Abstract
Until recently, the main diagnostic methods for bladder cancer (BC) are still voided urine cytology and cystoscopy, and many drawbacks persist. In this retrospective study, we evaluated the sensitivity and specificity of the CellDetect assay in the detection of BC with comparison to standard diagnostic methods. Between August 2020 and July 2022, B-ultrasonography or computed tomography (CT) scan was performed for patients with hematuria or irritative voiding symptoms. If no bladder mass was detected, the patient was excluded. A total of 148 patients with bladder mass formed the final study cohort. The patients’ urine samples were measured with CellDetect assay, followed by cystoscopy or diagnostic transurethral resection of bladder tumor. The patients were divided into two groups based on previous history of BC: group P and group R. The analysis included descriptive statistics and percentages. Finally, 115 cases had a positive CellDetect result, with 68 cases in group P and 47 in group R, respectively. And 134 cases revealed malignant tumor pathologically. The overall sensitivity and specificity for all patients were 82.1% and 64.2%, respectively. Concerning the subgroups, the respective sensitivity and specificity were: in group P- 81.0% and 50.0%; and in group R- 85.2% and 83.3%, respectively. In conclusion, CellDetect assay demonstrated significant performance for diagnosis of BC: it can identify BC patients at early stage with significant diagnostic performance and good reliability. This assay might develop novel methods and ideas for future clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80705-7.
Subject terms: Oncology, Urology
Introduction
Over the past decades, the management of BC has witnessed several advancements, such as immune checkpoint inhibitor (ICI) therapy, antibody-drug conjugates (ADCs), trimodality bladder-sparing therapy, gene therapy; and better outcomes have been obtained1. However, breakthrough progression has not been made in the diagnostic methods of BC, except some modifications based on urine cytology and cystoscopy. Therefore, early diagnosis of BC, which is of clinical importance for treatment decisions, remains a rather great challenge.
We are all aware that if BC patients are detected as early as being confined to the mucosa or lamina propria, they can receive appropriately treatment, resulting in better outcomes and quality of life (QOL)2. But if the diagnosis is delayed, patients would harbor higher stage and grade cancers. Currently, among patients who receive an initial diagnosis of BC, about 25% have muscle-invasive BC (MIBC) or metastatic disease, leading to worse prognosis3. Thus, BC should be detected at its earliest and most localized stage.
For nearly half a century, voided urine cytology has been the main clinical method for detecting BC. But studies have shown that its sensitivity is not very satisfactory4, thus it is usually seen as a basic and crude examination. Meanwhile, although cystoscopy is considered the gold standard of diagnosis for BC, the procedure has innate adverse outcomes, for example, inadvertent damage of urethra or bladder, infection, as well as uncomfortable feelings for patients.
Clinically, improvement in the accuracy of early diagnosis for BC is strongly required. More sensitive, non-invasive, and cost-effective examination method is an ongoing pursuit for urologists. Recently, some novel methods of urine-based cancer detection have been proposed, such as bladder tumor antigen, Xpert Bladder Cancer Detection, UroVysion, Oncuria5, nuclear matrix protein- 22. But for many reasons, such as increased cost, needing for trained personnel or expensive equipment, or not a point-of-care assay, the clinical applications were not quite satisfactory, and they could not replace cystoscopy completely.
A recently developed urine test technology- CellDetect- was introduced into clinical application, with the discriminative capacity of color and morphology between normal and neoplastic cells6, which are probably related to the increased metabolic activity in cancer cells. Hila et al. demonstrated that the overall sensitivity and specificity of CellDetect test for BC were 84% and 80%, respectively7. Particularly, the assay was found to have superior sensitivity for low grade (LG) BC. Several articles regarding this method have been published and showed promise for clinical use in non-invasive diagnosis and surveillance of BC.
At our hospital, we applied this assay for several years. We found it is an easy-to-use test with satisfactory results, especially its better ability to detect LG cancers. Herein, we described the routine procedures in our clinical practice about patients with suspicious BC. In this study, we aimed to evaluate the diagnostic performance of CellDetect assay in the detection of primary or recurrent BC for patients referred to our Urology Department, and sought to elucidate the potential usefulness of this assay in early diagnosis of BC.
Materials and methods
Patients
Between August 2020 and July 2022, the patients referred to our hospital for suspicion of BC were enrolled in the study. The inclusion criteria were: hematuria or irritative voiding symptoms, or imaging examination (B-ultrasonography/ CT scan) showing space-occupying mass in bladder when physical examination, suggestive of bladder tumor; ≥18 years of age. The exclusion criteria were: active tuberculosis; recent surgery or external injury of the bladder; radiotherapy or systemic chemotherapy during the past 3 months; urinary system instrumentation; concomitant with urinary malignancy other than the bladder. Patients who did not consent to the study design were also excluded.
Methods
In our hospital, we regularly performed B-ultrasonography or CT scan for patients with hematuria, especially gross hematuria, or irritative voiding symptoms. If no bladder mass was detected, the patient was further excluded. Then the patients with bladder mass formed the final study cohort. All patients received CT urography examination to rule out upper urinary tract tumor.
Before cystoscopy or diagnostic transurethral resection of bladder tumor (TURBt), at least 50 ml of voided mid-stream urine samples were collected from the second micturition of the morning. Then the urine samples were transported to clinical laboratory on ice within 2 h.
Subsequently, the patients underwent cystoscopy or diagnostic TURBt. During cystoscopy, the bladder was carefully inspected. In case of small suspicious lesions, biopsies were taken; while in patients with bigger lesions, a diagnostic TURBt was undertaken under anesthesia. Then histologic examination was performed for all biopsies or surgical specimens by two independent experienced pathologists. Based on the histologic reports, the patients were determined as having or not having BC. Histologic specimens with confirmed urothelial cancer were graded according to the 2004 World Health Organization (WHO) grading system, and staged according to TNM classification criteria8. The study protocol was approved by Institutional Review boards. All patients provided written informed consent, and the handling of patient data was in accordance with privacy laws (e.g., GDPR).
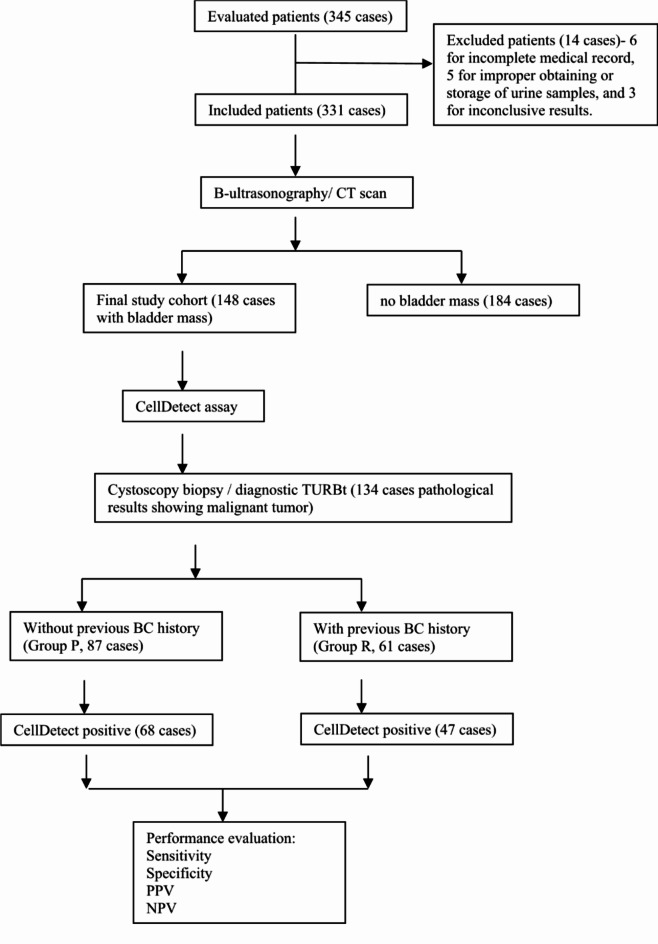
The patients were divided into two groups based on whether they had previous history of BC: group P- Primary group (without previous BC history), and group R- Recurrent group (with previous BC history). All patients’ data on medical history, sociodemographics were recorded. The Flow diagram of the study is shown in Fig. 1.
Fig. 1.
Flow diagram of the study procedures.
CellDetect assay
Cell staining was performed by use of CellDetect kit (Zetiq Techoogies Ltd., Tel Aviv, Israel), according to manufacturer instructions. In brief, urine samples were processed per the CellDetect instructions. After centrifugal separation and smears, the cellular staining was finished with the red and the green dyes in the kit, just as the detailed method described by Donghao Shang, et al.9.
With respect to CellDetect staining results, the following categories were included:
① negative: the nucleus of the normal urothelial cell is stained in green, blue or dark purple, with greenish cytoplasm. Inflammatory cells are stained in purple/ red, with well discrimination based on their morphology (Fig. 2A).
Fig. 2.
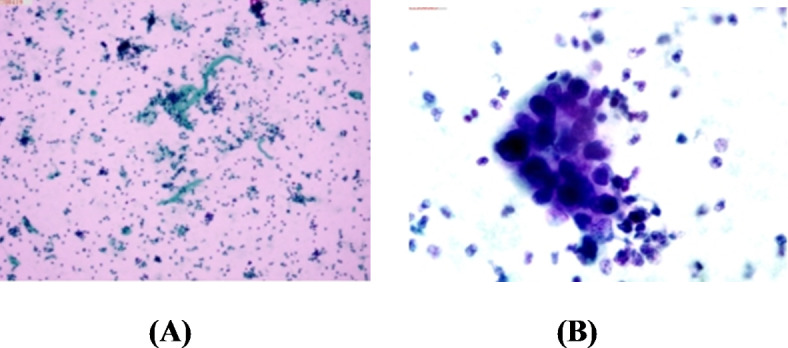
Urine smears stained with CellDetect assay (×30). (A) Urine smear of normal subject: the nucleus of the normal cell is stained in green, blue or dark purple, with greenish cytoplasm. (B) Urine smear of patient with BC: the nucleus of the malignant cell is stained in red/ purple, with the cytoplasm of cancer cells being stained in pink; in particular, the cells are arranged in clusters, with high nucleus/cytoplasm ratio.
② positive: the nucleus of the malignant cell is stained in red/ purple, with the cytoplasm of cancer cells being stained in pink, especially when cells are arranged in clusters, with high nucleus/ cytoplasm ratio. Regarding the atypical cells, their nuclei are stained in red/ purple, with cytoplasm being transparent, pink or greening (Fig. 2B).
Statistics
Histologic evaluation of the specimen was considered the gold standard to which CellDetect was compared. Cases with negative histology were considered negative. The diagnostic performance measures- sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) - were calculated for the overall cohort and for subsets of the patients. Data were meticulously recorded and analyzed using the software- statistical package for social science, version 18.0 (SPSS, IBM Corp., Armonk, NY, United States). The analysis included descriptive statistics and percentages. As this was an observational study without comparison between different groups, so we did not calculate p-values or confidence intervals.
Results
A total of 346 patients were enrolled in the study, among which 14 were excluded due to incomplete medical record, improper obtaining or storage of urine samples, or inconclusive results. Following B-ultrasonography or CT scan, 184 patients were further excluded for no bladder mass. Thus, a final cohort of 148 patients was eligible for this study. All received CellDetect assay and subsequent cystoscopy or diagnostic TURBT, with 134 cases showing malignant tumor after pathological confirmation.
Baseline characteristics of the enrolled patients are listed in Table 1. Of them, 87 patients had no previous BC history (group P) and 61 had previous BC history (group R). The ages ranged between 35 and 92 years and most patients were male (72.9%).
Table 1.
Patient demographics and baseline characteristics.
| Variable | Patients with bladder mass | Patients without bladder mass | ||
|---|---|---|---|---|
| Total | Group P | Group R | ||
| n | 148 | 87 | 61 | 184 |
| Age (years) | 35–92 | 35–86 | 45–92 | 31–87 |
| Male/ female | 108/40 | 66/21 | 42/19 | 137/54 |
| Main symptoms | ||||
| Microscopic hematuria | 48 | 29 (33.3%) | 19 (31.1%) | 52 |
| Gross hematuria | 62 | 35 (40.2%) | 27 (44.3%) | 94 |
| Irritative symptoms | 26 | 16(18.4%) | 10 (16.4%) | 38 |
| No symptoms: incidental finding when physical examination | 12 | 7 (8.0%) | 5 (8.2%) | 0 |
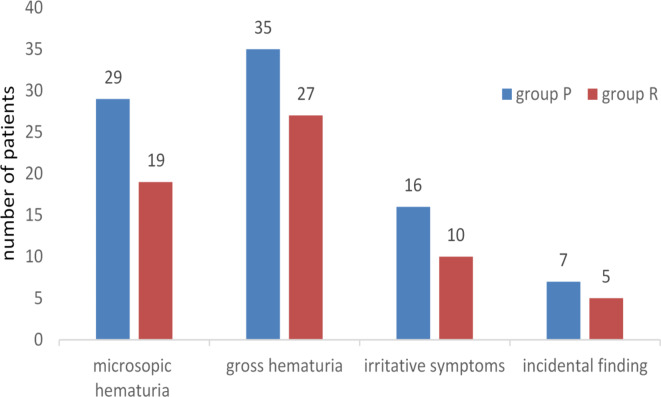
As to the main symptoms, 48 cases were due to microscopic hematuria (29 in group P and 19 in group R; accounting for 33.3% and 31.1%, respectively), 62 for gross hematuria (35 in group P and 27 in group R; 40.2% and 44.3%, respectively), 26 cases for irritative symptoms (16 in group P and 10 in group R; 18.4% and 16.4%, respectively), and 12 cases were for incidental finding at physical examination (7 in group P and 5 in group R; 8.0% and 8.2%, respectively) (seen in Table 1; Fig. 3). Further investigation demonstrated that among 134 patients with BC, 30 were LG cancer (17 in group P and 13 in group R; 19.5% and 21.3%, respectively), 104 high-grade (HG) cancer (65 in group P and 39 in group R; 74.7% and 63.9%, respectively). Thirteen patients were MIBC. Moreover, 3 cases were accompanied by carcinoma in situ (CIS) (1 in Group P and 2 in Group R; 1.1% and 3.3%, respectively), all of which were CellDetect positive.
Fig. 3.
Number of the patients with main symptoms at presentation: group P- Primary group (without previous BC history), and group R- Recurrent group (with previous BC history).
Overall, 115 out of 148 cases (77.7%) had a positive CellDetect result, with 68 cases in group P (78.2%) and 47 in group R (77.0%), respectively (Table 2). Table 3 provides the diagnostic value of CellDetect for the total patients with BC and different subgroups. The overall sensitivity and specificity for all patients were 82.1% and 64.2%, respectively. As far as the subgroups were concerned, the respective sensitivity and specificity were: in group P- 81.0% and 50.0%; and in group R- 85.2% and 83.3%, respectively. As seen in Table 3, the sensitivity and specificity in Group P and Group R differ significantly. We consider it is probably due to the differences in tumor grade or stage between the two groups. Other parameters- including PPV and NPV- were also shown in the table. As to the relatively low NPV values, especially for Group P, the results suggested that for patients with negative results but persistent symptoms, more tests would be better. Moreover, the detection sensitivity for LG BC was 76.7%; and 83.7% for HG BC. From this result, we can conclude that the screening ability of CellDetect in LG BC is inferior to in HG BC.
Table 2.
Grade and stage of 134 patients that were confirmed with BC, as well as CellDetect staining conditions in all the included 148 patients.
| Total | Group P | Group R | |
|---|---|---|---|
| Grade | |||
| Low grade | 30 | 17 (19.5%) | 13 (21.3%) |
| High grade | 104 | 65 (74.7%) | 39 (63.9%) |
| Stage | |||
| NMIBC | 121 | 75 (86.2%) | 46 (75.4%) |
| MIBC | 13 | 7 (8.0%) | 6 (9.8%) |
| CellDetect | |||
| Positive | 115 | 68 (78.2%) | 47 (77.0%) |
| Negative | 33 | 19 (21.8%) | 14 (23.0%) |
The differences between the two subgroups were not compared in this study, so the p-values were not calculated (NMIBC non-muscle invasive bladder cancer).
Table 3.
Overall results of the total patients with BC and different subgroups.
| sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Total | 82.1% | 64.2% | 95.6% | 27.3% |
| Group P | 81.0% | 50.0% | 94.1% | 21.0% |
| Group R | 85.2% | 83.3% | 97.8% | 35.7% |
Group P- Primary group (without previous BC history), and group R- Recurrent group (with previous BC history).
Discussion
Around the world, BC represents one of the most common malignancies in Urology, ranking as the 4th most common cancer in men and the 9th in women; as well as the 13th in terms of cancer mortality10. In 2019, “the report of Global Burden of Diseases, Injuries, and Risk Factors Study” declared that the global incidence of BC cases reached 524,300, occupying about 3% of new cancer patients. In that year, the highest incidence of BC occurred in China among 204 countries and territories; and in China, the incidence of BC ranks the 6th of solid tumor in men, and the 1st of urogenital system. With the development of aging population, the incidence of BC is increasing gradually; and it has been estimated that by 2030, there will be 192,390 new cases of BC in China11. During the past 15 years, the fatality rates of prostate cancer, breast cancer decreased by 20- 40%, but that of BC declined only by 5%. In particular, the therapeutic outcome of late stage BC is very poor, with a 5- year survival of merely 15%12.
It is well known that the typical clinical presentation of BC is hematuria (gross or microscopic); less common is persistent irritative urination. However, some patients might present with no obvious symptoms. Urothelial carcinomas account for most of the disease types. As shown in our study, 110 patients presented with hematuria, 26 with irritative symptoms, and 12 patients were incidentally found at physical examination. All these patients were urothelial cancer pathologically. Although the risk of urinary tract cancer exists for patients with such symptoms, the referral rate to urologists is still far from satisfactory, ranging between 13% and 47%13. This low referral rate could be attributed to numerous factors, for example, patient’s carelessness or ignorance; but the discomfort during cystoscopy would probably preclude patients from this maneuver. Unfortunately, the lack of evaluation for urothelial carcinoma in such patients would lead to delayed diagnosis, and further, worse prognosis. Hence, early, timely and accurate detection of BC, with subsequent intervention can drastically influence the outcomes14. The outcome of BC is closely associated with the initial stage at presentation. Patients with non-invasive BC at initial diagnosis have higher survival rates, lower risks of recurrence and progression as compared with those with invasive or metastatic BC. Moreover, the patients could receive curative intervention when diagnosed early15. It might, therefore, be therapeutically advantageous to detect urothelial carcinoma as early as possible. Besides, economic burden of BC diagnosed at early stage is much less than that diagnosed too late.
Currently, the routine detection methods for BC include cystoscopy and conventional urine cytology in clinical settings. Cystoscopy in conjunction with biopsy of suspicious lesions remain gold standard procedure; however, this technique bears inherent disadvantages16 - invasive, with risks for urethral injury, urinary tract infection or hematuria, thus influencing patients’ compliance negatively. Clinical research showed that BC patients’ physical and psychological QOL might be severely influenced by the necessity of repeated cytoscopic probes17. In addition, this technique is highly subjective: because the morphology of bladder tumors varies greatly, inexperienced urologist could miss small flat lesions, especially in the detection of CIS18, or even mistake atypical tumorous appearance as inflammatory lesions. It was reported that the cystoscopy examination has an overall false-negative rate of 10–20% for CIS. Furthermore, in China, patients usually receive cystoscopic tissue-sampling under local anesthesia, not general anesthesia. More importantly, cystoscopy is unfit for large-scale screening of BC. Thus, in clinical practice, the ideal condition would be cystoscopy only performed for appropriate patients, to decrease economic burden, increase diagnostic accuracy and minimize the invasive procedure.
As one of the major body fluids and one perfect source of biomarkers, urine is collected easily and non-invasively, and has long been considered as a typical representative of the prevailing concept of “liquid biopsy”19, rendering itself to widespread use in BC detection. Conventional urine cytology is highly specific for BC, but its low sensitivity -particularly for LG cancer- ranges from 20 to 53%, thus limiting its clinical value20. Hence, there is an increasing interest in the development of more accurate test, for example, Bladder tumor antigen assay, ImmunoCyt test, Nuclear matrix protein-22, UroVysion, fluorescence in situ hybridization (FISH), etc21,22. However, each has its own shortcomings- unsatisfactory accuracy, far more expensive, or manipulated complicatedly, so they have failed to be used widely in clinical practice to date. As an ideal marker, it should be “easier, better, faster and cheaper”23, even to replace or lengthen the interval between routine cystoscopic examinations. Theoretically, conventional urine cytology examines the cellular morphology, and its low sensitivity and high specificity suggest that the malignant cells identified are differentiated poorly, while the well- or moderately- differentiated malignant cells could not be identified, thus leading to false- negative result.
Consequently, the need for surpassing these detection modalities is urgent. The CellDetect assay, a novel histochemical staining technique was developed by Zetiq Technologies Ltd (Israel) for cancer screening and diagnosis24. It’s based on the principle that there are different metabolic processes and products between normal cells and cancerous cells, and the metabolism of cancer cells is increased significantly- the so called “Wahberg effect”. Using color and morphology as distinguishing method, the technique can discriminate between normal and malignant cells in one single urine specimen25. By applying bright field microscopy, this method highlights suspicious cells in red-purple, identifying malignant cells at a very early stage, even before they present the morphological traits of malignancy. Theoretically, it examines samples at a cellular level and is more concordant with pathologic results. In our study, we observed that CellDetect assay achieved a sensitivity of 82.1% and specificity of 64.2% for the overall patients in identifying BC. Moreover, unlike the urine cytology examination which detection sensitivity for LG BC is poor16, (and Springer et al. reported a sensitivity of urine cytology was 43%)26; our results for detection of LG BC revealed a sensitivity of 76.7%, with a 83.7% for HG BC, which is similar with the findings of Davis N, et al.27 and Yossepowitch et al.28. Our results suggested that CellDetect could identify not only poorly-differentiated cancer cells, but also well-differentiated cancer cells. By comparing with historical data regarding urine cytology examination, CellDetect assay demonstrated better effects and less likely produced false results (misdiagnosis).
It is known that the postoperative recurrence rate of BC is the highest among all cancers, with up to 50–70% of cases recurring; and many patients exhibited at least one recurrence within 5 years29. The life of sufferers is seriously endangered, also making it a great health challenge. At present, in clinical scenario, the detection methods of recurrent BC are the same as primary BC, and the need for avoiding or lengthening the intervals of cystoscpy is urgent. In our study, the sensitivity of CellDetect in recurrent BC was 85.2%, with a specificity of 83.3%. These results were similar with that of Donghao Shang et al.9 and different from that of Ömer Yüksel et al.25, but their results were based on patients regardless of prior BC history. In addition, in our study, the sensitivity of CellDetect for recurrent BC was some higher than primary BC in amount (85.2% vs. 81.0%). We speculated it was probably because higher concentrations of tumor cells are exfoliated into urine in recurrent BC with bladder mass. Based on the abovementioned results, we believe that for some selected patients, the CellDetect assay could provide an alternative method for lengthening the intervals of cystoscopy, reducing the number of cystoscopic procedures during follow up, or monitoring the relapse of BC. (But this is only our future ideas. As to the specific implementation plan, or for real clinical practice, we think this needs further clinical trials.)
Our study was not devoid of limitations. Firstly, CellDetect assay could only detect the exfoliated tumor cells in urine, without the ability to locate the site of the tumor nor to stage the tumor. This is the innate defect and main drawback of this assay. But we believe that patients might receive this assay to clarify whether to receive subsequent cystoscopy. Secondly, the topic of our study is to evaluate the clinical performance of CellDetect assay in patients with hematuria or irritative voiding symptoms for BC, and this is a preliminary study. In China, it is very difficult to persuade patients only with such symptoms and without bladder mass to undergo traumatic cystoscopy examination, especially taking biopsy and histologic examination. So, we exclude patients without suspicious sonographic and/or CT bladder masses but symptoms of hematuria and irritative voiding symptoms; because for these patients, we cannot confirm their pathologic results at present. In future, we hope to find new methods to undertake such clinical studies in the entire symptomatic patients. Thirdly, in our study, we conducted only an observational study without comparing with urine cytology; because in our hospital, urine cytology examination is seldom performed for shortage of reagents or other reasons. This study showed promising results of CellDetect assay, and we have applied this assay in routine practice. In future research, we will compare its performance with that of urine cytology and acquire our own center’s results. Fourthly, among the primary or recurrent patients, the results were not further evaluated or compared according to the respective stages of BC, and the results of PPV or NPV were not analyzed in detail. We hope to make up for these shortcomings in the future clinical studies. Lastly, the sample size of our study was small, so we plan to validate the results in a larger multi-institutional cohort of samples; and this assay has been also performed in another hospital in our city to expand sample sizes. The effect of CellDetect assay for predicting the recurrence of BC should be evaluated in future studies, especially in combination with other diagnostic tests (such as NMP22 or UroVysion). And exploring its use for monitoring treatment response or recurrence in BC patients is also a future clinical study. We expect better results. Moreover, there might be potential sources of bias in the results, for example, patient selection bias or inter-observer variability in the pathological evaluation. And we will try as much as possible to avoid such bias in future studies.
Conclusion
The typical clinical manifestation of BC is hematuria; less common is persistent irritative urination, while some patients might present with no obvious symptoms. Detection of BC in such patients is mainly by the means of cystoscopy and urine cytology at present; however, there are drawbacks in both methods. Our findings indicate that CellDetect assay could identify BC patients at an early stage with significant diagnostic performance, as well as with good reliability. Its significant benefit both in HG and LG BC implies that its clinical utility is reliable. This assay might provide an alternative method to prolong the intervals of cystoscopy, reduce the number of cystoscopic procedures during follow up, or monitor the relapse of BC for some selected patients, to avoid usage of the uncomfortable or traumatic cystoscopy examination. Moreover, this assay might indicate the treatment directions to some extent in clinical practice, for both doctors and patients; and facilitate the clinical decision-making process, especially in resource-limited settings where cystoscopy is not readily available. In future, more attention needs to validate the results in a larger multi-institutional cohort of samples, or in more studies comparing this assay to others in clinical use (e.g., FISH or NMP22), for better and wider application.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Dr. Jingjing Yu for her providing the pictures of CellDetect assay.
Author contributions
W.G. wrote, collected data and conceived this paper; Z. L. prepared and reviewed the manuscript.
Data availability
All data involved in this study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
The inclusion of patients complies with the Helsinki Declaration and has been approved by the Clinical Research Ethics Committee of Ningbo Urology and Nephrology Hospital. Written informed consent was obtained from all subjects involved in the study before their participation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Babjuk, M., Burger, M. & Capoun, O. European Association of Urology Guidelines on non-muscle-invasive bladder Cancer (Ta, T1, and carcinoma in situ). Eur. Urol.81, 75 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Shkolyar, E., Zhao, Q. & Mach, K. E. Bladder cancer risk stratification using a urinary mRNA biomarker panel - A path towards cystoscopy triaging. Uro Onc. 39, 497 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Chen, X. et al. Identification and validation of telomerase related lncRNAs signature to predict prognosis and tumor immunotherapy response in bladder cancer. Sci. Rep.13, 21816 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niko, K., Ivan, P., Andreja, Z. & Nadja, K. V. Clinical Evaluation of Two Non-invasive Genetic Tests for Detection and Monitoring of Urothelial Carcinoma: validation of UroVysion and xpert bladder Cancer detection test. Front. Genet.13, 1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yosuke, H. et al. Diagnostic performance of Oncuria, a urinalysis test for bladder cancer. J. Transl Med.19, 1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glickman, Y., Davis, N. & Nativ, O. Combining color and morphology to detect low grade urothelial cell carcinoma in urine specimens. Arch. Can. Res.4, 4 (2016). [Google Scholar]
- 7.Hila, K. S. et al. Performance of CellDetect for detection of bladder cancer: comparison with urine cytology and UroVysion. UrolOncol41, 6 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Guo, C. C., Bondaruk, J., Yao, H., Wang, Z. & Czerniak, B. Assessment of luminal and basal phenotypes in bladder cancer. Sci. Rep.10, 1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang, D., Liu, Y. & Xu, X. Diagnostic value comparison of CellDetect, fluorescent in situ hybridization (FISH), and cytology in urothelial carcinoma. Cancer Cell. Int.21, 465 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feber, A., Dhami, P. & Dong, L. UroMark-a urinary biomarker assay for the detection of bladder cancer. Clin. Epigenet.. 9, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, Q. et al. Secular trends of morbidity and mortality of prostate, bladder, and kidney cancers in China, 1990 to 2019 and their predictions to 2030. BMC Cancer. 22, 1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janev, A. et al. Human amniotic membrane inhibits migration and invasion of muscle-invasive bladder cancer urothelial cells by downregulating the FAK/PI3K/Akt/mTOR signalling pathway. Sci. Rep.13, 19227 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermanns, T., Savio, A. J. & Olkhov-Mitsel, E. A noninvasive urine-based methylation biomarker panel to detect bladder cancer and discriminate cancer grade. Uro Oncol.38, 601 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Pak, S. et al. Adjuvant chemotherapy versus observation after radical cystectomy in patients with node-positive bladder cancer. Sci. Rep.9, 8305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo, W. et al. LncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance through PROM2-activated iron export by sponging mir-129-5p in bladder cancer. Cell. Death Dis.12, 1043 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebret, T., Pignot, G. & Colombel, M. Artificial intelligence to improve cytology performances in bladder carcinoma detection: results of the VisioCyt test. BJU Int.129, 356 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Yu, S., Meng, Q., Hu, H. & Zhang, M. Correlation of anxa1 expression with drug resistance and relapse in bladder cancer. Int. J. Cli Exp. Patho. 7, 5538 (2014). [PMC free article] [PubMed] [Google Scholar]
- 18.Gu, L. et al. Observations of a pre-merger shock in colliding clusters of galaxies. Nat. Astron.3, 838 (2019). [Google Scholar]
- 19.Printz, C. Liquid biopsy test appears better at detecting bladder cancer than urine cytology. Cancer127, 663 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Dudley, J. C., Schroers-Martin, J. & Lazzareschi, D. V. Detection and surveillance of bladder Cancer using urine tumor DNA. Can. Dis.9, CD–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou, M. et al. Computational recognition of lncRNA signature of tumor-infiltrating B lymphocytes with potential implications in prognosis and immunotherapy of bladder cancer. Brief. Bioinform. 10.1093/bib/bbaa047 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Hussein, S. et al. SATB-1 and Her2 as predictive molecular and immunohistochemical markers for urothelial cell carcinoma of the bladder. Cancer Biomark.30, 249 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Song, Z. et al. A novel anoikis-related gene signature identifies LYPD1 as a novel therapy target for bladder cancer. Sci. Rep.14, 3198 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miniutti, G. et al. Nine-hour X-ray quasi-periodic eruptions from a low-mass black hole galactic nucleus. Nature573, 381 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Yüksel, Ö., Tosun, Ç. & Gümrükçü, G. What is the role of CellDetect® in detecting and monitoring bladder cancer? Urologia90, 261 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Springer, S. U., Chen, C. H. & Pena, M. D. Correction: non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. eLife Sci.7 . 10.7554/eLife.43237 (2018). [DOI] [PMC free article] [PubMed]
- 27.Davis, N., Shtabsky, A., Lew, S. A. & Novel Urine-based assay for bladder Cancer Diagnosis: multi-institutional validation Study.Eur urol focus, S2405456916301493. 10.1016/j.euf (2016). [DOI] [PubMed]
- 28.Yossepowitch, O. Colour and morphology combination for detection of low grade urothelial cancer cells Multicenter validation study. Eur. Urol. Suppl.15, e751 (2016). [Google Scholar]
- 29.Michelle, R. D., Katherine, L., Cynthina, K. B. & Girish, S. The impact of grading scheme on non-muscle invasive bladder cancer progression: potential utility of hybrid grading schemes. Pathology54, 425 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data involved in this study are available from the corresponding author on reasonable request.