioxidant assays
2,2′-diphenyl-1-picrylhydrazyl (DPPH) assay
The DPPH assay was used to determine the radical scavenging activity of C. papaya extracts.34,35 The crude extracts were dissolved in ethanol to yield extracts at various concentrations (0.1 – 10 mg/mL). A 20 μL extract was transferred to a 96-well plate, treated with 180 μL DPPH (150 μM DPPH in ethanol), and mixed for 60 s. The mixture was incubated in the dark at room temperature for 30 min. The absorbance was measured at 515 nm using a spectrophotometer (BMG Labtech FLUOstar Omega, Ortenberg, Germany). The ability of the extracts to scavenge DPPH was computed using the equation:
where Absblk is the absorbance of the blank and Absext is the absorbance of extract. The half maximal inhibitory concentration (IC50) was computed and the IC50 ratio of the extract and reference standard (ascorbic acid) was calculated to express the ascorbic acid equivalent antioxidant capacity (AEAC) using the following equation.
A higher AEAC value indicates a higher antioxidant activity.
Ferric reducing antioxidant power (FRAP) assay
The method for FRAP assay was adapted from Xiao et al.35 In a 96-well plate, a 20 μL of 10 mg/mL extract and 180 μL freshly prepared FRAP reagent [0.3 M acetate buffer pH 3.6, 10 mM 2,4,6 tripyridyl-s-triazine (TPTZ) solution in 40 mM HCl, and 20 mM ferric chloride (10:1:1)] was added. The mixture was shaken for 60 s and allowed to stand in the dark at 37°C for 15 min. A spectrophotometer was used to measure the absorbance at 595 nm. Ascorbic acid was used as a reference standard to express the ferric ionreducing power of the extracts as equivalent capacity (EC).
Total flavonoid content (TFC)
The determination of total flavonoid content was based on Sembiring et al.36 method with minor modifications. The extracts (10 mg/ ml, 50 μL) were placed in a 96-well plate and mixed with 10 μL 10% aluminum chloride and 180 μL 96% ethanol. After 60 s mixing, 10 μL 1 M sodium acetate was added. The mixtures were left in the dark at room temperature for 40 min. The absorbance of the extracts was measured at 510 nm against a blank. The total flavonoid content was expressed as mg quercetin equivalent (QE) per g of extract computed based on the standard calibration curve of quercetin.
Total phenolic content (TPC)
The total phenolic content of each extract was determined by the Folin-Ciocalteu method adapted from Chaiyana et al.34 with minor modifications. The extract (10 mg/mL, 20 μL) was transferred to a 96-well plate and added with 180 μL Folin–Ciocalteu reagent (1:10). The mixture was incubated for 4 min at room temperature, followed by the addition of 100 g/L sodium carbonate. After mixing, the solution was left at room temperature for another 2 h then the absorbance was measured at 750 nm. The total phenolic content was calculated as mg gallic acid equivalent (GAE) per g of extract using the gallic acid standard calibration curve.
In vitro 5α-reductase inhibitory assay
Animals
Adult ICR mice (20 – 30 g) were procured from the Research Institute for Tropical Medicine, Muntinlupa City, and maintained in the National Institutes of Health Central Laboratory of the University of the Philippines Manila. The mice were housed in separate cages, acclimatized for 1 week, and subjected to a 12-h light/dark cycle at 50–60% humidity at 22–26°C. Sufficient maintenance diet and water were given throughout the acclimatization period. The protocol for the animal studies was approved by the University of the Philippines Manila Institutional Animal Care and Use Committee, with approval number 2019-034.
Liver microsome preparation
The method of Lee et al.2 was used for the preparation of liver microsomes, with modifications on the source of animal liver. Three healthy ICR mice were fasted overnight and euthanized using Tiletamine + Zolazepam (Zoletil®") followed by cervical dislocation. The liver was harvested, washed with ice-cold homogenizing buffer (0.32 M sucrose, 1 mM dithiothreitol, and 20 mM potassium phosphate pH 6.5), homogenized, centrifuged twice at 10,000 g for 10 min, and washed twice with 2 volumes of homogenizing buffer to collect the pellet. The series of washing, homogenization, and centrifugation was performed twice. The collected supernatant from the washings was subjected to another round of centrifugation at 105,000 g for 1 h. The obtained microsome pellets from the centrifugation process were then suspended in homogenizing buffer followed by centrifugation at 105,000 g for 1 h. The produced microsome suspensions were divided into small aliquots and stored at -80°C for further analysis.
Protein content determination
The modified Lowry method was used for the determination of the protein content of the isolated microsome.37 Aliquots of the isolated microsome were added with 0.9 mL of 0.2 g/L KNaC4H4O6 and 100 g/L Na2CO3 in 0.5 M NaOH mixture, and 1 mL H2O then incubated for 10 min at 50°C. The resulting mixture was cooled down to room temperature and then added with 1 mL of 0.2 g/L KNaC4H4O6 and 0.1 g/L CuSO4 in a 0.1M NaOH mixture. The mixture was left to stand for 10 min at room temperature followed by the addition of 3 mL Folin-Ciocalteu phenol reagent in H2O (1:16 v/v) and subsequent incubation at 50°C for 10 min. The absorbance of the mixture was determined at 600 nm and plotted against the standard curve using bovine serum albumin.
Testosterone determination
The microsomes were diluted with 40 mM phosphate buffer pH 6.5 immediately before use to obtain a 30 μg freshly prepared protein concentration. The extracts' inhibitory activity followed Morikawa et al.38 method with slight modifications.
Reaction solution containing 1 mM dithiothreitol (25 μL), 1 mg/mL testosterone (75 μL), and 1.54 mg/mL NADPH (125 μL) was pre-incubated with the extracts (50 μL). The resulting mixture was added with 250 μL microsome followed by vortex mixing for 1 min and subsequent incubation for 10 min at 37°C. The reaction was stopped by the addition of 1 mL ethyl acetate. The internal standard (125 μL 1 mg/mL propylparaben) was added then the mixture was centrifuged at 105,000 g for 4 min. The organic layer was collected and evaporated to dryness while the residue was dissolved in 3 mL methanol, syringed filtered, and transferred to vials for quantification of testosterone using the developed high-performance liquid chromatography-photodiode array (HPLC-PDA, Waters Alliance, Manchester, United Kingdom) method. Additional reactions were prepared: complete reaction (rxn) containing 2% DMSO in place of the extract and an enzyme blank (blk) that was mixed with ethyl acetate before the addition of microsome. The % inhibition against 5α-reductase was calculated using the testosterone content (T) following the equation:
Dutasteride served as the positive control. The IC50 value for dutasteride was determined, and based on its inhibitory equation, the dutasteride equivalent (DE) of each extract was calculated. A high DE value signifies a strong 5α-reductase inhibitory potential.
Metabolite profiling of the extract
The extract with the highest 5α-reductase inhibitory and antioxidant activities was subjected to metabolite profiling to determine the putative compounds. Briefly, the extract was diluted with methanol to a final concentration of 0.5 mg/mL and passed through a 0.2 um PTFE syringe filter. A 5 μL filtered extract was injected into Waters Acquity ultraperformance liquid chromatography (UPLC) I-Class/Xevo with Xevo G2-XS quadrupole time of flight (QTOF) mass spectrometer (MS) and separated on an Acquity HSS T3 2.1 x 100 mm C18 column, with column temperature of 40°C. The mobile phase used was a mixture of water + 0.1% formic acid (A) and acetonitrile + 0.1% formic acid (B). A 0.4 mL/min linear gradient of 95% A for 0.5 min was initiated, followed by 95% A → 5% A (10 min), 5% A (4.5 min hold), 5% A → 1% A (2.5 min), 1% A → 95% A (2.5 min).
The Waters Xevo G2-XS QToF MSE mode was used for the detector, with the following MS parameters: capillary voltage - 1.0 kV (ESI+), source temperature - 120°C, desolvation temperature - 550°C, cone voltage – 40 V, cone glass flow – 40 L/h, desolvation gas flow – 950 L/h, and collision energy - high energy ramp 15 to 50 eV. The samples were scanned at 0.150 s, with a scan range of 100 – 1200 m/z. Leucine enkephalin was used as a reference for mass correction. All the data were processed using the UNIFI Scientific Information System, with matching of the distinct peaks against the Traditional Chinese Medicine Library. Parent compounds with ppm error of ≤7.5 and in-silico fragmentation patterns detected in the MSE fragmentation sample database are considered as identified with confidence.
Statistical analysis
All data were reported as mean ± standard error of mean (SEM). All experiments were done in triplicate. Individual differences in the extracts were evaluated using GraphPad Prism 8 one-way analysis of variance (one-way ANOVA) followed by Tukey’s post hoc test. Mean values were considered statistically significant when p<0.05.
RESULTS
Determination of percent yield and antioxidant activity
Among the C. papaya extracts, the ethyl acetate extract produced the highest yield, followed by ethanol extract, and hexane extract. Similarly, ethyl acetate extract showed the highest activity in the DPPH and FRAP assay, in terms of the AEAC and EC, respectively (Table 1). Significant differences were observed between ethyl acetate and ethanol extracts (p=0.0121), and between ethyl acetate and hexane extracts (p=0.0038) for DPPH activity. For FRAP activity, hexane extract demonstrated significant differences with ethanol (p=0.0021) and ethyl acetate extracts (p=0.00083).
Table 1.
Percent Yield and Antioxidant Activity of C. papaya Extracts
| C. papaya extracts | % Yield | AEACDPPH | ECFRAP |
|---|---|---|---|
| Ethanol | 2.12 | 0.000705 ± 0.00037a | 0.4343 ± 0.0770b |
| Hexane | 0.79 | 0.000418 ± 0.00036a | 0.1892 ± 0.0339 |
| Ethyl acetate | 7.62 | 0.001755 ± 0.00092 | 0.4842 ± 0.0936b |
Results are presented as mean ± SEM (n = 3) calculated using one-way ANOVA followed by Tukey’s post hoc test. Significant differences with ethyl acetate (a) and hexane (b) at p<0.05.
AEACDPPH = Ascorbic acid equivalent antioxidant capacity; ECFRAP = mg Ascorbic acid/g extract
Assessment of total flavonoid (TFC) and total phenolic contents (TPC)
The equation of calibration curve of quercetin standard was y = 6.8184x + 0.0491, r2= 0.9919. This equation was used to determine the TFC of C. papaya extracts in terms of QE per g extract. Ethyl acetate extract showed the highest TFC, although it was not statistically significant with other extracts (Figure 2).
Figure 2.
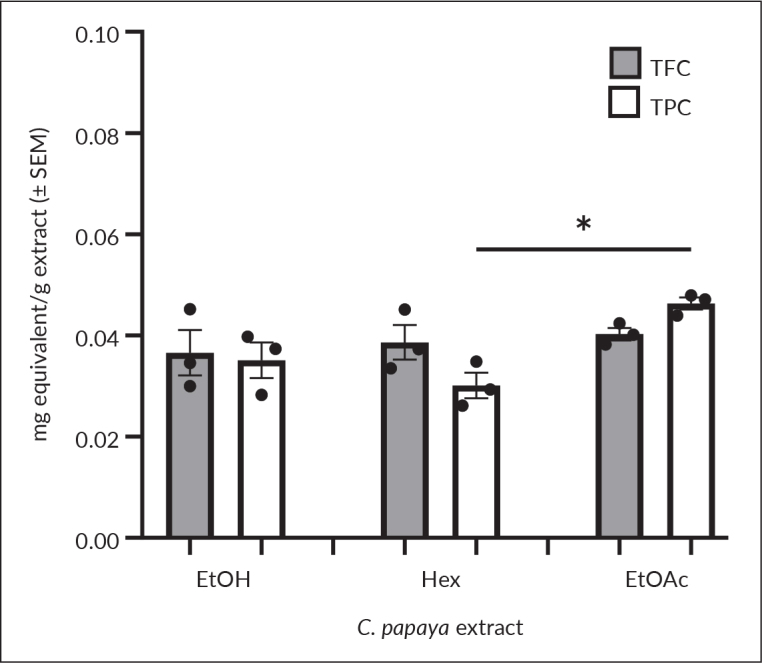
Total flavonoid and total phenolic contents of C. papaya extracts are expressed as mg quercetin equivalent per g extract and mg gallic acid equivalent per g extract, respectively. Results are presented as mean ± SEM (n = 3). Significant difference at p<0.05 (*).
For TPC, the GAE per C. papaya extract was computed based on the equation, y = 8.8825 – 0.0245, r2=0.9925. Among the extracts, the ethyl acetate revealed the highest TPC, followed by ethanol (p=0.050), and hexane (p=0.010) extracts (Figure 2).
Inhibition of 5α-reductase
The standard curve of the 5α-reductase inhibitory activity of dutasteride, with the equation y = 0.1856x + 29.622 (r2=0.9975), was used to determine the activity of the extracts. The IC50 of dutasteride was found to be 109.75 ± 4.53 μg/mL. All the extracts, except hexane, inhibited 5α-reductase, with values ranging from 79.36 to 115.18 mg dutasteride equivalent/g extract (Figure 3). Ethyl acetate extract showed highly significant inhibitory activity compared to ethanol extract (p=0.0016).
Figure 3.
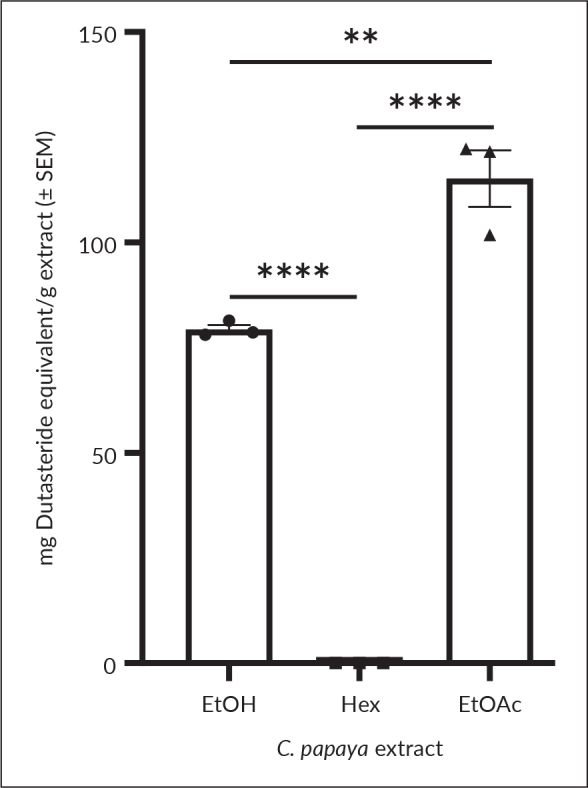
The 5α-reductase inhibitory activity of C. papaya extracts expressed as mg dutasteride equivalent per g extract. Results are presented as mean ± SEM (n = 3). Significant difference at p<0.01 (**) and p<0.0001 (****).
UPLC/MS-QToF analysis of the active extract
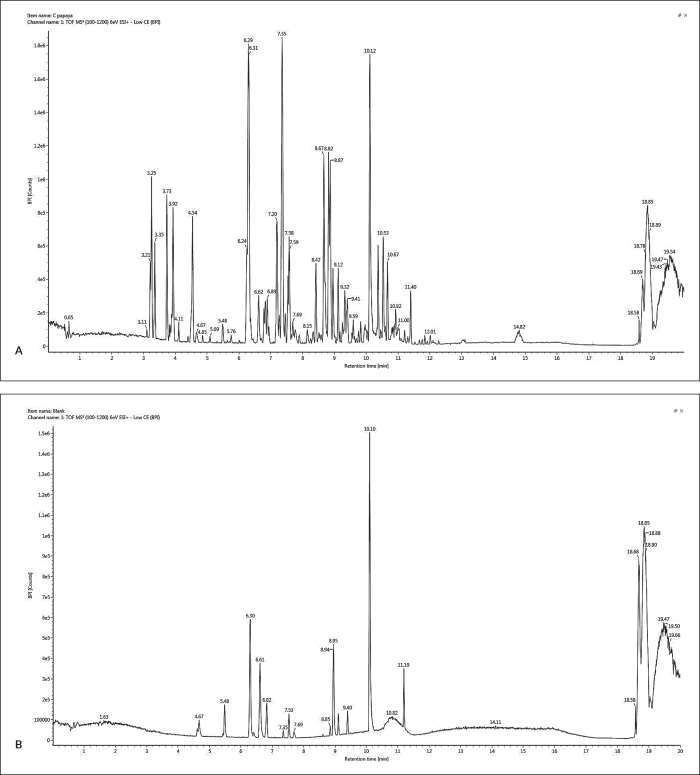
Identification of the putative compounds present in the C. papaya ethyl acetate extract was conducted via UPLC/MS-QToF. Representative chromatograms of the extract and blank diluent are provided in Figure 4. A total of 49 components were annotated, with seven phytochemicals identified based on the Traditional Chinese Medicine Library. The identified constituents are two fatty acids (9-hydroxy-10,12-pentadecadienoic acid, 9,12,15-octadecatrienoic acid), a hydroxyflavone (5-methylkaempferol), an alkaloid (allomatrine), a dipeptide derivative (aurantiamide acetate), bufotalinin, and 6β-acetoxy-5-epilimonin (Table 2).
Figure 4.
Representative chromatograms of 0.5 mg/mL C. papaya ethyl acetate extract (A) and methanol as the blank diluent (B). Blank peaks were discriminated from the sample peaks.
Table 2.
Putative Compounds Identified in C. papaya Ethyl Acetate Extract
| Component name | Formula | Retention time, min | Response | Neutral mass (Da) | Observed neutral mass (Da) | Observed m/z | Error | Adducts |
|---|---|---|---|---|---|---|---|---|
| CM 202.1018 | 3.11 | 21595 | - | - | 202.1018 | - | ||
| 5-Methyl kaempferol | C 16 H 12 O 6 | 3.21 | 20162 | 300.0634 | 300.0708 | 301.0781 | 7.459 | +H |
| CM 258.1988 | 3.25 | 642554 | - | - | 258.1988 | - | ||
| CM 252.1519 | 3.35 | 349514 | - | - | 252.1519 | - | ||
| CM 479.3775 | 3.73 | 616576 | - | - | 479.3775 | - | ||
| CM 300.2214 | 3.82 | 107994 | - | - | 300.2214 | - | ||
| CM 542.3883 | 3.88 | 19881 | - | - | 542.3883 | - | ||
| CM 479.3930 | 3.93 | 577928 | - | - | 479.393 | - | ||
| CM 701.5102 | 4.10 | 272190 | - | - | 701.5102 | - | ||
| CM 286.2214 | 4.54 | 528310 | - | - | 286.2214 | - | ||
| CM 308.2207 | 4.86 | 86143 | - | - | 308.2207 | - | ||
| CM 328.2457 | 5.09 | 68949 | - | - | 328.2457 | - | ||
| Allomatrine | C 15 H 24 N 2 O | 5.76 | 70272 | 248.1889 | 248.1818 | 249.1891 | 7.0308 | +H |
| CM 316.2854 | 6.79 | 182915 | - | - | 316.2854 | - | ||
| CM 367.2095 | 6.94 | 140224 | - | - | 367.2095 | - | ||
| CM 318.3004 | 7.19 | 628930 | - | - | 318.3004 | - | ||
| CM 358.2953 | 7.27 | 227550 | - | - | 358.2953 | - | ||
| CM 376.2612 | 7.36 | 1742667 | - | - | 376.2612 | - | ||
| Aurantiamide acetate | C 27 H 28 N 2 O 4 | 7.46 | 352725 | 444.2049 | 444.2088 | 467.1981 | 3.9367 | +Na, +H, +K |
| Bufotalinin | C 24 H 30 O 6 | 7.54 | 733625 | 414.2042 | 414.2083 | 437.1975 | 4.0318 | +Na, +H, +K |
| CM 360.3133 | 7.58 | 453601 | - | - | 360.3133 | - | ||
| CM 256.3004 | 7.78 | 156766 | - | - | 256.3004 | - | ||
| CM 344.3158 | 7.90 | 101813 | - | - | 344.3158 | - | ||
| CM 472.3995 | 8.15 | 44726 | - | - | 472.3995 | - | ||
| CM 379.2450 | 8.34 | 157879 | - | - | 379.245 | - | ||
| 6β-Acetoxy-5-epilimonin | C 28 H 32 O 10 | 8.42 | 578786 | 528.1996 | 528.2013 | 529.2086 | 1.7392 | +H |
| CM 574.3949 | 8.51 | 207832 | - | - | 574.3949 | - | ||
| CM 395.2396 | 8.67 | 982155 | - | - | 395.2396 | - | ||
| CM 371.2652 | 8.82 | 20901 | - | - | 371.2652 | - | ||
| 9-Hydroxy-10,12-pentadecadienoic acid | C 15 H 26 O 3 | 9.12 | 265525 | 254.1882 | 254.1909 | 277.1801 | 2.6669 | +Na |
| CM 277.2152 | 9.17 | 106868 | - | - | 277.2152 | - | ||
| CM 419.2461 | 9.27 | 301029 | - | - | 419.2461 | - | ||
| CM 381.2646 | 9.33 | 346161 | - | - | 381.2646 | - | ||
| CM 365.2711 | 9.56 | 123801 | - | - | 365.2711 | - | ||
| CM 424.3084 | 9.60 | 144931 | - | - | 424.3084 | - | ||
| CM 324.2931 | 9.76 | 106170 | - | - | 324.2931 | - | ||
| CM 366.3037 | 9.83 | 96212 | - | - | 366.3037 | - | ||
| 9,12,15-Octadecatrienoic acid | C 18 H 30 O 2 | 9.97 | 208758 | 278.2246 | 278.2253 | 279.2326 | 0.7502 | +H, +Na |
| 9,12,15-Octadecatrienoic acid | C 18 H 30 O 2 | 10.38 | 320172 | 278.2246 | 278.2243 | 279.2316 | -0.2711 | +H |
| CM 466.3157 | 10.45 | 163636 | - | - | 466.3157 | - | ||
| CM 809.5277 | 10.53 | 105972 | - | - | 809.5277 | - | ||
| CM 256.2648 | 10.67 | 272980 | - | - | 256.2648 | - | ||
| CM 501.3418 | 10.84 | 169456 | - | - | 501.3418 | - | ||
| CM 313.2751 | 10.90 | 111447 | - | - | 313.2751 | - | ||
| CM 370.2674 | 11.40 | 234014 | - | - | 370.2674 | - | ||
| CM 346.2829 | 11.85 | 66642 | - | - | 346.2829 | - | ||
| CM 427.3254 | 12.01 | 11366 | - | - | 427.3254 | - | ||
| CM 399.3422 | 12.10 | 196738 | - | - | 399.3422 | - | ||
| CM 633.1320 | 12.28 | 132037 | - | - | 633.132 | - | ||
| CM 359.2420 | 14.82 | 24777 | - | - | 359.242 | - |
CM = Candidate Mass
DISCUSSION
The use of C. papaya as a therapeutic component in skin care has gained commercial acceptance because it is rich in antioxidants. Ethnobotanical reports in the Philippines showed its potential to treat several communicable and noncommunicable diseases, but not hair growth. In this study, we used different extracts of C. papaya flowers to investigate their potential antioxidant and 5α-reductase inhibitory activities, which are biological mechanisms associated with hair loss. We believe that this study is necessary to support the traditional claim of the hair-growth promoting properties of C. papaya flower in other countries and to identify the putative compounds responsible for this effect.
Numerous experimental findings support the concept that oxidative stress plays a significant role in the aging process. As we age, we generate more free radicals while our body’s defense mechanism gradually weakens. Consequently, this ongoing imbalance has deleterious effects on various cellular structures and extracellular biomolecules, including the hair. The outcome is often manifested as hair aging, characterized by a decrease in hair production or hair graying.8,39 In this sense, antioxidants may have an important role in preventing oxidative stress. Among the extracts used in the study, C. papaya ethyl acetate extract displayed the highest antioxidant activity, both in its ability to scavenge DPPH free radicals and reduce ferric ions. This activity might be attributed to the richness of phenolic compounds in the C. papaya ethyl acetate extract. In the Philippines, C. papaya male flowers collected from Nueva Ecija contain phenolic compounds, such as flavonoids, and polyphenols, in agreement with the current study.22 The antioxidant properties of phenolic compounds are primarily due to their redox characteristics.40 The structure of phenolic compounds is composed of an aromatic ring backbone with one or more hydroxyl groups as substituents. Through these hydroxyl groups, the phenolic compounds can elicit their reducing power and radical scavenging effect.41 However, the total phenolic content of the potent C. papaya ethyl acetate extract was approximately six-fold lower compared to C. papaya flower extracts collected from other countries.29,42
The 5α-reductase is an enzyme that reduces 3-oxo-4 steroidal substances, such as testosterone, progesterone, and corticosterone, into more potent androgens. In humans, this enzyme catalyzes the conversion of testosterone into DHT, which is essential for normal male growth. High DHT expression, on the other hand, stimulates androgen-related diseases including androgenic alopecia.43,44 As a result, several studies target the inhibition of this enzyme to promote hair growth. In our study, the capacity to inhibit 5α-reductase varied among the C. papaya extracts tested – only ethanol and ethyl acetate displayed potent 5α-reductase inhibitory activity. These varying activities may be affected by the polarity of the extracting solvent, which influences the composition and types of compounds in the extract.
We also identified the putative metabolites present in C. papaya ethyl acetate extract via UPLC/MS-QToF including free fatty acids, hydroxy flavone, alkaloid, and dipeptide derivative. These components may be responsible for the C. papaya ethyl acetate extract’s potent 5α-reductase inhibitory activity. Several studies have documented that plants' free fatty acid-rich extracts effectively inhibit 5α-reductase activity,45–48 with saturated fatty acids containing C12-C16 chains and carbon-carbon double bonds improve the activity in vitro49. Hydroxy flavones, particularly kaempferol, also exemplified inhibitory activity against 5α-reductase.39,45 Kaempferol derivatives, kaempferol, kaempferol 3-O-α-L-rhamnopyranoside, kaempferol 3-O-β-D-glucopyranoside, kaempferol 3-O-α-L-arabinopyranoside, were also found in the ethyl acetate fraction of C. papaya male flowers collected from Quang Nam - Da Nang, Vietnam.28 There was no reported literature on the ability of allomatrine to inhibit 5α-reductase although an alkaloid derivative isolated from Piper nigrum displayed inhibitory activity.50 Likewise, the potential antioxidant and 5α-reductase inhibitory effect of dipeptidyl derivatives and other identified components are still unknown and inconclusive. Hence, the potential of these putative compounds as 5α-reductase inhibitors and antioxidants may be further explored.
CONCLUSION
In this study, we verified that the crude ethyl acetate extract of C. papaya flower has 5α-reductase inhibitory and antioxidant activities, which are associated mechanisms for hair growth promotion. These activities may possibly be attributed to the phenolic compounds, free fatty acids, and hydroxy flavone that were putatively identified in the potent extract. Further tests are needed to fully characterize the major metabolite/s responsible for 5α-reductase inhibitory and antioxidant activities. Moreover, it is worth exploring alternative mechanisms through which these compounds promote hair growth.
Acknowledgments
The authors express their gratitude to the Philippine Institute of Traditional and Alternative Health Care (PITAHC) for funding this study.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
Author Disclosure
All authors declared no conflicts of interest.
REFERENCES
- 1.Novak MA, Meyer JS. Alopecia: possible causes and treatments, particularly in captive nonhuman primates. Comp Med. 2009. Feb;59(1):18–26. PMID: 19295051; PMCID: . [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HH, Ho CT, Lin JK. Theaflavin-3,3’-digallate and penta-O-galloyl-β-D-glucose inhibit rat liver microsomal 5α-reductase activity and the expression of androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2004. Jul;25(7):1109–18. doi: 10.1093/carcin/bgh106. PMID: 14963012. [DOI] [PubMed] [Google Scholar]
- 3.Salman KE, Altunay IK, Kucukunal NA, Cerman AA. Frequency, severity, and related factors of androgenetic alopecia in dermatology outpatient clinic: hospital-based cross-sectional study in Turkey. An Bras Dermatol. 2017. Jan-Feb;92(1):35–40. doi: 10.1590/abd1806-4841.20175241. PMID: 28225954; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asfour L, Cranwell W, Sinclair R. Male Androgenetic Alopecia. In: Feingold K, editor. Endotext [Internet]. MDText.com, Inc.; 2023. [cited 2022 Dec 7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278957/ [Google Scholar]
- 5.Ustuner ET. Cause of androgenic alopecia: crux of the matter. Plast Reconstr Surg Glob Open. 2013. Nov;1(7):e64. doi: 10.1097/GOX.0000000000000005. PMID: 25289259; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallob AL, Sadick NS, Unger W, Lipert S, Geissler LA, Gregoire SL, et al. The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. J Clin Endocrinol Metab. 1994. Sep;79(3):703–6. doi: 10.1210/jcem.79.3.8077349. PMID: 8077349. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998. Oct;39(4 Pt 1):578-89. doi: 10.1016/s0190-9622(98)70007-6. PMID: 9777765. [DOI] [PubMed] [Google Scholar]
- 8.Trueb RM. Oxidative stress in ageing of hair. Int J Trichology. 2009. Jan;1(1):6-14. doi: 10.4103/0974-7753.51923. PMID: 20805969; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trüeb RM. Oxidative stress and its impact on skin, scalp and hair. Int J Cosmet Sci. 2021. Nov;43 Suppl 1:S9–S13. doi: 10.1111/ics.12736. PMID: 34424547. [DOI] [PubMed] [Google Scholar]
- 10.Terry RL. Further evidence on components of facial attractiveness. Percept Mot Skills. 1977;45(1):130. doi: 10.2466/pms.1977.45.1.130. [DOI] [Google Scholar]
- 11.Tucker P. Bald is beautiful?: the psychosocial impact of alopecia areata. J Health Psychol. 2009. Jan;14(1):142–51. doi: 10.1177/1359105308097954. PMID: 19129346. [DOI] [PubMed] [Google Scholar]
- 12.Hunt N, McHale S. The psychological impact of alopecia. BMJ. 2005. Oct;331(7522):951-3. doi: 10.1136/bmj.331.7522.951. PMID: 16239692; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cash TF. The psychosocial consequences of androgenetic alopecia: a review of the research literature. Br J Dermatol. 1999. Sep;141(3):398-405. doi: 10.1046/j.1365-2133.1999.03030.x. PMID: 10583042. [DOI] [PubMed] [Google Scholar]
- 14.Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017. Jul;77(1):136–41.e5. doi: 10.1016/j.jaad.2017.02.054. PMID: 28396101. [DOI] [PubMed] [Google Scholar]
- 15.Boersma IH, Oranje AP, Grimalt R, Iorizzo M, Piraccini BM, Verdonschot EH. The effectiveness of finasteride and dutasteride used for 3 years in women with androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2014. Nov-Dec;80(6):521–5. doi: 10.4103/0378-6323.144162. PMID: 25382509. [DOI] [PubMed] [Google Scholar]
- 16.Shanshanwal SJS, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017. Jan-Feb;83(1):47–54. doi: 10.4103/0378-6323.188652. PMID: 27549867. [DOI] [PubMed] [Google Scholar]
- 17.Davis MG, Piliang MP, Bergfeld WF, Caterino TL, Fisher BK, Sacha JP, et al. Scalp application of antioxidants improves scalp condition and reduces hair shedding in a 24-week randomized, double-blind, placebo-controlled clinical trial. Int J Cosmet Sci. 2021. Nov;43 Suppl 1:S14–S25. doi: 10.1111/ics.12734. PMID: 34424558. [DOI] [PubMed] [Google Scholar]
- 18.Davis MG, Piliang MP, Bergfeld WF, Caterino TL, Fisher BK, Sacha JP, et al. Scalp application of the antioxidant piroctone olamine reduces hair shedding in an 8-week randomized, double-blind, placebo-controlled clinical study. Int J Cosmet Sci. 2021. Nov;43 Suppl 1:S26–S33. doi: 10.1111/ics.12737. PMID: 34424549. [DOI] [PubMed] [Google Scholar]
- 19.Boisvert WA, Yu M, Choi Y, Jeong GH, Zhang YL, Cho S, et al. Hair growth-promoting effect of Geranium sibiricum extract in human dermal papilla cells and C57BL/6 mice. BMC Complement Altern Med. 2017. Feb;17(1):109. doi: 10.1186/s12906-017-1624-4. PMID: 28193226; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe R, Ohtani K. An ethnobotanical study of medicinal plants and traditional therapies on Batan Island, the Philippines. J Ethnopharmacol. 2013. Jan;145(2):554–65. doi: 10.1016/j.jep.2012.11.029. PMID: 23183086. [DOI] [PubMed] [Google Scholar]
- 21.Tantiado RG. Survey on ethnopharmacology of medicinal plants in Iloilo, Philippines. International Journal of Bio-Science and Bio-Technology. 2012. Dec;4(4):11–26. [Google Scholar]
- 22.Susaya-Garcia J, Borja N, Sevilla-Nastor JB, Villanueva JD, Peyraube N. An ethnobotanical study of medicinal plants and perceptions on plant biodiversity conservation in Leyte, Philippines. J Hum Ecol. 2018;7(1):26–42. [Google Scholar]
- 23.Fabie-Agapin JS. Medicinal plants used by traditional healers in Pagadian City, Zamboanga del Sur, Philippines. Philipp J Sci. 2020. Mar;149(1):83–9. [Google Scholar]
- 24.Balangcod TD, Balancod KD. Ethnomedicinal plants in Bayabas, Sablan, Benguet Province, Luzon, Philippines. eJBio. 2015;11(3): 63–73. [Google Scholar]
- 25.Ong HG, Kim YD. Quantitative ethnobotanical study of the medicinal plants used by the Ati Negrito indigenous group in Guimaras Island, Philippines. J Ethnopharmacol. 2014. Nov;157:228–42. doi: 10.1016/j.jep.2014.09.015. PMID: 25240586. [DOI] [PubMed] [Google Scholar]
- 26.Balinado LO, Chan MA. An ethnomedicinal study of plants and traditional health care practices in District 7, Cavite, Philippines. International Conference on Chemical, Agricultural, Biological and Medical Sciences (CABMS-17). Manila, Philippines; 2017. [Google Scholar]
- 27.Tantengco OAG, Condes MLC, Estadilla HHT, Ragragio EM. Ethnobotanical survey of medicinal plants used by Ayta communities in Dinalupihan, Bataan, Philippines. Pharmacogn J. 2018;10(5):859–70. doi: 10.5530/pj.2018.5.145. [DOI] [Google Scholar]
- 28.Van DTT, Cuong DH, Lien GTK, Yen PH. Phytochemical study of the ethyl acetate extract of male Carica papaya flowers from Quang Nam-Da Nang. Vietnam J Chem. 2020. Apr;58(2):145-50. doi: 10.1002/vjch.201900029. [DOI] [Google Scholar]
- 29.Dwivedi MK, Sonter S, Mishra S, Patel DK, Singh PK. Antioxidant, antibacterial activity, and phytochemical characterization of Carica papaya flowers. Beni-Suef Univ J Basic Appl Sci. 2020;9:23. doi: 10.1186/s43088-020-00048-w. [DOI] [Google Scholar]
- 30.Bergonio KB, Perez MA. The potential of male papaya (Carica papaya L.) flower as a functional ingredient for herbal tea production. Indian J Tradit Knowle. 2016. Jan;15(1):41–9. [Google Scholar]
- 31.Premanand A, Ancy VB, Jeevanandam J, Rajkumari BR, Danquah MK. Chapter 14 - Phytochemicals as emerging therapeutic agents for alopecia treatment. In: Egbuna C, Kumar S, Ifemeje JC, Ezzat SM, Kaliyaperumal S, editors. Phytochemicals as Lead Compounds for New Drug Discovery [Internet]. Elsevier; 2020. p. 221–238. [cited 2022 Dec 7]. Available from: https://www.sciencedirect.com/science/article/pii/B9780128178904000147 [Google Scholar]
- 32.Annegowda HV, Bhat R, Min-Tze L, Karim AA, Mansor SM. Influence of sonication treatments and extraction solvents on the phenolics and antioxidants in star fruits. J Food Sci Technol. 2012. Aug;49(4):510-4. doi: 10.1007/s13197-011-0435-8. PMID: 23904662; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusama IW, Murdiyanto, Arung ET, Syafrizal, Kim Y. Antimicrobial and antioxidant properties of medicinal plants used by the Bentian tribe from Indonesia. Food Sci Hum Wellness. 2014. Sep-Dec;3(3-4):191-6. doi: 10.1016/j.fshw.2014.12.004. [DOI] [Google Scholar]
- 34.Chaiyana W, Punyoyai C, Somwongin S, Leelapornpisid P, Ingkaninan K, Waranuch N, et al. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex Vaucher extract as functional food and nutraceuticals ingredients. Nutrients. 2017. Oct;9(10):1105. doi: 10.3390/nu9101105. PMID: 28994714; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao F, Xu T, Lu B, Liu R. Guidelines for antioxidant assays for food components. Food Frontiers. 2020. Mar;1(1):60-9. doi: 10.1002/fft2.10. [DOI] [Google Scholar]
- 36.Sembiring EN, Elya B, Sauriasari R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn J. 2018. Jan-Feb;10(1):123-7.doi: 10.5530/pj.2018.1.22. [DOI] [Google Scholar]
- 37.Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972. Aug;48(2):422-7. doi: 10.1016/0003-2697(72)90094-2. PMID: 4115981. [DOI] [PubMed] [Google Scholar]
- 38.Morikawa T, Luo F, Manse Y, Sugita H, Saeki S, Chaipech S, et al. Geranylated coumarins from Thai medicinal plant Mammea siamensis with testosterone 5α-reductase inhibitory activity. Front Chem. 2020. Mar;8:199. doi: 10.3389/fchem.2020.00199. PMID: 32266216; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park HJ, Jin GR, Jung JH, Hwang SB, Lee SH, Lee BH. Hair growth promotion effect of Nelumbinis Semen extract with high antioxidant activity. Evid Based Complement Alternat Med. 2021. Mar;2021:6661373. doi: 10.1155/2021/6661373. PMID: 33790980; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flieger J, Flieger W, Baj J, Maciejewski R. Antioxidants: classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials (Basel). 2021. Jul;14(15):4135. doi: 10.3390/ma14154135. PMID: 34361329; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vuolo MM, Lima VS, Junior MRM. Chapter 2 - Phenolic Compounds: Structure, Classification, and Antioxidant Power. In: Campos MRS, editor. Bioact Compd [Internet]. Woodhead Publishing; 2019. [cited 2022 Dec 7]. p. 33–50. Available from: https://www.sciencedirect.com/science/article/pii/B9780128147740000025 [Google Scholar]
- 42.Halder S, Dutta S, Khaled KL. Evaluation of phytochemical content and in vitro antioxidant properties of methanol extract of Allium cepa, Carica papaya and Cucurbita maxima blossoms. Food Chem Adv. 2022. Oct;1:100104. doi: 10.1016/j.focha.2022.100104. [DOI] [Google Scholar]
- 43.Lee S, Kang H. Inhibition of 5α-reductase of de novo generation of short antioxidant peptides. Biomed Sci Letters. 2018;24:263-9. doi: 10.15616/BSL.2018.24.3.263. [DOI] [Google Scholar]
- 44.Kumar T, Chaiyasut C, Rungseevijitprapa W, Suttajit M. Screening of steroid 5alpha-reductase inhibitory activity and total phenolic content of Thai plants. J Med Plant Res. 2011;5(7):1265-71. [Google Scholar]
- 45.Hiipakka RA, Zhang HZ, Dai W, Dai Q, Liao S. Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem Pharmacol. 2002. Mar;63(6):1165-76. doi: 10.1016/s0006-2952(02)00848-1. PMID: 11931850. [DOI] [PubMed] [Google Scholar]
- 46.Niederprûm HJ, Schweikert HU, Zanker KS. Testosterone 5α-reductase inhibition by free fatty acids from Sabal serrulata fruits. Phytomedicine. 1994. Sep;1(2):127-33. doi: 10.1016/S0944-7113(11)80030-9. PMID: 23195885. [DOI] [PubMed] [Google Scholar]
- 47.Raynaud JP, Cousse H, Martin PM. Inhibition of type 1 and 2 5-alpha reductase activity by free fatty acids, active ingredients of Permixon. J Steroid Biochem Mol Biol. 2002. Oct;82(2-3):233-9. doi: 10.1016/s0960-0760(02)00187-5. PMID: 12477490. [DOI] [PubMed] [Google Scholar]
- 48.Liang T, Liao S. Inhibition of steroid 5 alpha-reductase by specific aliphatic unsaturated fatty acids. I Biochem J. 1992. Jul;285 (Pt 2):557-62. doi: 10.1042/bj2850557. PMID: 1637346; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Shimizu K, Kondo R. Anti-androgenic activity of fatty acids. Chem Biodivers. 2009. Apr;6(4):503-12. doi: 10.1002/cbdv.200800125. PMID: 19353546. [DOI] [PubMed] [Google Scholar]
- 50.Hirata N, Tokunaga M, Naruto S, Iinuma M, Matsuda H. Testosterone 5alpha-reductase inhibitory active constituents of Piper nigrum leaf. Biol Pharm Bull. 2007. Dec;30(12):2402-5. doi: 10.1248/bpb.30.2402. PMID: 18057734. [DOI] [PubMed] [Google Scholar]