Abstract
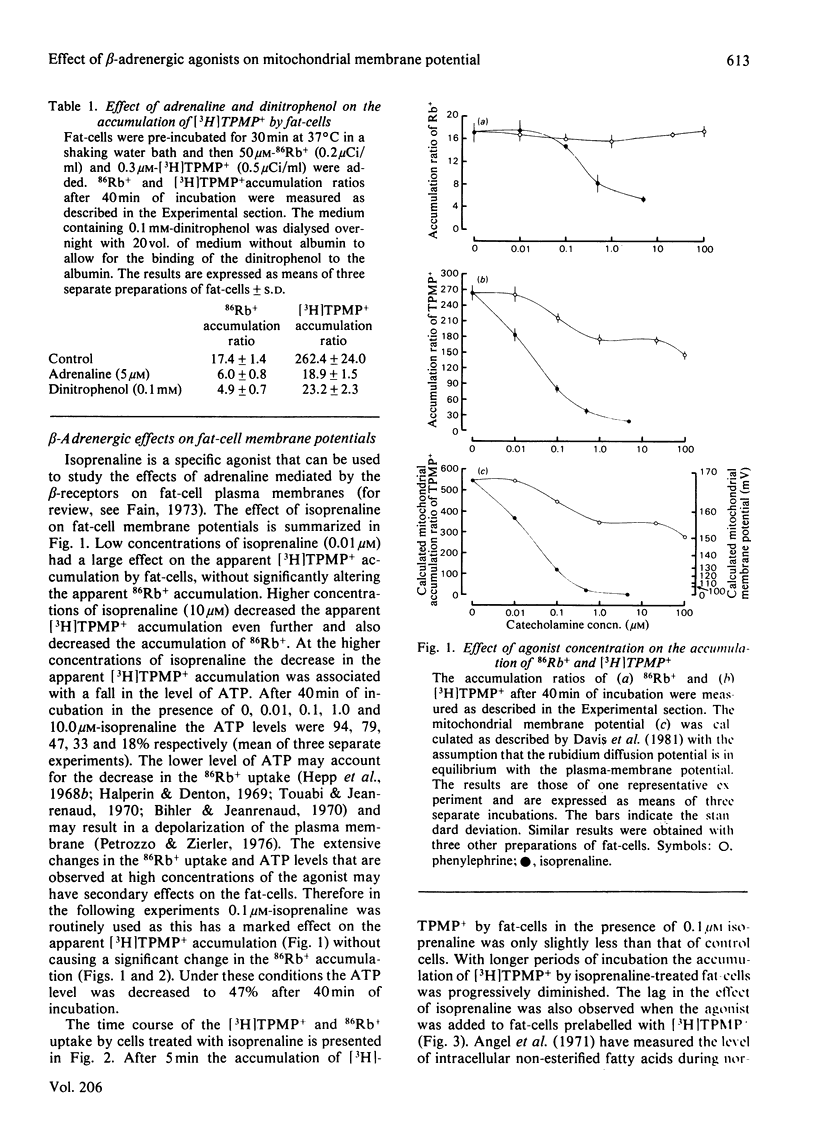
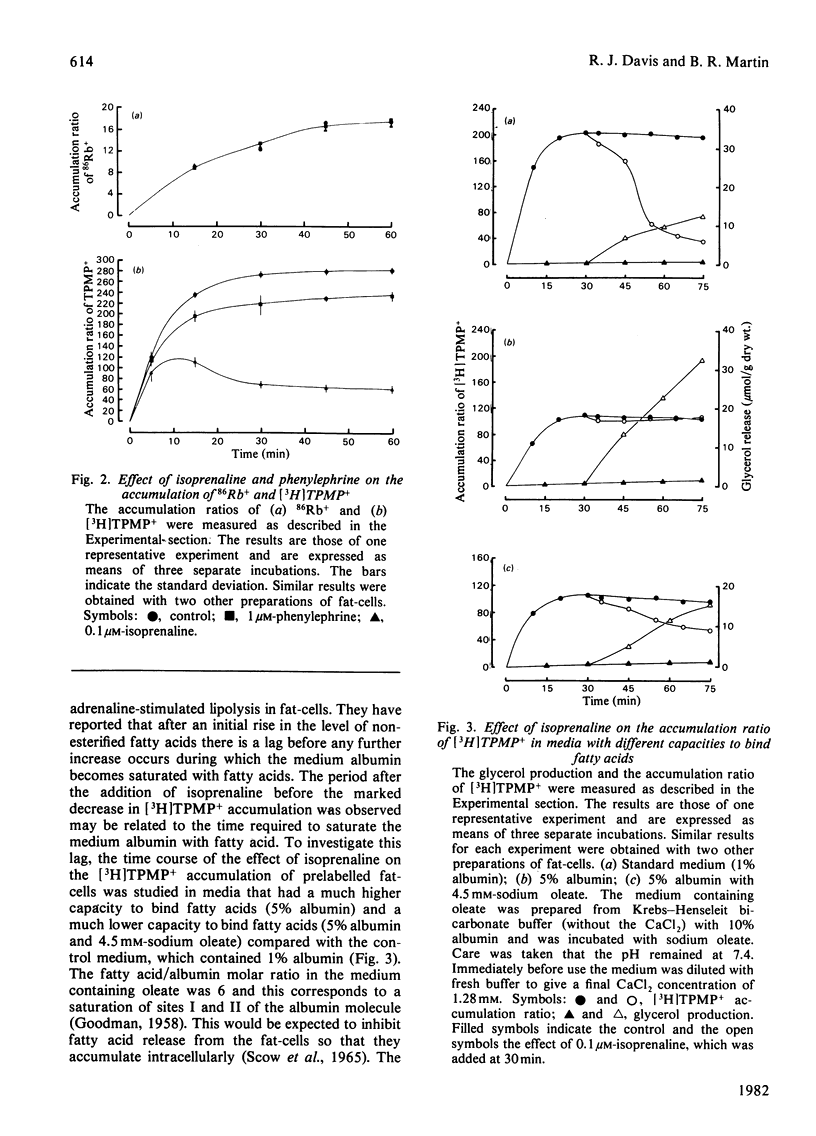
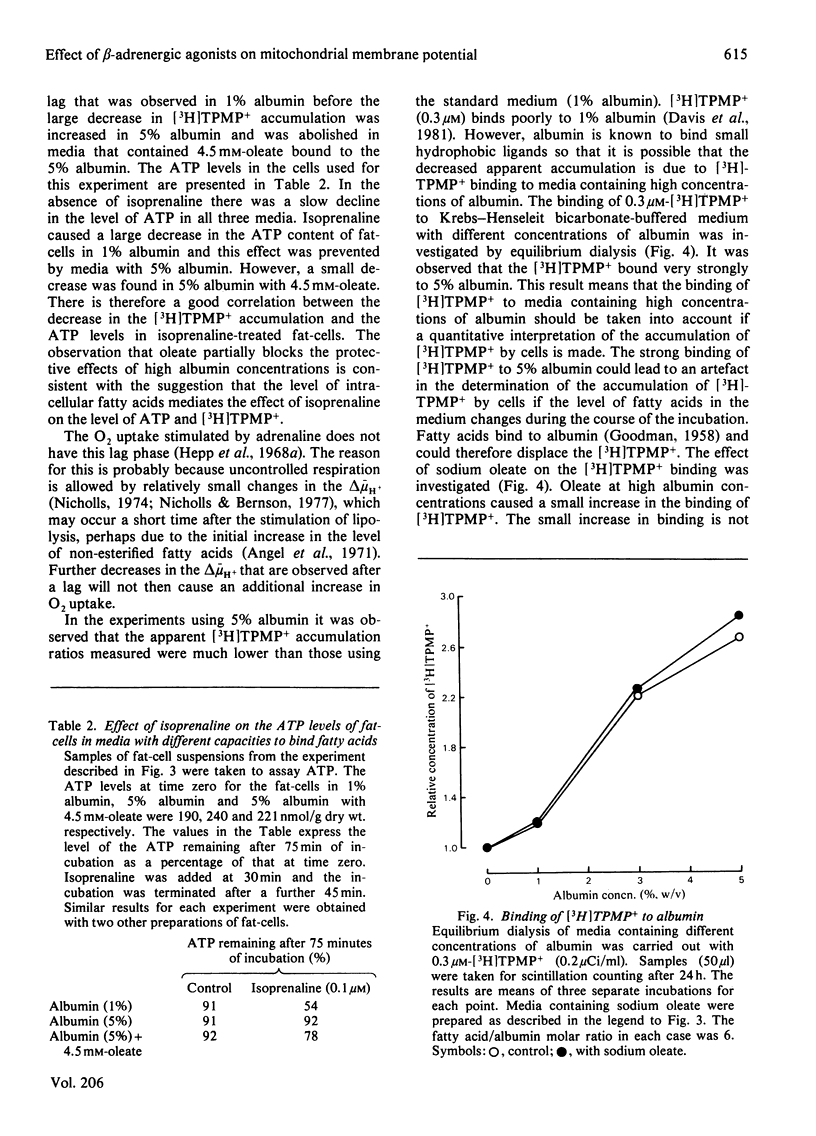
1. The accumulation of [3H]methyltriphenylphosphonium by isolated fat-cells was used to estimate the membrane potential of mitochondria in situ. 2. Adrenaline caused a large decrease in the accumulation of [3H]methyltriphenylphosphonium. Mitochondria in fat-cells incubated in the presence of adrenaline had a very low calculated membrane potential. This effect was also given by isoprenaline (a beta-adrenergic agonist) and was blocked by propranolol (a beta-adrenergic antagonist). 3. The effect of isoprenaline could be partially antagonized by the use of media with high albumin concentrations. Addition of sodium oleate to saturate the fatty acid-binding sites on the albumin reversed this antagonism. 4. It is proposed that the decrease in the calculated mitochondrial membrane potential is due to the uncoupling effect of the non-esterified fatty acids released by the stimulation of lipolysis observed in the presence of beta-adrenergic agonists.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Järvisalo J. O. Effects of ionophores and metabolic inhibitors on the mitochondrial membrane potential within isolated hepatocytes as measured with the safranine method. Biochem J. 1980 Oct 15;192(1):183–190. doi: 10.1042/bj1920183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman K. E., Nicholls D. G. Calcium transport by intact synaptosomes. Influence of ionophore A23187 on plasma-membrane potential, plasma-membrane calcium transport, mitochondrial membrane potential, respiration, cytosolic free-calcium concentration and noradrenaline release. Eur J Biochem. 1981 Mar 16;115(1):67–73. [PubMed] [Google Scholar]
- Akerman K. E. Qualitative measurements of the mitochondrial membrane potential in situ in Ehrlich ascites tumour cells using the safranine method. Biochim Biophys Acta. 1979 May 9;546(2):341–347. doi: 10.1016/0005-2728(79)90051-3. [DOI] [PubMed] [Google Scholar]
- Angel A., Desai K. S., Halperin M. L. Reduction in adipocyte ATP by lipolytic agents: relation to intracellular free fatty acid accumulation. J Lipid Res. 1971 Mar;12(2):203–213. [PubMed] [Google Scholar]
- BEIGELMAN P. M., HOLLANDER P. B. EFFECTS OF HORMONES UPON ADIPOSE TISSUE MEMBRANE ELECTRICAL POTENTIALS. Proc Soc Exp Biol Med. 1964 May;116:31–35. doi: 10.3181/00379727-116-29149. [DOI] [PubMed] [Google Scholar]
- BEIGELMAN P. M., HOLLANDER P. B. Effect of insulin and rat weight upon rat adipose tissue membrane resting electrical potential (REP). Diabetes. 1963 May-Jun;12:262–267. doi: 10.2337/diab.12.3.262. [DOI] [PubMed] [Google Scholar]
- BEIGELMAN P. M., HOLLANDER P. B. Effect of insulin upon resting electrical potential of adipose tissue. Proc Soc Exp Biol Med. 1962 Jul;110:590–595. doi: 10.3181/00379727-110-27588. [DOI] [PubMed] [Google Scholar]
- Beigelman P. M., Hollander P. B. Effect of insulin and insulin antibody upon rat adipose tissue membrane resting electrical potential (REP). Acta Endocrinol (Copenh) 1965 Dec;50(4):648–656. doi: 10.1530/acta.0.0500648. [DOI] [PubMed] [Google Scholar]
- Bihler I., Jeanrenaud B. ATP content of isolated fat cells. Effects of insulin, ouabain, and lipolytic agents. Biochim Biophys Acta. 1970 May 5;202(3):496–506. doi: 10.1016/0005-2760(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Cheng K., Groarke J., Osotimehin B., Haspel H. C., Sonenberg M. Effects of insulin, catecholamines, and cyclic nucleotides on rat adipocyte membrane potential. J Biol Chem. 1981 Jan 25;256(2):649–655. [PubMed] [Google Scholar]
- Cheng K., Haspel H. C., Vallano M. L., Osotimehin B., Sonenberg M. Measurement of membrane potentials (psi) of erythrocytes and white adipocytes by the accumulation of triphenylmethylphosphonium cation. J Membr Biol. 1980 Oct 31;56(3):191–201. doi: 10.1007/BF01869476. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Brand M. D., Martin B. R. The effect of insulin on plasma-membrane and mitochondrial-membrane potentials in isolated fat-cells. Biochem J. 1981 Apr 15;196(1):133–147. doi: 10.1042/bj1960133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J., Martin B. R. The effect of alpha-adrenergic agonists on the membrane potential of fat-cell mitochondria in situ. Biochem J. 1982 Sep 15;206(3):619–626. doi: 10.1042/bj2060619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C., Erecińska M., Werrlein R., Silver I. A. Cellular energy metabolism, trans-plasma and trans-mitochondrial membrane potentials, and pH gradients in mouse neuroblastoma. Proc Natl Acad Sci U S A. 1979 May;76(5):2175–2179. doi: 10.1073/pnas.76.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N. Biochemical aspects of drug and hormone action on adipose tissue. Pharmacol Rev. 1973 Mar;25(1):67–118. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Halperin M. L., Denton R. M. Regulation of glycolysis and L-glycerol 3-phosphate concentration in rat epididymal adipose tissue in vitro. Role of phosphofructokinase. Biochem J. 1969 Jun;113(1):207–214. doi: 10.1042/bj1130207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton G. M., Nicholis D. G. Hamster brown-adipose-tissue mitochondria. The role of fatty acids in the control of the proton conductance of the inner membrane. Eur J Biochem. 1976 Aug 16;67(2):511–517. doi: 10.1111/j.1432-1033.1976.tb10717.x. [DOI] [PubMed] [Google Scholar]
- Hepp D., Challoner D. R., Williams R. H. Respiration in isolated fat cells and the effects of epinephrine. J Biol Chem. 1968 May 10;243(9):2321–2327. [PubMed] [Google Scholar]
- Hepp D., Challoner D. R., Williams R. H. Studies on the action of insulin in isolated adipose tissue cells. I. Stimulation of incorporation of 32P-labeled inorganic phosphate into mononucleotides in the absence of glucose. J Biol Chem. 1968 Aug 10;243(15):4020–4026. [PubMed] [Google Scholar]
- Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980 Feb 25;255(4):1458–1464. [PubMed] [Google Scholar]
- Joel C. D. Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J Biol Chem. 1966 Feb 25;241(4):814–821. [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Larner J. Evidence for alpha adrenergic activation of phosphorylase and inactivation of glycogen synthase in rat adipocytes. Effects of alpha and beta adrenergic agonists and antagonists on glycogen synthase and phosphorylase. Mol Pharmacol. 1977 Nov;13(6):1060–1075. [PubMed] [Google Scholar]
- Locke R. M., Nicholls D. G. A re-evaluation of the role of fatty acids in the physiological regulation of the proton conductance of brown adipose tissue mitochondria. FEBS Lett. 1981 Dec 7;135(2):249–252. doi: 10.1016/0014-5793(81)80793-4. [DOI] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G., Bernson V. S. Inter-relationships between proton electrochemical gradient, adenine-nucleotide phosphorylation potential and respiration, during substrate-level and oxidative phosphorylation by mitochondria from brown adipose tissue of cold-adapted guinea-pigs. Eur J Biochem. 1977 May 16;75(2):601–612. doi: 10.1111/j.1432-1033.1977.tb11560.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Brown adipose tissue mitochondria. Biochim Biophys Acta. 1979 Jul 3;549(1):1–29. doi: 10.1016/0304-4173(79)90016-8. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Crompton M. Mitochondrial calcium transport. FEBS Lett. 1980 Mar 10;111(2):261–268. doi: 10.1016/0014-5793(80)80806-4. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Perry M. C., Hales C. N. Factors affecting the permeability of isolated fat-cells from the rat to [42K] potassium and [36Cl] chloride ions. Biochem J. 1970 Apr;117(3):615–621. doi: 10.1042/bj1170615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. C., Hales C. N. Rates of effux and intracellular concentrations of potassium, sodium and chloride ions in isolated fat-cells from the rat. Biochem J. 1969 Dec;115(5):865–871. doi: 10.1042/bj1150865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Scott I. D., Nicholls D. G. Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J. 1980 Jan 15;186(1):21–33. doi: 10.1042/bj1860021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scow R. O., Stricker F. A., Pick T. Y., Clary T. R. Effect of ACTH on FFA release and diglyceride content in perfused rat adipose tissue. Ann N Y Acad Sci. 1965 Oct 8;131(1):288–301. doi: 10.1111/j.1749-6632.1965.tb34797.x. [DOI] [PubMed] [Google Scholar]
- Siegel J., Olefsky J. M. Role of intracellular energy in insulin's ability to activate 3-O-methylglucose transport by rat adipocytes. Biochemistry. 1980 May 13;19(10):2183–2190. doi: 10.1021/bi00551a029. [DOI] [PubMed] [Google Scholar]
- Touabi M., Jeanrenaud B. Lipolysis and potassium accumulation in isolated fat cells. Effect of insulin and lipolytic agents. Biochim Biophys Acta. 1970 May 5;202(3):486–495. doi: 10.1016/0005-2760(70)90119-0. [DOI] [PubMed] [Google Scholar]


