Abstract
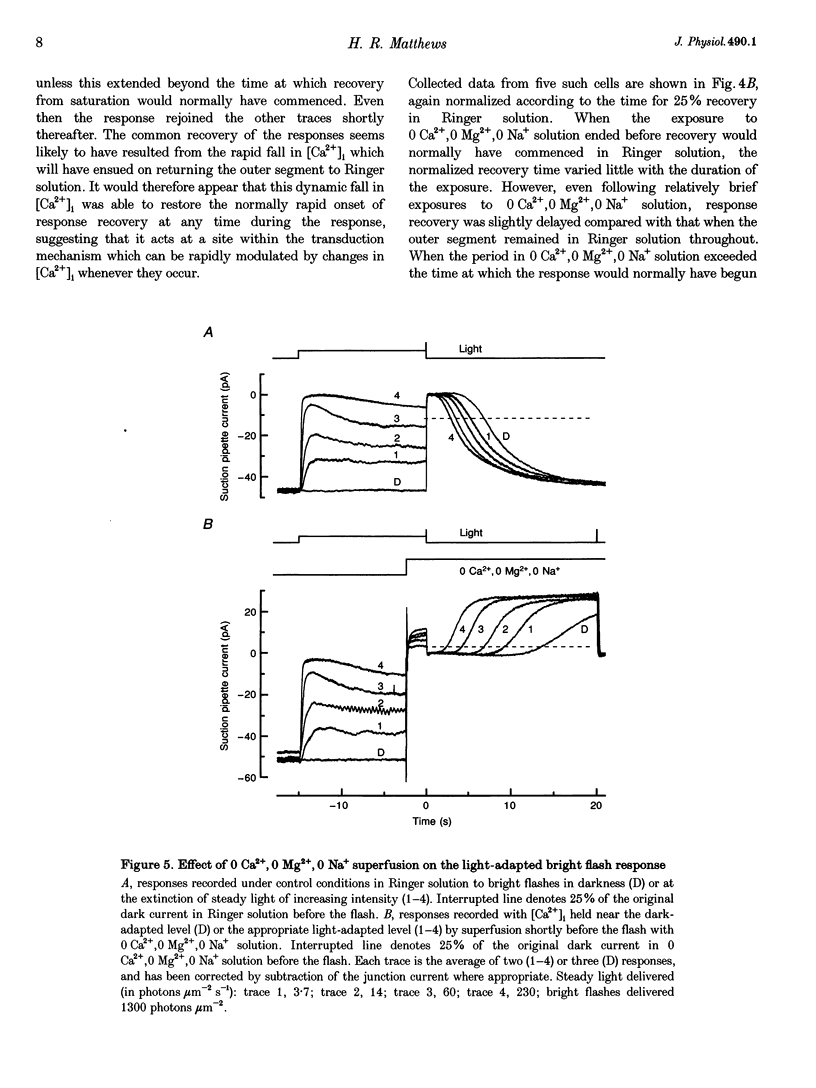
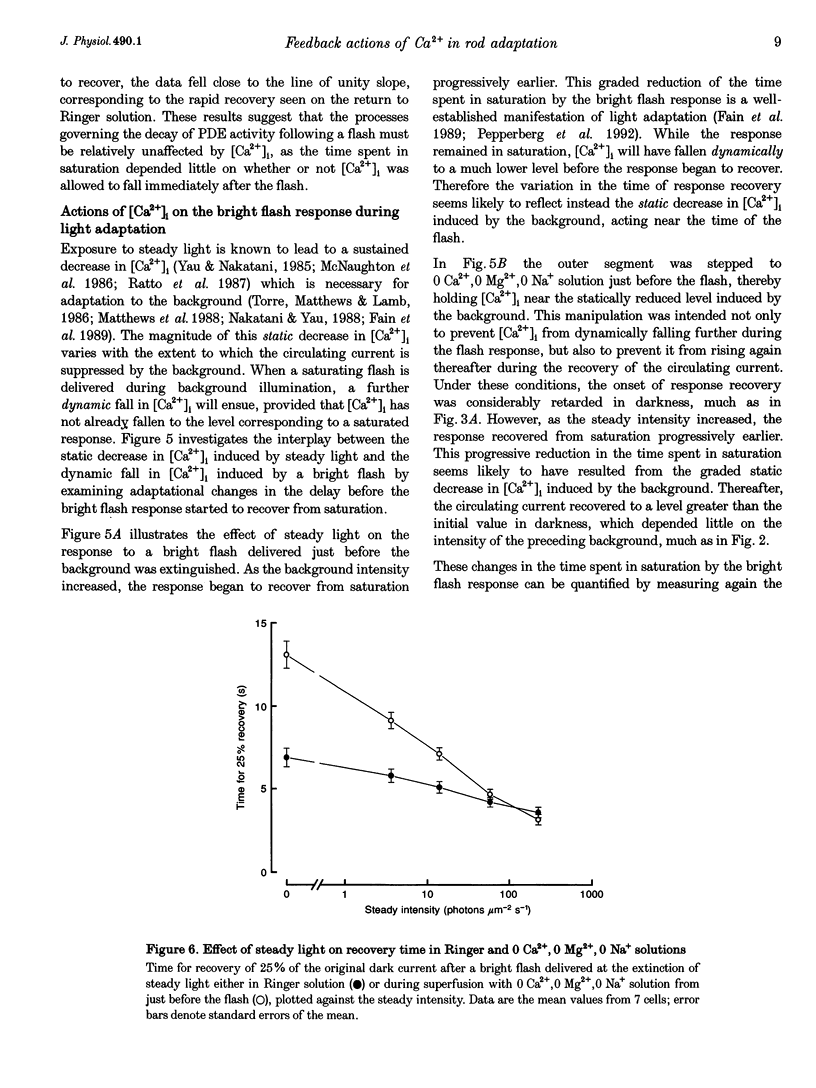
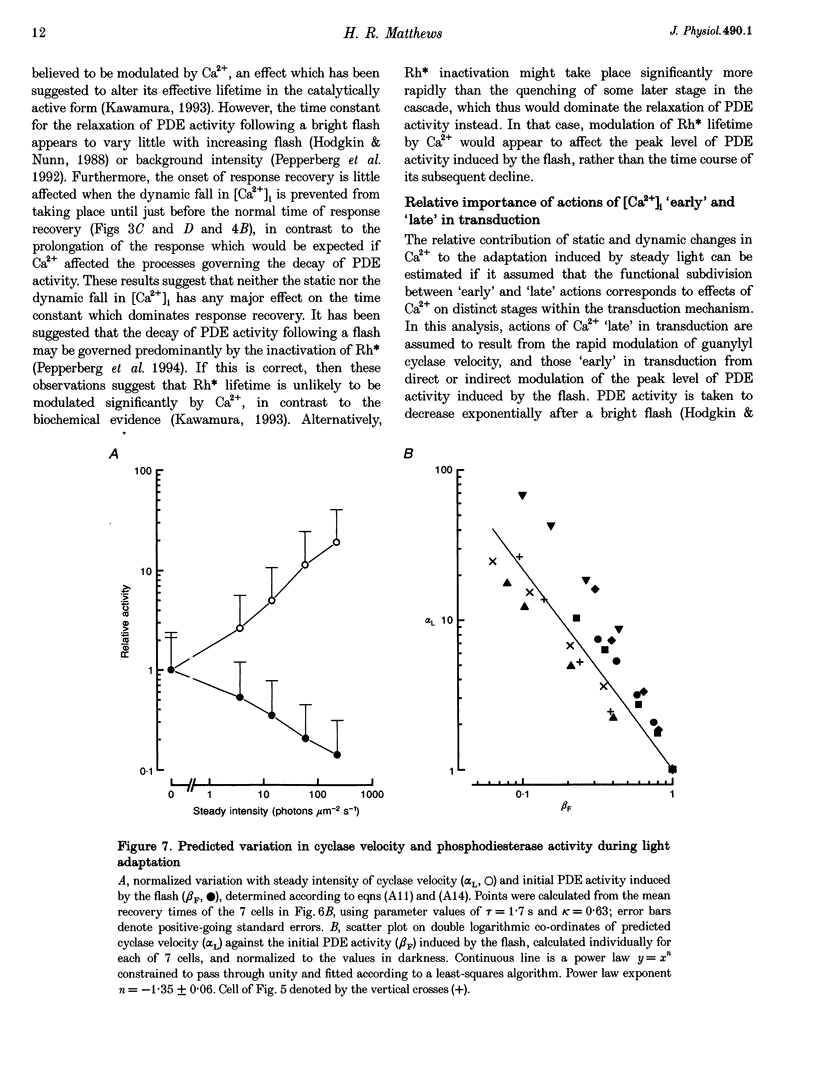
1. In order to study the relative contribution to light adaptation of the various actions of Ca2+ in rod photoreceptors, changes in cytoplasmic calcium concentration ([Ca2+]i) were opposed by manipulating the calcium fluxes across the outer segment membrane at different times during the response to a bright flash. 2. When the outer segment was superfused with 0 Ca2+, 0 Mg2+,0 Na+ solution just before a bright flash, the period of response saturation was greatly prolonged. But if instead the solution change was made at progressively increasing times after the flash, the delay before the response recovered from saturation declined exponentially towards its value in Ringer solution with a time constant of around 1 s. In contrast, recovery time was little affected by stepping to 0 Ca+,0 Mg2+,0 Na+ solution before the flash and returning to Ringer solution shortly before the normal time of recovery from saturation. 3. When a bright flash was delivered just before the extinction of steady light, the response recovered from saturation progressively earlier as this steady intensity was increased. If, instead, the outer segment was transferred to 0 Ca2+,0 Mg2+,0 Na+ solution just before the bright flash then the time spent in saturation by the response was prolonged in darkness, but this additional delay progressively decreased as the steady intensity increased. 4. These results are consistent with the notion that the light-induced reduction of the time spent in saturation by the bright flash response in Ringer solution resulted from the static decrease in [Ca2+]i induced by the background, while the additional delay in the recovery from saturation when further changes in [Ca2+]i were prevented stemmed from the abolition of the dynamic fall in [Ca2+]i during the flash response. 5. Analysis of the effects of steady light on the time spent in saturation by the bright flash response under these conditions suggests that actions of [Ca2+]i at, or soon after, the time of the flash are largely responsible for the graded changes which take place in the bright flash response during light adaptation, while rapid actions of [Ca2+]i at the time of response recovery also play a role in the adaptation of the steady response to background light itself. 6. These data have been interpreted in terms of differential actions of [Ca2+]i on 'early' stages (e.g. events leading to phosphodiesterase activation) and 'late' stages (e.g. guanylyl cyclase) in the transduction mechanism. A quantitative model is presented which suggests that actions of [Ca2+]i on 'late' stages play a proportinately larger role in background adaptation than actions on 'early' stages.
Full text
PDF


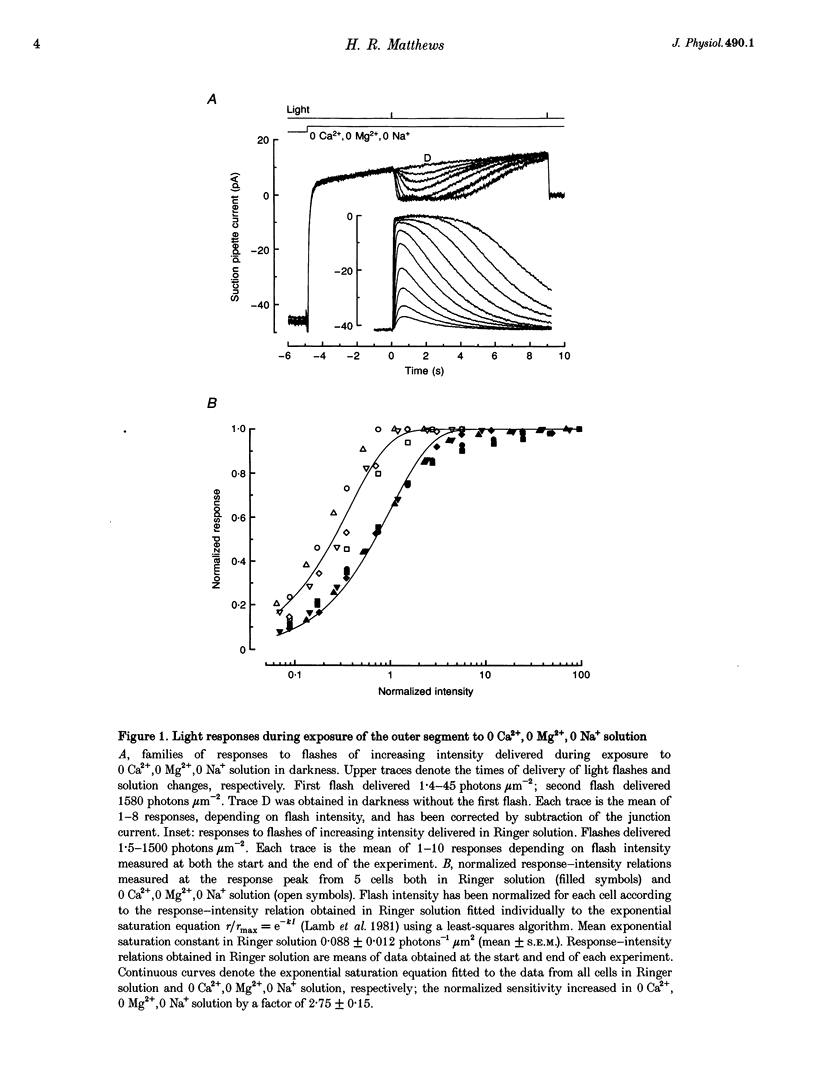
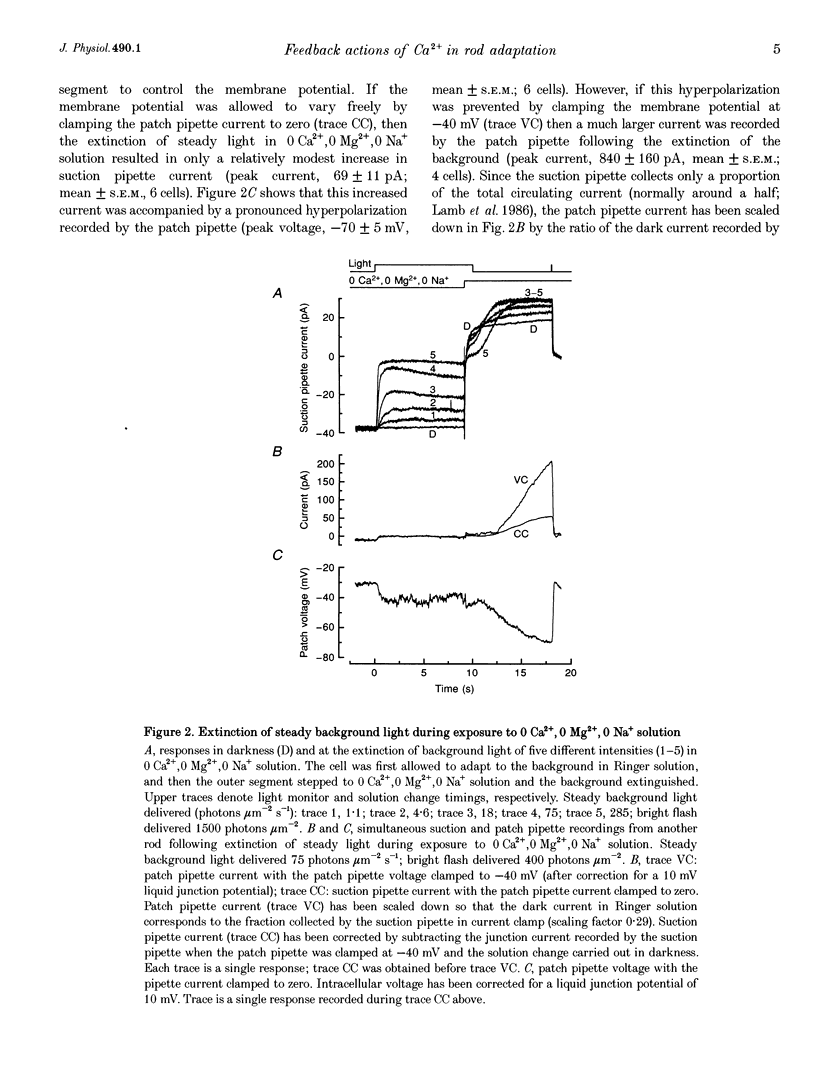
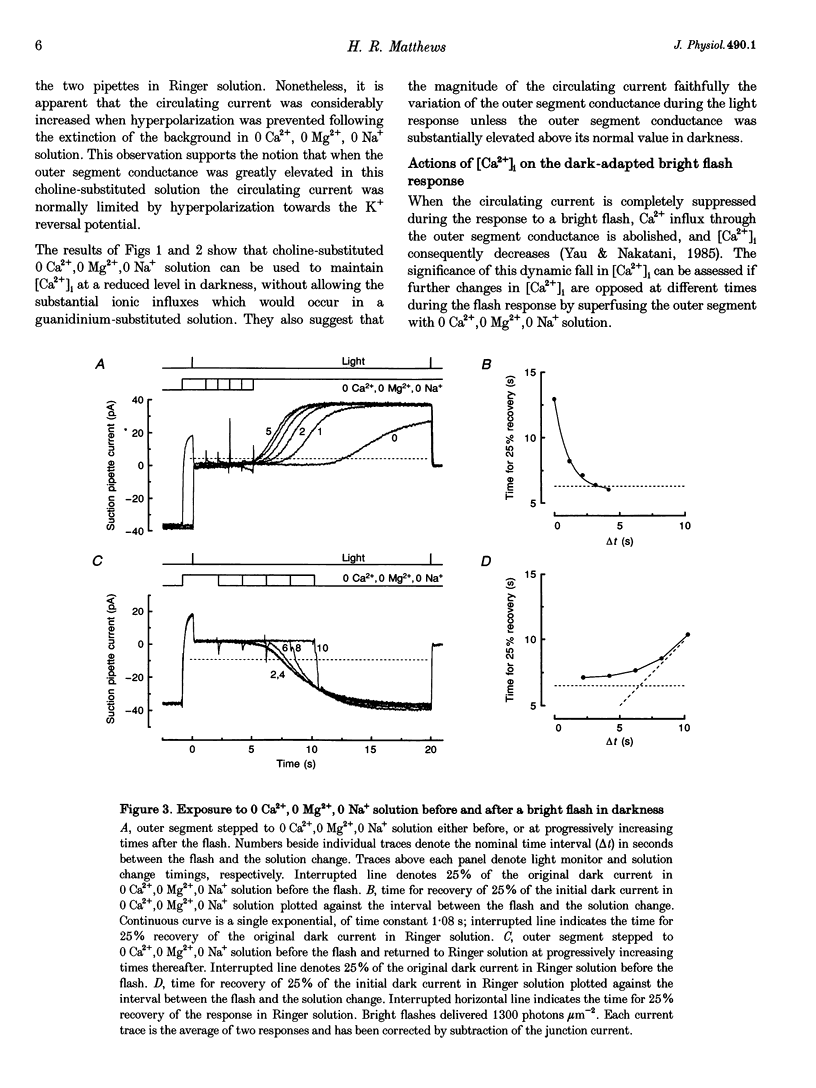
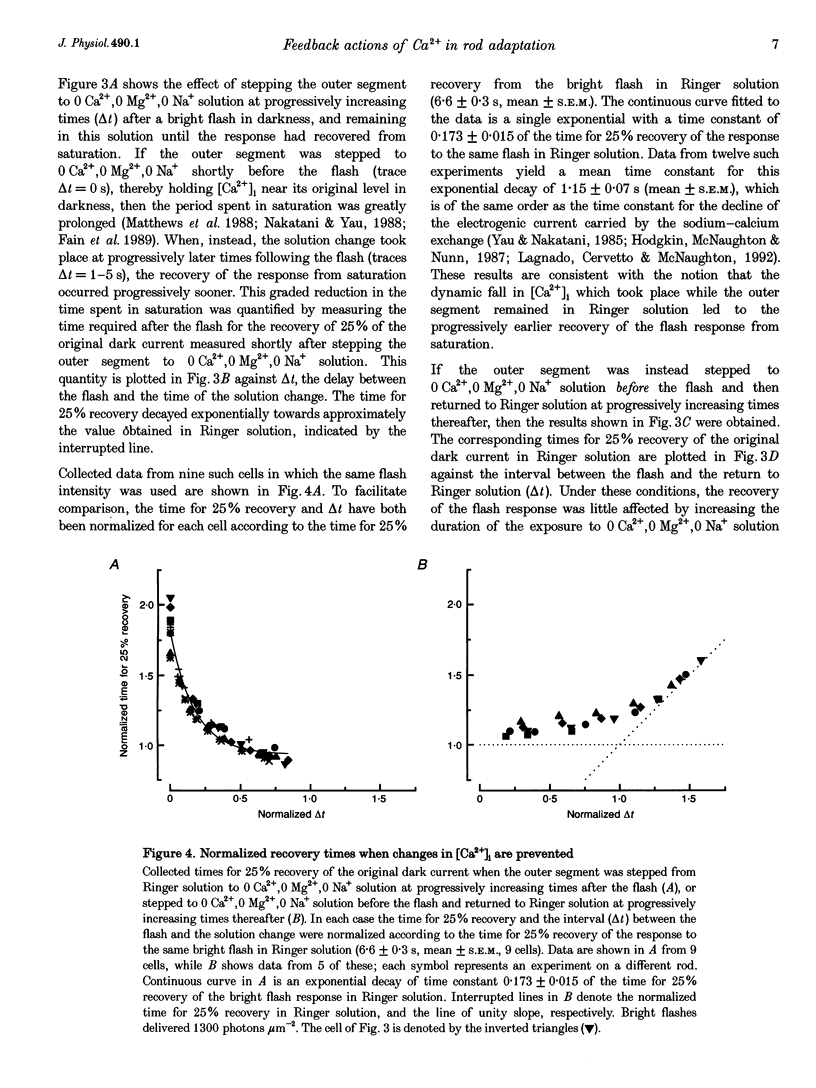





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornwall M. C., Fain G. L. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol. 1994 Oct 15;480(Pt 2):261–279. doi: 10.1113/jphysiol.1994.sp020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote R. H., Brunnock M. A. Intracellular cGMP concentration in rod photoreceptors is regulated by binding to high and moderate affinity cGMP binding sites. J Biol Chem. 1993 Aug 15;268(23):17190–17198. [PubMed] [Google Scholar]
- Dawis S. M., Graeff R. M., Heyman R. A., Walseth T. F., Goldberg N. D. Regulation of cyclic GMP metabolism in toad photoreceptors. Definition of the metabolic events subserving photoexcited and attenuated states. J Biol Chem. 1988 Jun 25;263(18):8771–8785. [PubMed] [Google Scholar]
- Fain G. L., Lamb T. D., Matthews H. R., Murphy R. L. Cytoplasmic calcium as the messenger for light adaptation in salamander rods. J Physiol. 1989 Sep;416:215–243. doi: 10.1113/jphysiol.1989.sp017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca W. A., Gray-Keller M. P., Detwiler P. B., Palczewski K. Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4014–4018. doi: 10.1073/pnas.91.9.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. Control of light-sensitive current in salamander rods. J Physiol. 1988 Sep;403:439–471. doi: 10.1113/jphysiol.1988.sp017258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. T., Molday R. S. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993 Jan 7;361(6407):76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Murakami M. Calcium-dependent regulation of cyclic GMP phosphodiesterase by a protein from frog retinal rods. Nature. 1991 Jan 31;349(6308):420–423. doi: 10.1038/349420a0. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Murakami M. In situ cGMP phosphodiesterase and photoreceptor potential in gecko retina. J Gen Physiol. 1986 May;87(5):737–759. doi: 10.1085/jgp.87.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993 Apr 29;362(6423):855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. A. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994 Jan 20;367(6460):273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992 Sep;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R. External and internal actions in the response of salamander retinal rods to altered external calcium concentration. J Physiol. 1988 Sep;403:473–494. doi: 10.1113/jphysiol.1988.sp017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R., Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986 Mar;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. J Physiol. 1995 Apr 15;484(Pt 2):267–286. doi: 10.1113/jphysiol.1995.sp020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- McCarthy S. T., Younger J. P., Owen W. G. Free calcium concentrations in bullfrog rods determined in the presence of multiple forms of Fura-2. Biophys J. 1994 Nov;67(5):2076–2089. doi: 10.1016/S0006-3495(94)80691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Korenbrot J. I. Kinetics of light-dependent Ca fluxes across the plasma membrane of rod outer segments. A dynamic model of the regulation of the cytoplasmic Ca concentration. J Gen Physiol. 1987 Sep;90(3):397–425. doi: 10.1085/jgp.90.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Cornwall M. C., Kahlert M., Hofmann K. P., Jin J., Jones G. J., Ripps H. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis Neurosci. 1992 Jan;8(1):9–18. doi: 10.1017/s0952523800006441. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Jin J., Jones G. J. Modulation of transduction gain in light adaptation of retinal rods. Vis Neurosci. 1994 Jan-Feb;11(1):53–62. doi: 10.1017/s095252380001110x. [DOI] [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispoli G., Sather W. A., Detwiler P. B. Visual transduction in dialysed detached rod outer segments from lizard retina. J Physiol. 1993 Jun;465:513–537. doi: 10.1113/jphysiol.1993.sp019691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Matthews H. R., Lamb T. D. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]


