Abstract
Despite wide availability of prevention and treatment services, including the ongoing roll-out of pre-exposure prophylaxis (PrEP), the HIV epidemic is not under control in Belgium. Hence, there is a recognized need to improve case finding and early diagnosis to curb the further spread of HIV more effectively. The objective of the present study was to improve insight into the profiles of persons recently infected with HIV-1 and on their prevention trajectory. Between May 2018 and December 2022, we selected persons diagnosed in Belgium within three months of the presumed infection date. We then analyzed information collected using a questionnaire covering topics on HIV testing, sexually transmitted infections (STIs), PrEP use, sexual behavior, partner notification and substance use. The data obtained were analyzed alongside information derived from phylogenetic cluster analysis of the viral source of infection. A total of 93 persons with a recent HIV-1 infection completed the questionnaire, the majority (74%) being MSM, 14% were heterosexual women and 12% were heterosexual men. Nearly one-third of participants engaged in sexual activity with an average of 2 to 5 casual partners around the presumed time of infection. A significant percentage reported frequent substance use during sexual activity (65%), being previously diagnosed with STI (65%) and using condoms infrequently (44%). 63% reported a testing frequency of at least one HIV test per year before being diagnosed and 46% notified their previous sex partner(s) after being diagnosed. Over 20% of respondents (including 11 MSM, 4 heterosexual men and 5 heterosexual women) reported exclusive sexual activity with their steady partner. Eight participants (9%, all MSM, 75% born outside of Belgium) reported PrEP use in the past. No significant differences in behavioral characteristics were found between persons who were part of a local transmission cluster (48%) and persons that were not part of a cluster (47%). The study results revealed that the majority of persons diagnosed early with HIV-1 infection in Belgium exhibited characteristics corresponding to a high-at-risk population and were aware of this risk, as evidenced by a high testing frequency. However, partner notification rates were low and use and awareness of PrEP limited. A notable group of persons not corresponding to the high-risk profiles was also identified. This information may help to expose missed opportunities for prevention and contribute to enhancing the implementation of future prevention measures.
Keywords: HIV- recent infections-transmission clusters-behavior-prevention
Introduction
Over the past decade, there has been a consistent decline in the number of new HIV diagnoses, attributed to the effectiveness of antiretroviral therapy (ART) and a combination of preventive measures such as the promotion of consistent condom use, increased attention for testing of HIV and sexually transmitted infections (STIs) and the implementation of pre-exposure prophylaxis (PrEP) [1, 2]. Despite progress in recent years, the HIV epidemic in Belgium is not yet under control. In 2019, 675 HIV infections were newly diagnosed. After a notable drop in 2020 with 503 new HIV diagnoses, due to the impact of the COVID-19 epidemic and associated isolation measures, the number of new HIV diagnoses increased again in 2021 and 2022 with respectively 523 and 597 new diagnoses [3].
Since the start of the HIV epidemic in Belgium, two key populations have been particularly affected, namely men who have sex with men (MSM) of Belgian nationality, and heterosexual men and women from Sub-Saharan Africa [4]. However, the proportion of populations with other profiles has increased in recent years and the epidemic in Belgium is now more diversified. In 2022, 51% of newly registered HIV infections occurred in heterosexuals, 43% of whom had sub-Saharan African nationalities; 43% of newly registered HIV infections occurred in MSM, 49% of whom were Belgians; 2.5% of diagnoses occurred in transgender women, mainly Latin Americans. Overall, just over a third of those newly diagnosed persons were of a nationality other than Belgian or Sub-Saharan. Three-quarters of newly diagnosed persons aged between 20 and 49 years. Late diagnoses are more common in heterosexuals from Sub-Saharan Africa, while diagnoses in the early stages of infection are more frequent among Belgian MSM [3]. Regarding HIV diagnoses in people of foreign nationality, it is important to note that a substantial proportion of infections occurred before arrival in Belgium. In this way, it was estimated that between 26% and 33% of migrants diagnosed in Belgium in 2007–2016 were infected post-migration [5]. In addition to those with a new HIV diagnosis, based on ECDC’s HIV modelling tool [6], there were an estimated 627 infected but undiagnosed people living with HIV in Belgium in 2022 [3].
Previous research indicated that more than half of people living with HIV diagnosed in Belgium are infected with a virus localized in a phylogenetic transmission cluster, indicating local transmission of these strains [7]. Early detection of and a swift response to growing transmission clusters may contribute to reducing this local transmission [8]. To optimize strategies for prevention of transmission however, a deeper understanding of the current circumstances of infection is essential.
The present study was initiated to shed light on the factors driving ongoing HIV transmission in Belgium and to identify the population(s) most susceptible to infection. For that purpose, we recruited persons diagnosed within 3 months of infection to complete a questionnaire on their behavior around the time of infection.
Materials and Methods
Setting
All 12 Belgian HIV references centers, specialized outpatient clinics for HIV care, were contacted and provided with detailed information about the study protocol. Four of these 12 agreed to participate. Recruitment of HIV-1 infected persons for this study took place over the period May 2018 till December 2022. The 8 HIV reference centers not willing to participate mainly reported practical problems as the cause (lack of time, lack of staff). The study was approved by the institutional review board of Ghent University Hospital (Belgian Registration number B670201732785) and by the medical ethical committees of all participating centers.
Participants
Between May 2018 and February 2021, all newly diagnosed persons with a recent HIV infection were selected. The study inclusion criteria were (a) persons with a HIV infection, most likely acquired less than 3 months before, based on the results of the HIV confirmation assays and/or an additionally performed incidence assay, and (b) aged over 18 years. Exclusion criteria were (a) recently infected HIV persons who did not visit one of the participating centers after diagnosed, (b) persons who did not have sufficient knowledge of Dutch, French or English and (c) persons who experience psychiatric problems.
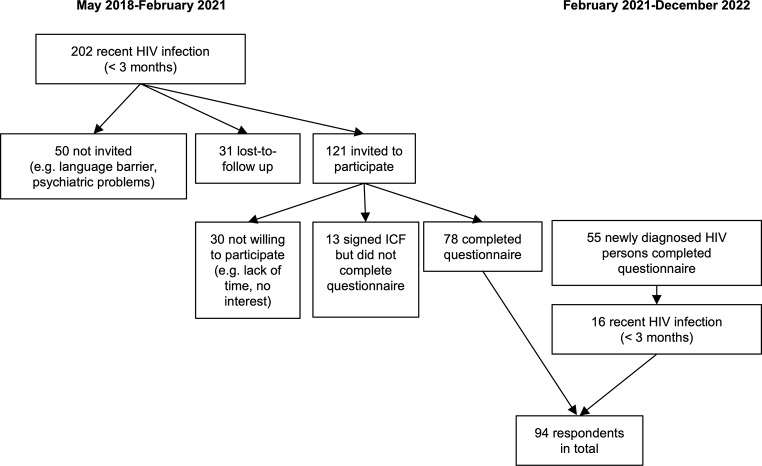
From February 2021 till December 2022, the general recruitment was extended to also include late diagnoses (presumed infection of more than 3 months before diagnosis) as part of a follow-up project. For the 55 persons who completed the questionnaire in this follow-up study, the time of infection was defined and only those with a presumed infection of less than 3 months were included in the analysis presented. Figure 1 gives a flowchart of the selection process of the study population.
Fig. 1.
Flowchart describing the selection process of the study population
Participants were categorized by the combination of reported ‘gender ((transgender) male or (transgender) female))’ and ‘most probable way of infection (via sex with men or via sex with women)’. Sixty-five persons were categorized as MSM (male or transgender male and transmission through sex with men), 9 persons as heterosexual men (male or transgender male and transmission through sex with women) and 11 as heterosexual women (female or transgender female and transmission through sex with men). Two participants indicated ‘injecting drug use’ as the transmission route but this option was reported in combination with ‘sexual transmission’. However, as multiple sex partners and unprotected sex were reported, the sexual transmission route (one MSM, one hetero) was prioritized over injecting drug use for these two male cases. For 7 participants, the transmission route was unknown. To classify those participants, we considered the gender, the reported sexual orientation, and the reported gender of the steady partner. Bisexual men with male steady partner(s) and 2–5 casual sex partners were categorized as MSM (N = 2), heterosexual female with male steady partner as heterosexual women (N = 2), gay, male, with no steady partners and with multiple casual partners, as MSM (N = 1) and male, heterosexual, with female steady partner and no casual partners as heterosexual men (N = 1). One participant did not ‘fit’ any of these categories: male, bisexual, no steady partner, one casual partner and transmission by a puncture incident. This participant was excluded, leaving 93 persons for final analysis.
Infection Timing
The time point of infection was estimated based on the results of the HIV confirmation assays and an additional incidence assay, the Sedia HIV-1 Lag Avidity enzyme immunoassay (Sedia Biosciences Corporation). The latter was performed at the Aids Reference Laboratory of Ghent using an aliquot of left-over plasma collected from the participants at or immediately after diagnosis. Persons were considered as being infected less than 3 months before diagnosis if the results of the Sedia HIV-1 Lag Avidity enzyme immunoassay were indicative for a recent infection (median OD of triplicate measurements < 1.5) or if they were diagnosed during seroconversion (detectable p24 antigen or HIV RNA and a negative or indeterminate result in either the INNO-LIA HIV I/II Score (Fujirebio Europe) or the Geenius HIV ½ Confirmatory Assay (Bio-Rad)). The recency window for the Sedia HIV-1 Lag Avidity enzyme immunoassay was previously defined at 108 days [9]. Because there is a higher chance for false recency predictions in advanced infections, persons with a CD4 count of less than 100 cells/mm3 were excluded from the study.
Phylogenetic Analysis
Since 2013, part of the routine practices in the monitoring of newly diagnosed HIV infected persons in Belgium is baseline resistance testing. The sequences resulting from this baseline resistance testing can serve to construct phylogenetic trees that illustrate the genetic relationship between viruses and allow to visualize transmission histories of the virus. While phylogenetic analysis cannot define the direction of transmission or rule out the presence of intermediary or common transmission links, it can reliably establish epidemiological linkage. A phylogenetic tree was generated with the sequence data from all persons newly diagnosed with HIV-1 in Belgium between 2013 and 2022 who received baseline resistance testing. The maximum likelihood (ML) approach implemented in PhyML 3.0 was used and Branch support was obtained by approximate likelihood-ratio test (aLRT, SH-like). Sequences clustering with at least one other sequence with aLRT of ≥ 0.97 and a mean pairwise distance ≤ 0.015 were considered as genetically linked. For the persons who completed the questionnaire, the position in the phylogenetic tree was defined and used to classify them as ‘isolated’ (located isolated, no close genetic link), ‘paired’ (belonging to a cluster of 2 closely related persons) or ‘clustered’ (being part of a cluster of 3 or more closely related persons).
Procedure
All persons fulfilling the selection criteria were invited by the treating physician or study nurse of the local HIV reference center to participate in the study. After giving informed consent, participants were free to fill in the questionnaire either online or on paper. The questionnaire contained 50 items on HIV testing and STI diagnoses (reasons, where, frequency), PEP & PrEP use, sexual behavior (type of partners, status, condom use, meeting places), alcohol and substance use, depression and partner notification. The questionnaire assessed behavior during the three months prior to HIV diagnosis to correspond with the estimated infection time. Questionnaires were available in English, Dutch and French.
Data Analysis
Demographic descriptive analyses were done on the collected behavioral data to describe behavioral characteristics of the recently infected HIV persons.
Results
Participant Characteristics
Table 1 provides a comprehensive overview of the socio-demographic profile of the 93 respondents. The majority were MSM (N = 69, 74%; 95%CI = [0.65%; 0.83%]) followed by heterosexual women (N = 13, 14%; 95%CI = [0.07%; 0.21%]) and heterosexual men (N = 11, 12%; 95%CI = [0.05%; 0.18%])). Three quarters of the respondents (N = 71; 76%; 95%CI = [0.68%; 0.85%]) had the Belgian nationality. Slightly more than half of the participants completed higher education (N = 50; 54%; 95%CI = [0.44%; 0.64%]) and the majority (N = 59; 63%; 95%CI = [0.54%; 0.73%])) were employed full-time.
Table 1.
Socio-demographic profile of the 93 respondents
| Heterosexual men (N = 11) | Heterosexual women (N = 13) |
MSM (N = 69) |
Total (N = 93) |
|
|---|---|---|---|---|
| Gender | ||||
| Male | 11 (100%) | - | 68 (99%) | 79 (85%) |
| Female | - | 11 (85%) | - | 11 (12%) |
| Transgender female | - | 2 (15%) | - | 2 (2%) |
| Transgender male | - | - | 1 (1%) | 1 (1%) |
| Age (in years) | ||||
| 19–29 | 1 (9%) | 2 (15%) | 16 (24%) | 19 (21%) |
| 30–39 | 6 (55%) | 4 (31%) | 25 (37%) | 35 (38%) |
| 40–49 | 3 (27%) | 4 (31%) | 9 (13%) | 16 (17%) |
| 50–59 | 1 (9%) | 3 (23%) | 14 (21%) | 18 (20%) |
| 60+ | - | - | 4 (6%) | 4 (4%) |
| Nationality | ||||
| Belgian | 11 (100%) | 8 (62%) | 52 (75%) | 71 (76%) |
| Other European | - | 2 (15%) | 8 (12%) | 10 (11%) |
| Sub-Saharan African | - | - | 3 (4%) | 3 (3%) |
| Other | - | 3 (23%) | 6 (9%) | 9 (10%) |
| Education level | ||||
| Primary education | - | 1 (8%) | 5 (7%) | 6 (6%) |
| Secondary education | 6 (55%) | 8 (62%) | 23 (33%) | 37 (40%) |
| Higher education | 5 (45%) | 4 (31%) | 41 (60%) | 50 (54%) |
| Occupation | ||||
| Employed full-time | 11 (100%) | 6 (46%) | 42 (61%) | 59 (63%) |
| Employed part-time | - | 3 (23%) | 7 (10%) | 10 (11%) |
| Student | - | 1 (8%) | 6 (9%) | 7 (8%) |
| Unemployed | - | 3 (23%) | 8 (12%) | 11 (12%) |
| Not able to work | - | - | 1 (1%) | 1 (1%) |
| Not allowed to work | - | - | 1 (1%) | 1 (1%) |
| Retired | - | - | 3 (4%) | 3 (3%) |
| Other | - | 1 (1%) | 1 (1%) | |
| Self-reported sexual orientation | ||||
| Homosexual/lesbian | - | 2 (15%)a | 51 (74%) | 53 (57%) |
| Heterosexual/straight | 11 (100%) | 11 (85%) | 1 (1%) | 23 (25%) |
| Bisexual | - | - | 16 (23%) | 16 (17%) |
| Other | - | - | 1 (1%) | 1 (1%) |
| Type of partners | ||||
| Only casual partner(s) | 2 (18%) | 2 (15%) | 28 (41%) | 32 (34%) |
| Only steady partner | 4 (36%) | 5 (39%) | 11 (16%) | 20 (22%) |
| Both steady and casual partner(s) | 5 (46%) | 5 (39%) | 24 (39%) | 34 (37%) |
| No partners | - | 1 (7%) | 6 (8%) | 7 (8%) |
aRespondents were categorized by the combination of the reported ‘gender’ and ‘most probable way of infection’, without considering the self-reported sexual orientation. Transgender women were therefore categorized as ‘heterosexual women’ as they reported being infected through male sex although they identified themselves as ‘homosexual’
Type of Partners and Meeting Places
Almost three quarters (N = 66; 71%; 95%CI = [0.62%; 0.80%]) of all respondents reported vaginal, anal or oral sexual intercourse with casual sex partner(s) in the 3 months before their diagnosis (Table 1). Twenty-nine of these 66 (44%; 95%CI = [0.32%; 0.56%]) reported sexual activity with two to five casual sex partners; 11 (17%; 95%CI = [0.08%; 0.66%]) had 5 to 10 casual sex partners and 10 (15%; 95%CI = [0.07%; 0.24%])) reported sex with more than 10 partners.
Forty-six (70%; 95%CI = [0.59%; 0.81%]) of the participants that reported sexual activity with casual partners accessed websites or apps to contact new sex partners. They were in majority MSM (N = 40/46; 87%; 95%CI = [0.77%; 0.97%]).
The most common place for MSM to meet casual sex partners was at home (N = 27/52; 52%; 95%CI = [0.38%; 0.66%]), while heterosexual men and women most frequently met their partners in a bar (N = 6/14; 43%; 95%CI = [0.17%; 0.69%]).
Participants aged between 19 and 29 years old reported more frequently having had sexual activity with casual sex partners (N = 16/20; 80%; 95%CI = [0.62%; 0.98%]) compared to participants aged between 30 and 49 years old (N = 32/45; 71%; 95%CI = [0.58%; 0.84%]) and participants aged above 50 (N = 15/22; 68%; 95%CI = [0.49%; 0.88%]).
Vaginal, anal or oral sexual intercourse with a steady partner was reported by more than half of the participants (N = 54; 59%, 95%CI = [0.48%; 0.68%]). Sex with a steady partner was more frequently reported by heterosexual men and heterosexual women compared to MSM, see Table 1 and more frequently in the age group of 30–49 years (N = 31/45; 69%; 95%CI = [0.55%; 0.82%]) compared to the age groups of 19–29 years (N = 9/20; 45%; 95%CI = [0.23%; 0.67%]) and aged above 50 (N = 11/22, 50%; 95%CI = [0.29%; 0.71%]). Twenty persons (11 MSM, 4 heterosexual men and 5 heterosexual women) reported that they only had sex with a steady partner.
For most participants with a steady sex partner, this partner was of European origin (N = 35/54; 65%; 95%CI = [0.52%; 0.78%]). Seven persons (N = 7/54; 13%; 95%CI = [0.04%; 0.22%]) had a steady partner known to be HIV positive, fifteen persons (28%) reported not knowing the HIV status of their steady partner.
Seven participants (6 MSM of which 3 aged above 50 and 1 heterosexual women aged above 50) reported no sexual activity with any partner in the three months before their HIV diagnosis. Five of them (all MSM) however indicated ‘having had sex with a man’ as the most likely mode of infection and among these five, two were diagnosed before seroconversion clearly indicating acute infection. Two of the five, one heterosexual woman and one MSM, reported not knowing how they got infected. One person reported being tested for HIV at least five times a year and that he had participated in a PrEP study.
Condom Use
The overall frequency of condom use with a steady sex partner was lower for heterosexuals (N = 1/19; 5%; 95%CI = [-0.04%; 0.15%]) than for MSM (N = 9/35; 26%; 95%CI = [0.11%; 0.40%]). Of the 7 participants who had an HIV positive steady partner, 3 always used a condom. Of the persons who reported only having had sex with their steady partner, less than half used a condom ((N = 8/20; 40%; 95%CI = [0.19%; 0.61%]) (N = 6/20; 30% ; 95%CI = [0.10%; 0.50%] ‘most of the time’ and N = 2/20; 10%; 95%CI = [-0.03%; 0.23%]‘all the time’)).
The overall frequency of condom use with casual sex partner(s) was slightly higher (N = 25/52; 48%; 95%CI = [0.34%; 0.62%] in MSM and N = 4/14; 29%; 95%CI = [0.05%; 0.52%] in heterosexuals). Half of the participants having had sex with casual sex partner(s) was not aware of the HIV status of this/those partner(s) (N = 34/66; 52%; 95%CI = [0.39%; 0.64%]). Of these 34 persons, 13 (38%; 95%CI = [0.22%; 0.55%]) used condoms ‘all the time’ or ‘most of the time’. For an overview, see Table 2. No differences were found in frequency of condom use with casual sex partner(s) between the different age categories.
Table 2.
Overview of condom use with casual sex partner(s) in function of the frequency of informing about the HIV status of casual sex partner(s)
| Condom use with casual sex partner(s) | |||||
|---|---|---|---|---|---|
| Inform about HIV status casual sex partner(s) | All the time | Most of the time | Sometimes | Never | Total |
| Heterosexual men (N = 7) | |||||
| Never | 1 | - | - | 2 | 3 |
| Sometimes | - | 1 | - | 1 | 2 |
| Most of the time | - | - | 1 | 1 | 2 |
| Heterosexual women (N = 7) | |||||
| Never | - | 1 | 1 | 3 | 5 |
| Sometimes | - | 1 | - | - | 1 |
| Most of the time | - | - | - | - | - |
| All time | - | - | - | 1 | 1 |
| MSM (N = 52) | |||||
| Never | 2 | 9 | 10 | 5 | 26 |
| Sometimes | 1 | 3 | 4 | - | 8 |
| Most of the time | 2 | 2 | 4 | - | 8 |
| All time | 2 | 4 | 1 | 3 | 10 |
| Total | 8 | 21 | 21 | 16 | 66 |
The most frequently reported reasons for not (always) using a condom with respectively steady and casual sex partner(s) were ‘less enjoyable sex’ (N = 15/44; 34%; 95%CI = [0.20%; 0.48%] and N = 26/59; 44%; 95%CI = [0.31%; 0.57%]) and ‘not considering a risk’ (N = 20/44; 45%; 95%CI = [0.31%; 0.60%] and N = 22/59; 37%; 95%CI = [0.25%; 0.50%]). ‘Being under the influence of alcohol or drugs’ was given as a reason for inconsistent condom use with casual sex partner(s) by 18 persons in the MSM group (N = 18/52; 35%; 95%CI = [0.29%; 0.56%]).
Testing History, Reasons for Testing and Partner Notification
A majority of participants (N = 85; 91%; 95%CI = [0.86%; 0.97%]) have tested for HIV before being diagnosed. Among them, 59 (69%; 95%CI = [0.60%; 0.79%]) were tested at least once a year, with 34 MSM (40%; 95%CI = [0.30%; 0.50%]) tested at least twice a year (see Fig. 2). Sixty-seven persons (72%; 95%CI = [0.63%; 0.81%]) were diagnosed within a year after the last negative test.
Fig. 2.
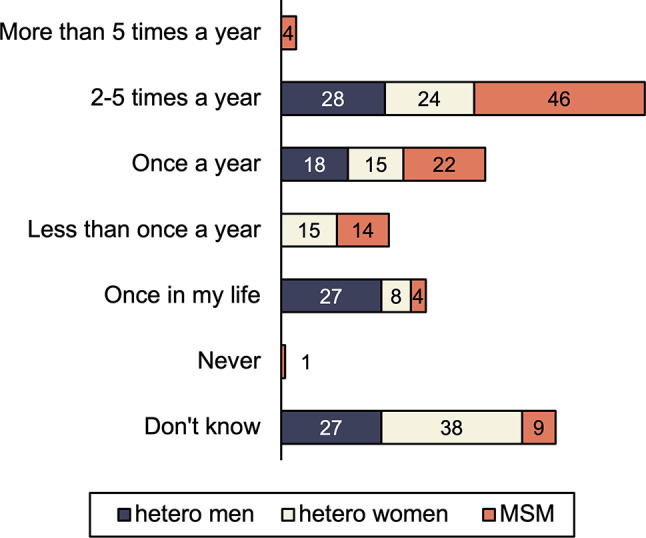
HIV testing in function of sexual transmission category. The numbers depicted in the bars represent the proportions of respondents who had indicated a specific answer
Of all participants, 43 (46%; 95%CI = [0.36%; 0.56%]) informed their sex partner(s) following their HIV diagnosis but 22 (24%; 95%CI = [0.15%; 0.32%]), mainly MSM (N = 15/22; 95%CI = [0.49%; 0.88%]) did not inform any of their partners, while the remaining 28 (30%; 95%CI = [0.21%; 0.39%]) informed only some of their sex partners. Participants aged between 30 and 49 years old notified their sex partners more frequently after their HIV diagnosis (N = 24/35; 53%; 95%CI = [0.53%; 0.84%]) than participants aged between 19 and 29 years old (N = 8/20; 40%;95%CI = [0.19%; 0.61%]) and participants aged above 50 (N = 7/22; 32%; 95%CI = [0.12%; 0.51%]).
Requests for testing were primarily initiated by the general practitioner (N = 63; 68%; 95%CI = [0.58%; 0.77%]), followed by the emergency unit of the hospital (N = 14; 15%; 95%CI = [0.08%; 0.22%]) or the HIV reference center (N = 13; 14%; 95%CI = [0.07%; 0.21%]).
Participants reported various reasons for seeking HIV testing, including ‘I was feeling ill’ (N = 43, 46%; 95%CI = [0.36%; 0.56%]), ‘I was tested on a regularly basis’ (N = 16; 17%; 95%CI = [0.10%; 0.25%]), ‘I thought I might have been exposed to HIV’ (N = 11; 12%; 95%CI = [0.05%; 0.18%]), ‘My partner was found to be HIV positive’ (N = 5, 5%; 95%CI = [0.01%; 0.10%]), ‘I had a new partner’ (N = 4; 4%; 95%CI = [0.01%; 0.08%]), ‘I was informed that a sex partner had been diagnosed with a sexually transmitted infection’ (N = 6; 6%; 95%CI = [0.01%; 0.11%]) or ‘other reasons’ (N = 8; 9%; 95%CI = [0.03%; 0.14%]).
Sexually Transmitted Infections (STIs)
Sixty participants (65%; 95%CI = [0.55%; 0.74%]), equally divided between MSM, heterosexual men and heterosexual women and all age categories, reported a history of STIs before their HIV diagnosis. Four persons reported not knowing about their STI history. Syphilis, chlamydia and gonorrhea were mostly mentioned by MSM, chlamydia and anal or genital herpes most often by heterosexual men and heterosexual women (see Fig. 3).
Fig. 3.
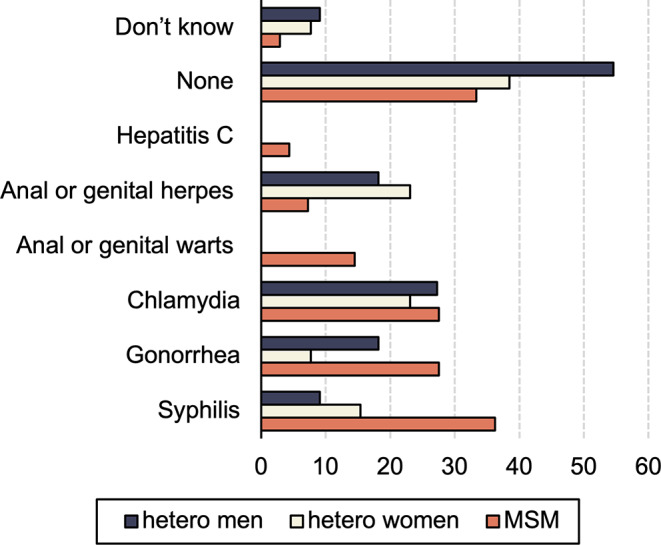
Sexually transmitted infections divided by sexual transmission category. The numbers depicted in the bars represent the proportions of respondents who had indicated a specific answer
Substance Use
Sixty participants (65%; 95%CI = [0.55%; 0.74%]) used substances (other than alcohol) before or during sex in the three months prior to their HIV diagnosis. Most frequently used substances were amyl nitrates and poppers, followed by cannabis, cocaine and ecstasy (see Fig. 4). Sexualized drug use is commonly reported by MSM (N = 48/69; 70%; 95%CI = [0.59%; 0.80%]) and heterosexual men (N = 8/11; 73%; 95%CI = [0.46%; 0.99%]) and slightly more reported in participants aged between 19 and 29 (N = 17/20; 85%; 95%CI = [0.69%; 1%]) and 30 to 49 years old (N = 37/45; 82%; 95%CI = [0.71%; 0.93%]) compared to participants aged above 50 (N = 16/22,72%; 95%CI = [0.54%; 0.91%]).
Fig. 4.
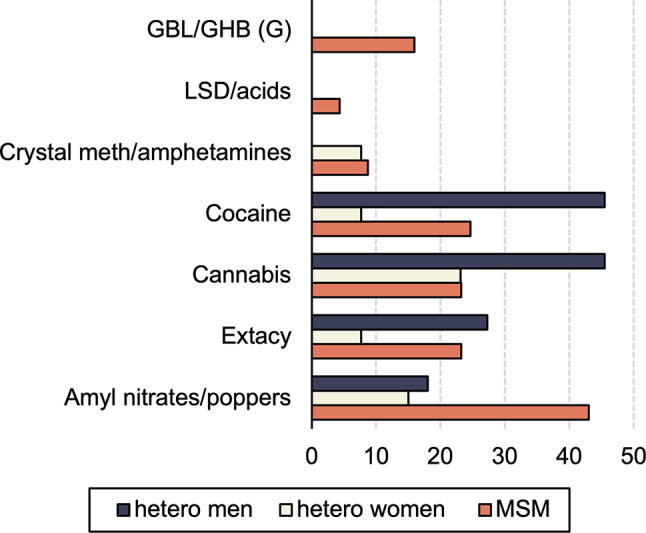
Substance use before or during sex divided by sexual transmission category. The numbers depicted in the bars represent the proportions of respondents who had indicated a specific answer. GBL/GHB = gamma butyrolactone/gamma hydroxybutyrate; LSD = lysergic acid diethylamide
Respondents having sex with casual partner(s) or with both casual and steady partners were more likely to use substances before or during sex (respectively N = 29/32; 91%; 95%CI = [0.80%; 1%] and N = 21/34; 62%; 95%CI = [0.45%; 0.78%]) than those having sex with their steady partner only (N = 8/20; 40%; 95%CI = [0.19%; 0.61%]).
PrEP and PEP Use
Sixty participants (66%, 95%CI = [0.55%; 0.74%] 56 MSM, 3 heterosexual men and one heterosexual women) were aware of the existence of PrEP. Among those unaware, there were no differences in numbers between age categories and between those infected only shortly after the reimbursement of PrEP in Belgium (HIV infection in 2018 and 2019; N = 18; 55%; 95%CI = [0.38%; 0.72%]) and those infected in 2020 or later (N = 15; 45%; 95%CI = [0.28%; 0.62%]).
Eight participants (9%; 95%CI = [0.03%; 0.14%]), all MSM, used PrEP in the past. Six of them did not have the Belgian nationality; 2 of these 6 reported that they were probably infected abroad. All except one of these previous PrEP users used substances before or during sex in the three months prior to their diagnosis and 7 persons had reported one or more STIs in the past. Half of the PrEP users notified their sex partner(s) after being diagnosed.
Fourteen participants (15%; 95%CI = [0.08%; 0.22%] 12 MSM and 2 heterosexual women, mainly aged between 30 and 49 years old (N = 9/14; 64%; 95%CI = [0.39%; 0.89%])) had taken PEP in the past because of suspected HIV exposure. Half of these persons were not of Belgian nationality. Three of the PEP users, all MSM, had also taken PrEP in the past.
Risks Factors for Being Part of a Transmission Cluster
Viral sequences for phylogenetic analysis were available for 89 of the 93 participants. Forty-one (46%; 95%CI = [0.36%; 0.56%]) were localized in a phylogenetic cluster, 12 were part of a pair (14%, 95%CI = [0.06%; 0.21%]) and 36 (40%; 95%CI = [0.30%; 0.51%]) were isolated, see Table 3. Six of the phylogenetic clusters contained more than one study participant (one with 4 participants included, one with 3 participants included and 4 with 2 participants included).
Table 3.
Overview of cluster information
| Heterosexual men (N = 11) |
Heterosexual women (N = 13) |
MSM (N = 65) |
Total (N = 89) |
|
|---|---|---|---|---|
| Clustered | 5 | 3 | 33 | 41 |
| Paired | 1 | 4 | 7 | 12 |
| Isolated | 5 | 6 | 25 | 36 |
The percentage of MSM localized in a phylogenetic cluster was only slightly higher than the percentage of heterosexuals in a phylogenetic cluster (MSM, 51%; heterosexual men, 46%; heterosexual women, 23%;). While men were more frequently part of a cluster, women were more frequently part of a pair (31% vs. 9% for heterosexual men and 11% for MSM). No differences were observed between clustered and isolated or paired persons concerning type of sex partners, number of casual sex partners, history of STIs, alcohol and substance use, use of condoms with casual partners and age categories. Of the 8 persons who used PrEP in the past, only two were part of a cluster, 5 occurred isolated or paired. For one person no sequence data were available. Of the clustered persons, 88% reported Belgium as the most probable country of infection, isolated or paired persons more frequently indicated being infected abroad, see Fig. 5.
Fig. 5.
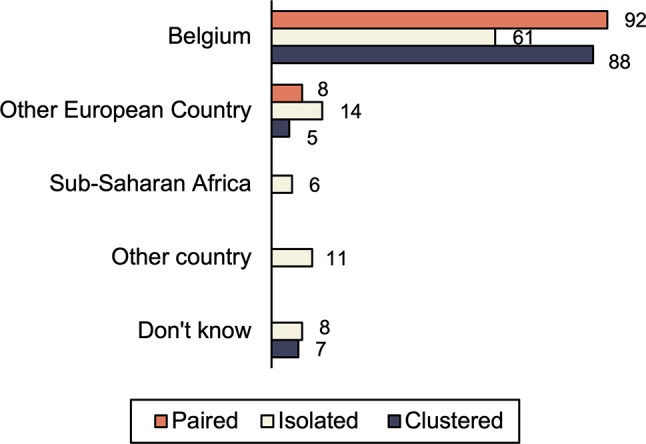
Most probable country of infection in function of cluster information. The numbers depicted in the bars represent the proportions of respondents who had indicated a specific answer
Discussion
The study combined behavior data with phylogenetic cluster analysis to assess the characteristics of the population most at risk for HIV infection in Belgium and to identify potential behavior correlates of clustering as well as gaps in prevention. There has been a previous report on the combination of risk behavior and cluster data [10]. The strength of our study however, is its focus on the recent infections. Persons diagnosed with an HIV-1 infection of less than 3 months completed a questionnaire on their behavior around the time of infection. Sequence data from drug resistance analysis were used to place each of these persons in the phylogenetic tree of the diagnosed cases in Belgium between 2013 and 2022.
In comparison to the 2018–2022 national HIV surveillance data of persons with a diagnosis of recent infection (< 6 months), the sample was representative of most socio-demographic characteristics. However, among the study participants there were proportionally slightly more MSM and Belgians, and fewer persons with Sub-Saharan African nationalities. About three-quarters of the study participants were MSM, primarily of Belgian origin, a known key-affected population. A high rate of high-risk sexual behavior was derived from the survey, and most respondents were also aware of the risk taking, reflected in the fact that most of them underwent frequent HIV testing. More than half even tested for HIV several times a year. A substantial proportion of the respondents reported multiple sex partners and often engaging in unprotected sex. Overall, alcohol and substance use before or during sexual activity was high, and most participants were diagnosed with one or more sexually transmitted infections.
In line with previous findings [7, 11], almost half of the study participants were part of a transmission cluster, and a smaller proportion was part of a transmission pair. This confirms the previous observations that an important part of local HIV transmission in our country, especially among MSM, can be allocated to phylogenetic clusters [12, 13]. It is also in-line with the findings reported for other Western European countries [14–16].
As a result, there is growing interest in identifying clusters of sexual transmission and the corresponding social networks to target testing and prevention initiatives to this population. The rapid detection and response to growing HIV clusters is one of the pillars of the Ending the HIV Epidemic initiative of the US Department of Health and Human Services that aims to reduce new HIV infections in the US by 90% in 2030 [17]. But evidence-based metrics showing the effectiveness of cluster prioritization in prevention is still missing [8] and, despite the wealth of studies suggesting cluster driven interventions, potential implementation strategies have not been well defined [18, 19].
Although most participants were at high HIV-risk, the study also identified people with a less pronounced risk profile. These included Belgian heterosexuals and people aged over 50. Also, just over half of the participants reported having had only sexual intercourse, with a steady partner; this was more often reported by heterosexuals than by MSM. These findings support the national surveillance data that reveal increasing diversity of the HIV epidemic in Belgium, as evidenced by the profile of newly diagnosed individuals and those infected who have not yet been diagnosed. For example, of the estimated 627 people with undiagnosed HIV in Belgium in 2022, almost 40% were Belgian heterosexuals. It is therefore clear that, to reduce transmission, prevention strategies must reach beyond the known high-risk populations. Given the long-term and holistic patient-physician relationship, primary care may lend itself to provide personalized sexual health information and repeated testing opportunities [20]. People over 50 need special attention for prevention and testing, as health workers and older people themselves underestimate their risk of HIV infection and early signs and symptoms of an HIV infection may be attributed to diseases of ageing [21, 22].
PEP use was limited, as was knowledge about and use of PrEP in the study sample. Even though a small majority were aware of the availability of PrEP, only a minority had effectively engaged in PrEP use. Since June 2017, physicians at an HIV reference center can prescribe PrEP and it is reimbursed for persons who have medical insurance in Belgium. Eligibility criteria included being at least 16 years old and reporting one or more of the following: (1) unprotected sexual intercourse with a least 2 partners, (2) multiple STIs in the past year, (3) use of PEP in the past year, (4) using psychoactive substances during sex, and/or (5) other reasons of being at high risk of acquiring HIV (e.g. people who inject drugs and share needles, sex workers, partners of HIV-positive people with viral suppression). However, no differences in the frequency of PrEP use were observed for persons diagnosed in the period immediately after the start of PrEP reimbursement in Belgium and those diagnosed when the roll-out of PrEP was already more consolidated. Previous research indicated that an interplay of several underlying determinants and barriers such as younger age, lower education levels, socio-economic vulnerabilities and migration related stressors impact on PrEP uptake and effective use, illustrating a social gradient in PrEP care [23–25]. To ensure that people who could benefit from PrEP also use PrEP, further investment in the accessibility of PrEP care is recommended. Moreover, prevention strategies for people stopping PrEP are required, since some people remain at high risk for HIV after having stopped PrEP [26], as also shown in this study.
Finally, an important observation from our study was that less than half of the study participants informed all their sex partners of their diagnosis and almost one fourth, mainly MSM, did not inform any of their sex partners. These are missed opportunities for testing and prevention. The full potential of prevention efforts cannot be realized as long as a significant number of people remain unaware of their HIV infection and unknowingly transmit HIV. This finding therefore calls for more involvement of the healthcare provider to accelerate and intensify case finding. Adequate partner notification may be of particular importance for the persons that are localized in a cluster. Six of the phylogenetic clusters identified contained more than one study participant. Timely identification of these clusters and the associated sexual network may have prevented some of these infections by earlier testing and offering of PrEP. However, for optimal efficiency of cluster-driven interventions, it will be crucial to be able to perform the cluster analyses in real time. This real-time integration, analysis and interpretation of phylogenetic data remains challenging [27]. Future studies are also needed to support the effectiveness of this approach. A recent study on HIV-infected persons in Chicago led to the conclusion that partner services initiated from clients in a molecular cluster did not result in a higher number of new diagnoses than partner services initiated from clients not in a cluster [28]. It is likely that much will depend on how well the phylogenetic analysis covers the HIV infected population.
Limitations
Several limitations must be considered. The sample size is small, and the composition may present some bias. A quarter of individuals were not invited to participate for several reasons (language barrier, psychiatric problems, not followed at the one of the participating centers after diagnosis). The selective inclusion of Reference Centers may partly explain the relatively small number of Sub-Saharan African migrants as the profiles of people living with HIV in follow-up differ between HIV Reference Centers (i.e. surveillance data show that non-Belgian nationalities among heterosexuals were proportionally more present in the Brussels region than in the other regions) and some of the Belgian HIV Reference Centers did not participate in the study, mainly because of practical considerations (lack of time, lack of staff). Moreover, migrants from sub-Saharan Africa are also less likely to be diagnosed during acute infection, as a large proportion of them were already infected before arriving in Belgium. Apart from that however, it also remains difficult to motivate people to participate in scientific studies, especially people from migrant backgrounds. Experiencing more HIV-related stigma among Sub-Saharan African migrants would be a plausible explanation why they are less motivated to participate in HIV research [29]. The small sample size hampered possible sub-analyses and one should be careful when generalizing the results to the overall population of recently infected persons living with HIV.
Next, as already mentioned, phylogenetic analysis is limited to infections newly diagnosed in Belgium; clusters extending country borders may have been missed. Also, a significant number of persons with a high-risk profile, especially MSM, currently use PrEP. As shown recently in a study of the Dutch HIV cohort, this may impact cluster size and rate of expansion [30].
As for informing the partner, they were not further asked about the reasons why they did not do so. Indeed, it is important to distinguish between ‘not wanting to inform their sex partner(s)’ versus ‘not being able to inform their sex partner(s) in case of anonymous sex partner(s)’. It would therefore be valuable for future prevention initiatives to explore these reasons in more detail.
The COVID-19 pandemic forced us to extend the study period and may have affected participants’ sexual behavior. In line with findings from other studies [31], persons infected during the COVID-19 pandemic reported less casual sex partners compared to those infected before the pandemic. No other differences in sexual risk behavior were found between pre and post COVID-19 diagnoses.
Another limitation is that this study used questionnaires to examine behavior, questions and answers could not be explored further. Although anonymity was emphasized, one should be aware of socially desirability in answering. Memory biases can influence questionnaire responses. This may have been the case for the 7 participants who reported not having had sex partners in the 3 months before their HIV diagnosis, but who reported sexual activity in the same period in response to other questions.
Recommendations
The findings of the current study show that the blind spot of individuals not currently reached by existing prevention strategies may be larger than thought.
These results point out the need for prevention measures that consider the diverse profile of persons at risk of HIV.
There is an urgent need to address prevention gaps, particularly missed testing and partner notification opportunities.
It is recommended to further invest in the accessibility of PrEP and to develop strategies to enhance persistence of PrEP user.
Real-time phylogenetic cluster analysis may help to trace undiagnosed infections and provide targeted prevention, but further research is needed to estimate the full potential impact of such an approach.
Further research is also needed to better understand how people’s embedding in social networks may increase or decrease their ability to engage in sexual risk-taking and HIV-preventive behavior.
Conclusions
Most participants recently infected with HIV-1 reported having engaged in high-risk behavior and were aware of their risk-taking; a substantial number of participants was found in transmission clusters. However, the study also identified people with a less pronounced risk profile. These findings emphasize that, to reduce HIV transmission, prevention strategies must extend beyond the key-affected populations. Furthermore, gaps in prevention pathways need to be better understood and filled. Finally, cluster-driven preventive interventions, including testing and partner notification, should be piloted so that best practices can be operationalised.
Acknowledgements
The authors want to thank the COLIBRI study team (Prof dr. E. Florence, Prof dr. S. De Wit, Prof dr. J-C Goffard, Prof dr. P. Lacor, Prof dr. M-L Delforge, dr. C. Necsoi, dr. J. Van Praet, L. Mertens, F. Vanroye, dr. E. Van Cutsem, K. Stoffels, L. Debaisieux and V. Mortier) for their help with the recruitment of the participants. Furthermore, we appreciate the work of M. Schauvliege, D. Staelens and V. Mortier for incidence and phylogenetic analysis. We thank Gilead Sciences and ViiV Healthcare for financial support.
Funding
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Declarations
Conflict of Interest
The authors declare no conflict of interest
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chou R, Evans C, Hoverman A, Sun C, Dana T, Bougatsos C et al. Preexposure prophylaxis for the prevention of HIV infection: evidence report and systematic review for the US preventive services task force. JAMA. 2019;321(22):2214-30. 10.1001/jama.2019.2591 PMID: 31184746. [DOI] [PubMed]
- 2.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2023–2022 data. Stockholm: ECDC; 2023. [Google Scholar]
- 3.Deblonde J, Serien B, De Rouck M, Montourcy M, Van Beckhoven D. Epidemiologie van Hiv in België. Toestand Op 31 December 2022. Belgium 10.25608/24dn-es90
- 4.Van Beckhoven D, Florence E, Ruelle J, Deblonde J, Verhofstede C, Callens S, et al. Good continuum of HIV care in Belgium despite weaknesses in retention and linkage to care among migrants. BMC Infect Dis. 2015;15:496. 10.1186/s12879-015-1230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Z, Brown AE, Rice BD, Marrone G, Sönnerborg A, Suligoi B, et al. Post-migration acquisition of HIV: estimates from four European countries, 2007 to 2016. Eurosurveillance. 2021;26(33). 10.2807/1560-7917.ES.2021.26.33.2000161. [DOI] [PMC free article] [PubMed]
- 6.European Centre for Disease Prevention and Control. HIV Modelling Tool. 2015. https://www.ecdc.europa.eu/en/publications-data/hiv-modelling-tool
- 7.Verhofstede C, Mortier V, Dauwe K, Callens S, Deblonde J, Dessilly G, Delforge ML, Fransen K, Sasse A, Stoffels K, Van Beckhoven D, Vanroye F, Vaira D, Vancutsem E, Van Laethem K. Exploring HIV-1 Transmission Dynamics by combining phylogenetic analysis and infection timing. Viruses. 2019;11(12):1096. 10.3390/v11121096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennis AM, Mobley V. Interrupting HIV transmission networks: how can we design and implement timely and effective interventions? Expert Rev Anti Infect Ther. 2023;9:1–3. 10.1080/14787210.2023.2221850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhofstede C, Van Den Fransen K, Van Laethem K, Ruelle J, Vancutsem E, Stoffel K, Van den Wijngaert S, Delforge M-, Vaira D, Hebberecht L, Schauvliege M, Mortier V, Dauwe K, Callens S. Decision tree for accurate infection timing in individuals newly diagnosed with HIV-1 infection. BMC Infect Dis. 2017;17:738. 10.1186/s12879-017-2850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilbourn B, Saafir-Callaway B, Jair K, Wertheim JO, Laeyendeker O, Jordan JA, Kharfen M, Castel A. Characterization of HIV risk behaviors and clusters using HIV-Transmission Cluster Engine among a cohort of persons living with HIV in Washington, DC. Aids Res Hum Retrov. 2021;37(9):706–15. 10.1089/aid.2021.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Wijhe M, Fischer TK, Fonager J. Identification of risk factors associated with national transmission and late presentation of HIV-1, Denmark, 2009 to 2017. Surveillance. 2021:26. 10.2807/1560-7917.ES.2021.26.47.2002008 [DOI] [PMC free article] [PubMed]
- 12.Chalmet K, Staelens D, Blot S, Dinakis S, Pelgrom J, Plum J, Vogelaers D, Vandekerckhove L, Verhofstede C. Epidemiological study of phylogenetic transmission clusters in a local HIV-1 epidemic reveals distinct differences between subtype B and non-B infections. BMC Infect Dis. 2010;10:262. 10.1186/1471-2334-10-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruelle J, Ingels MG, Jnaoui K, Ausselet N, Vincent A, Sasse A, Verhofstede C, Goubau P. Transmission network of an HIV type 1 strain with K103N in young Belgian patients from different risk groups. AIDS Res Hum Retroviruses. 2013;10:1306–9. 10.1089/aid.2013.0108. [DOI] [PubMed] [Google Scholar]
- 14.Gil H, Delgado E, Benito S, Georgalis L, Montero V, Sanchez M, Canada-Garcia JE, Garcia-Bodas E, Diaz A, Thomson MM. Transmission clusters, predominantly Associated with men who have sex with men, play a main role in the propagation of HIV-1 in Northern Spain (2013–2018). Front Microb. 2022;13. 10.3389/fmicb.2022.782609. [DOI] [PMC free article] [PubMed]
- 15.Parczewski M, Leszczyszyn-Pynka M, Witak-Jedra M, Szetela B, Gasiorowski J, Knysz B, Bociaga-Jasik M, Skwara P, Grzeszczuk A, Jankowska M, Baralkiewicz G, Mozer-Lisewska I, Lojewski W, Koziel K, Grabczewska E, Jablonowska E, Urbanska A. Expanding HIV1 subtype B transmission networks among men who have sex with men in Poland. PLoS ONE. 2017;12(2). 10.1371/journal.pone.0172473. [DOI] [PMC free article] [PubMed]
- 16.Yebra G, Holguín Á, Pillay D, Hué S. Phylogenetic and demographic characterization of HIV-1 transmission in Madrid, Spain. Infect Genet Evol. 2013;14:232–9. 10.1016/j.meegid.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services. What is Ending the HIV Epidemic: A plan for America? 2020. https://www.hiv.gov/federal-response/endingthe-hiv-epidemic/overview, accessed 14th of November, 2023.
- 18.Gore DJ, Schueler K, Ramani S, et al. HIV Response interventions that Integrate HIV Molecular Cluster and Social Network Analysis: a systematic review. AIDS Behav. 2022;26:1750–92. 10.1007/s10461-021-03525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oster A, Lyss SB, McClung RP, Watson M, Panneer N, Hernandez AL, Buchacz K, Robilotto SE, Curran KG, Hassan R, Bañez Ocfemia MC, Linley L, Perez SM, Phillip S Jr, France SA. HIV Cluster and Outbreak Detection and Response: the Science and Experience am. J Prev Med. 2021;61:130–42. 10.1016/j.amepre.2021.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Deblonde J, Van Beckhoven D, Loos J, Boffin N, Sasse A, Nöstlinger C, et al. HIV testing within general practices in Europe: a mixed-methods systematic review. BMC Public Health. 2018;18(1):1191. 10.1186/s12889-018-6107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Justice AC, Goetz MB, Stewart CN, Hogan BC, Humes E, Luz PM, et al. Delayed presentation of HIV among older individuals: a growing problem. Lancet HIV. 2022;9(4):e269–80. 10.1016/S2352-3018(22)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprague C, Brown SM. Local and global HIV aging demographics and research. In: Brennan-Ing M, DeMarco DF, editors. HIV and Aging. Volume 42. Karger; 2017. pp. 1–10. 10.1159/000448532. [DOI] [PubMed]
- 23.Buffel V, Reyniers T, Masquillier C, Thunissen E, Nöstlinger C, Laga M, et al. Awareness of, willingness to take PrEP and its actual use among Belgian MSM at high risk of HIV infection: secondary analysis of the Belgian European MSM Internet Survey. AIDS Behav. 2022;26(6):1793–807. 10.1007/s10461-021-03526-z. [DOI] [PubMed] [Google Scholar]
- 24.Van Landeghem E, Dielen S, Semaan A, Rotsaert A, Vanhamel J, Masquillier C, et al. Insights into barriers and facilitators in PrEP uptake and use among migrant men and transwomen who have sex with men in Belgium. BMC Public Health. 2023;23(1):712. 10.1186/s12889-023-15540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velter A, Champenois K, Girard G, Roux P, Mercier A. Prophylaxie pré-exposition (PrEP) de L’infection Au VIH parmi les hommes ayant des rapports sexuels avec des hommes répondant à l’enquête Rapport Au Sexe 2023: qui sont les éligibles ? Qui sont les usagers ? Bull Épidémiol Hebd. 2023;(24–25):542–52.http://beh.santepubliquefrance.fr/beh/2023/24-25/2023_24-25_5.html
- 26.Koppe U, Marcus U, Albrecht S, et al. Barriers to using HIV pre-exposure prophylaxis (PrEP) and sexual behaviour after stopping PrEP: a cross-sectional study in Germany. BMC Public Health. 2021;21:159. 10.1186/s12889-021-10174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howison M, Gillani FS, Novitsky V, Steingrimsson JA, Fulton J, Bertrand T, et al. An Automated Bioinformatics Pipeline Informing Near-Real-Time Public Health Responses to New HIV diagnoses in a Statewide HIV Epidemic. Viruses. 2023;15(3):737. 10.3390/v15030737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider JA, Hayford C, Hotton A, Tabidze I, Wertheim JO, Ramani S, et al. Do partner services linked to molecular clusters yield people with viremia or new HIV? AIDS. 2022;36(6):845–52. 10.1097/QAD.0000000000003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manirankunda L, Wallace A, Ddungu C, Nöstlinger C. Stigma mechanisms and outcomes among sub-saharan African descendants in Belgium-Contextualizing the HIV Stigma Framework. Int J Environ Res Public Health. 2021;18(16):8635. 10.3390/ijerph18168635. PMID: 34444384; PMCID: PMC8393566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prins HAB, Rokx C, Verbon A, van Sighem A, de Bree GJ, Dijkstra M, Prins JM, Reiss P, van Kampen JJA. D A M C. HIV transmission among acutely infected participants of a Dutch cohort study 2015–2021 is not associated with large, clustered outbreaks. AIDS. 2023;37:299–303. 10.1097/QAD.0000000000003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyniers T, Rotsaert A, Thunnissen E, Buffel V, Masquillier C, Van Landeghem E, Vanhamel J, Nöstlinger C, Wouters E, Laga M, Vuylsteke B. Reduced sexual contacts with non-steady partners and less PrEP use among MSM in Belgium during the first weeks of the COVID-19 lockdown. Results of an online survey. Sex Transm Infect. 2020;0:1–6. 10.1136/sextrans-2020-054756. [DOI] [PMC free article] [PubMed] [Google Scholar]