Abstract
Individuals diagnosed with advanced cancer often experience stress and depression, factors linked to worse survival. Curability belief—defined as the hope and expectation of cure through treatment, based on affective forecasting—may differ from the patient’s actual life expectancy (i.e., likelihood estimation) and has shown variable associations with cancer survival. In this study, multivariate Cox regression analyses were used to examine the effect of curability belief and depression on 1-year survival after adjustment for physical factors. Additionally, regularized partial correlations among physical and psychological factors were assessed using mixed graphical models to elucidate their roles in mediating the relationship between curability belief and 1-year survival. This multi-center cohort study, conducted across 13 tertiary hospitals (including four ranked among the ‘World’s Best Specialized Hospitals 2025’ in oncology), involved 382 adults with stage IV advanced cancer and an oncologist-estimated survival of more than 6 months. Baseline data included demographics, primary tumor site, number of metastatic sites, symptom burdens (EORTC QLQ-C15-PAL), performance status (ECOG-PS), depression levels (PHQ-9), anti-cancer treatment type, patient’s life expectancy estimation, and curability belief. Follow-up data included 1-year survival and end-of-life care (place of death) for deceased patients. Multivariate Cox proportional hazards models were used to assess adjusted hazard ratios (aHRs) for curability belief, depression, and their interaction on 1-year survival, adjusting for significant demographic and clinical factors from univariate Cox regressions. The Kaplan–Meier method was used to plot survival probability by curability belief and depression interaction. Mixed graphical models estimated regularized partial correlations among 1-year survival, curability belief, patient’s life expectancy, depression, primary tumor site, anti-cancer treatment type, performance status, and symptom burden. In terms of healthcare utilization, patients with curability belief were more likely to receive standard or advanced anti-cancer therapy, while those without curability belief tended to suspend or discontinue therapy (P < 0.001). Among patients who did not survive the 1-year follow-up (N = 161), end-of-life care settings differed significantly between those with curability belief (predominantly nursing homes and home settings) and those without (primarily hospice and tertiary/secondary hospitals; P = 0.036). In multivariate Cox regression, curability belief (P = 0.003), depression (PHQ-9 score ≥ 10; P = 0.003), and their interaction (P = 0.040) were significantly associated with 1-year survival, after adjusting for sex, residential area, primary tumor site, performance status, anti-cancer treatment type, and symptom burdens (fatigue and appetite loss). The relationship between curability belief and 1-year survival was significant only in patients without depression [PHQ-9 score < 10; aHR (95% CI) = 2.20 (1.31–3.70); P = 0.003]. In the mixed graphical model, node predictability values for curability belief, depression, and 1-year survival were 0.68, 0.50, and 0.70, respectively, with curability belief showing partial correlations with depression (r = 0.30) and patients’ life expectancy (r = 0.20); depression correlated with fatigue (r = 0.53), anorexia (r = 0.16), life expectancy (r = 0.24), performance status (r = 0.23), and curability belief; and 1-year survival correlated with suspended/stopped anti-cancer treatment (r = 0.45), primary tumor site (r = 0.24), and performance status (r = 0.15). Partial correlations of performance status with depression and discontinued treatment mediated the association between curability belief and 1-year survival. Curability belief among stage IV advanced cancer patients with an oncologist-estimated survival of over 6 months was associated with depression levels and patients’ perceived life expectancy estimations. Performance status, depression, and anti-cancer treatment status mediate the relationship between curability belief and improved 1-year survival in patients without depression. Further research using longitudinal modeling of depression, performance status, and healthcare utilization, with curability belief and primary tumor site as covariates, is warranted.
Trial registration: Clinical Trial Number (ClinicalTrials.gov): NCT03222258; Study Registration Dates (First submitted: 2017-06-05; First submitted following the QC criteria: 2017-07-16; First posted: 2017-07-19).
Keywords: Advanced cancer, Curability belief, Depression, 1-year survival, Cox proportional hazard regression model, Mixed graphical model
Subject terms: Prognosis, Cancer, Psychiatric disorders, Psychology, Oncology
Introduction
Curability belief in advanced cancer does not always align with patients’ life expectancy
Prognostic awareness refers to a patient’s recognition of their shortened and limited life expectancy in advanced cancer1. This awareness generally persists throughout the course of the illness2,3. However, a patient’s curability belief—defined as the hope or expectation that they “will be cured” or “may be cured if treatment is successful,” rooted in affective forecasting—does not always match their perceived life expectancy estimate, which is a likelihood assessment based on information received. Curability beliefs of patients with advanced cancer frequently differ not only from their life expectancy estimates but also from the prognosis provided by their physicians or the explicit goals of their treatment. In fact, only 49.1% of advanced cancer patients report alignment between their personal prognostic beliefs and those of their physicians4. Interestingly, prognostic accuracy tends to be lower among patients with stage IV gastrointestinal cancer compared to those with stage I–III, and is also reduced in those with unresectable cancers compared to resectable ones3. Generally, patients who have recently been informed by their healthcare providers of the incurable nature of their illness have more realistic expectations about their prognosis5. Additionally, advanced care planning and discussions around end-of-life care play critical roles in shaping patients’ prognostic beliefs6. Conversely, a subgroup of patients with metastatic cancer has been shown to maintain a personal goal of cure, even when their oncologists have communicated that the objective of treatment is not to achieve disease cure7.
Curability belief, physical functioning, healthcare utilization, and survival in advanced cancer patients
The potential association between curability beliefs and patient survival may be partly mediated by patterns of healthcare utilization and physical functioning in daily life. First, prognostic awareness can affect treatment adherence and healthcare utilization. For instance, patients with advanced cancer who participate in clinical trials, including phase I trials, exhibit more optimism regarding their survival, curability, and treatment outcomes, have a preference for life-extending therapies, and are less likely to have a do-not-resuscitate order8. Conversely, patients who are aware of the incurable nature of their disease do not prefer life-prolonging treatment5, are more likely to use home hospice care3, and are less likely to be hospitalized in the last 30 days of life3. Second, a lower quality of life in the physical domain is associated with awareness of disease incurability9. Better physical functioning predicts longer survival; for instance, among patients who underwent metastatic spine tumor surgery, preserved physical functioning enabling ambulation and self-care was related to a higher probability of survival for up to 90 days without an unplanned hospital readmission10. Patients with advanced cancer may consider a fair level of physical functioning predictive of longer survival, even during hospice residency11. Third, decreased levels of self-efficacy and motivation, hopelessness, and anxious preoccupation in cancer patients with comorbid depression12 can weaken the “agency thinking components” of curability beliefs, which represents the mental energy required to initiate and maintain goal-oriented behaviors even during health-related distress13. A subgroup of patients with advanced cancer who possess energy and motivation and are able to engage in goal-orientated behaviors have longer survival than those who experience hopelessness14,15.
Association of prognostic awareness and depressive symptoms in advanced cancer patients
Prognostic awareness can be a significant source of distress in patients with advanced cancer6. Firstly, patients, along with their families and clinicians, often face considerable uncertainty regarding treatment outcomes and survival duration. Among patients with stage IV solid tumors, those who are aware of or uncertain about their prognosis tend to experience heightened anxiety and depression, greater symptom burden, reduced quality of life, and diminished spiritual well-being compared to those who believe their cancer is curable16. Conversely, patients with a limited life expectancy (median: < 12 months) and their caregivers encounter numerous challenges, including severe physical symptoms, disability, uncertainty around survival, and decreased self-efficacy3. Furthermore, while more conservative prognostic awareness in caregivers can enhance the quality of life and mood of patients with advanced cancer, it may lead to a decrease in the quality of life and mood for the caregivers themselves17. Consequently, disclosing the incurable nature of the disease to patients with advanced cancer should be accompanied by appropriate psychological support. Moreover, patients are often required to make personal and healthcare decisions based on their prognostic awareness, engaging in prognosis-based decision-making8. Since beliefs about survival or curability can profoundly influence feelings of hope or hopelessness and impact health-related behaviors in advanced cancer patients18–20, investigating the relationship between curability beliefs and survival in this patient population is essential.
Study aim and hypothesis
In unresectable or metastatic advanced cancers, physical factors influencing survival—such as primary and metastatic tumor sites, treatment type, and physical capability—are not modifiable21. This study aimed to analyze possible associations between curability beliefs and healthcare utilization, physical functioning in daily life, comorbid depression, and length of survival in patients with advanced cancer. Multivariate Cox regression analyses were utilized to examine the effect of the interaction between curability belief and depression on 1-year survival after adjustment for physical factors. Furthermore, mixed graphical models (MGMs) were used to estimate partial correlations among physical and psychological factors that may mediate the association of curability belief with 1-year survival. We hypothesized that curability beliefs serve as a protective factor promoting survival in advanced cancer patients, depending on the presence or absence of comorbid depression.
Materials and methods
Study design and participants
In the current study, we performed a secondary analysis of data from a prospective multicenter cohort study of patients with advanced cancer [Clinical Trial Registry (ClinicalTrials.gov); Clinical Trial Number: NCT03222258; Study Registration Dates (First submitted: 2017-06-05; First submitted following the QC criteria: 2017-07-16; First Posted: 2017-07-19)]. The study examined the potential associations among survival, curability beliefs, and depressive symptoms in patients with advanced cancer. Some of the results of the prospective cohort study related to the effect of prognostic awareness on quality of life in patients with advanced cancer were published recently22. The current study recruited participants who were aware of their cancer diagnosis from 13 tertiary hospitals in the Republic of Korea between 17 December 2016 and 17 August 2018. Four of the 13 hospitals in this study were ranked among the “World’s Best Specialized Hospitals, 2025” in oncology: Asan Medical Center (3rd), Seoul National University Hospital (8th), Seoul National University-Bundang Hospital (57th), and Chonnam National University-Hwasun Hospital (116th) (https://r.statista.com/en/healthcare/worlds-best-specialized-hospitals-2025/ranking/).
The inclusion criteria were as follows: (1) age ≥ 19 years; (2) diagnosis of stage IV advanced breast, colon, gastric, pancreatobiliary, lung, or liver cancer, or malignant hematologic neoplasm; (3) anti-cancer treatment that included ongoing standard chemotherapy, interrupted standard chemotherapy, ongoing advanced anti-cancer treatment (such as immunotherapy or clinical trials) following standard chemotherapy, or cessation of all anti-cancer therapy; and (4) oncologist-estimated survival of > 6 months at baseline. Patients with poor cognitive capacity, an inability to read or understand Korean, or an inability to complete the survey due to poor physical condition were excluded. All oncologists participating in this study as attending physicians are active members of the Korean Society of Medical Oncology (KSMO; http://eng.ksmo.or.kr/main.html) and have comparable levels of clinical expertise. The study protocol was approved by the Institutional Review Board of Seoul National University College of Medicine and Hospital (approval no.: 1602-142-745). The study was performed in accordance with the 1975 Declaration of Helsinki and its 2013 amendment. Informed consent was obtained from all participants.
Measures: sociodemographic and physical health status
At baseline, demographic factors (age, sex, marital status, religion, and place of residence), socioeconomic status (monthly household income and educational achievement), physical health (primary tumor site, physical performance, type of cancer treatment, and number of metastatic sites), and mental health (depressive symptoms) were evaluated using self-administered questionnaires during face-to-face interviews at an outpatient or inpatient facility.
With regard to cancer-related physical health status, physical functioning in daily life was assessed using the Eastern Cooperative Oncology Group performance status (ECOG-PS) scale23. This scale categorizes functioning in daily life into six classes: fully active and able to carry out all pre-disease activities without restriction (0), restricted in physically strenuous activities but ambulatory and able to carry out light or sedentary work, such as light house work or office work (1), ambulatory and capable of all self-care but unable to carry out any work activities, with the patient spending > 50% of waking hours “up and about” (2), capable of only limited self-care, confined to bed or chair for > 50% of waking hours (3), completely disabled, unable to perform self-care, and totally confined to bed or chair (4), and death (5).
Cancer-related symptom burden was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 Palliative (EORTC QLQ-C15-PAL) scores24–26. The EORTC QLQ-C15-PAL includes 15 items covering seven symptom domains—fatigue, pain, dyspnea, insomnia, appetite loss, constipation, and nausea/vomiting—as well as three functional scales assessing quality of life, physical functioning, and emotional functioning26. Most items are rated on a 4-point Likert scale (1 = “not at all,” 2 = “a little,” 3 = “quite a bit,” 4 = “very much”), while overall quality of life is evaluated on a scale from 1 (very poor) to 7 (excellent)26. Ten sub-scores are calculated and linearly transformed to a 0–100 scale26,27. Higher scores on the symptom scales indicate greater symptom severity, whereas higher scores on the functional and overall quality of life scales reflect better health-related quality of life26,27.
Measures: curability belief, patient’s perceived life expectancy, and depression
Curability belief was assessed by asking patients, “Do you think your illness will be cured?”22,28 Response options included: (1) “my cancer will be cured,” (2) “my cancer may be cured if the treatment is successful,” (3) “my cancer cannot be cured but treatment will control it,” (4) “my cancer cannot be cured and additional treatment is unsuitable,” and (5) “not sure28.” Curability belief was indicated by responses 1 or 2 only.
Patient life expectancy was evaluated with the item, “How much longer do you think you might live?”28 Participants selected one of the following options: (1) “similar to a healthy person of my age,” (2) “more than a few years,” (3) “more than a few months,” (4) “within a few months,” or (5) “not sure.” For comparisons between groups (Table 1) and MGM analysis (Fig. 2), responses were reclassified into two categories: “ ≥ several years” (options 1 and 2) and “ ≤ several months” (options 3–5).
Table 1.
Sociodemographic and clinical characteristics of patients with and without curability beliefs.
| Variable | Category | Without curability beliefs [N = 239] (“Cannot be cured but I will try to control the cancer”, “Cannot be cured and additional cancer treatment is not suitable for me”, or “do not know”) |
Having curability beliefs [N = 143] (“will be cured” or “may be cured if the treatment is successful”) |
P-value |
|---|---|---|---|---|
| Age (years) | Mean (SD) | 58.5 (11.0) | 61.69 (11.2) | 0.01 |
| Sexa | Male/Female | 122 (56.0%)/115 (71.0%) | 96 (44.0%)/47 (29.0%) | 0.003 |
| Educational achievement | < High school/ ≥ High school | 83 (62.4%)/156 (62.7%) | 50 (37.6%)/93 (37.4%) | 0.96 |
| Monthly income | < 3,000 USD/ ≥ 3,000 USD | 174 (61.9%)/65 (64.4%) | 107 (38.1%)/36 (35.6%) | 0.7 |
| Residential areab | Rural or suburban/Metropolitan area | 123 (57.8%)/113 (68.5%) | 90 (42.3%)/52 (31.5%) | 0.03 |
| Marital statusb | Unmarried/Married | 57 (63.0%)/179 (61.9%) | 32 (36.0%)/110 (38.1%) | 0.72 |
| Religious practice | No/yes | 104 (64.6%)/135 (61.1%) | 57 (35.4%)/86 (38.9%) | 0.48 |
| Primary tumor sitec | Breast/lung/stomach-colon-hepato-biliary-pancreatic/hematologic | 54 (65.1%)/55 (63.2%)/114 (61.0%)/13 (65.0%) | 29 (34.9%)/32 (36.8%)/73 (39.0%)/7 (35.0%) | 0.92 |
| Number of metastatic sitesa | 0/1/2/3/4/5 | 13 (54.2%)/82 (64.1%)/69 (56.6%)/44 (65.7%)/22 (75.9%)/8 (72.7%) | 11 (45.8%)/46 (35.9%)/53 (43.4)/23 (34.3%)/7 (24.1%)/3 (27.3%) | 0.34 |
| Type of cancer treatmenta | Standard chemotherapy ongoing | 77 (59.2%) | 53 (40.8%) | 0.0001* |
| Standard chemotherapy suspended | 17 (60.7%) | 11 (39.3%) | ||
| Advanced cancer treatment (including immunotherapy and clinical trials) | 57 (50.4%) | 56 (49.6%) | ||
| All anti-cancer treatment stopped | 87 (79.1%) | 23 (20.9%) | ||
| Performance status (ECOG-PSb) | 0 (Fully active and can carry out all pre-disease activities without restriction)/1 (Restricted in physically strenuous activities, but remains ambulatory and able to carry out work of a light or sedentary nature)/2 (Ambulatory and capable of all self-care, but unable to carry out any work activities; “up and about” > 50% of waking hours)/3 (Capable of only limited self-care; confined to bed or chair > 50% of waking hours)/4 (Completely disabled; cannot carry out any self-care; totally confined to bed or chair) | 9 (40.9%)/85 (51.8%)/68 (73.1%)/55 (73.3%)/22 (75.6%) | 13 (59.1%)/79 (48.2%)/25 (26.9%)/20 (26.7%)/6 (21.4%) | < 0.0001* |
| Symptom burden (EORTC QLQ-C15-PAL)d | QOL | 42.6 (22.9) | 57.0 (23.3) | < 0.0001* |
| Physical functioning | 48.9 (25.8) | 60.1 (23.5) | < 0.0001* | |
| Emotional functioning | 67.5 (29.5) | 79.6 (23.3) | < 0.0001* | |
| Appetite loss | 44.1 (35.2) | 29.3 (29.8) | < 0.0001* | |
| Fatigue | 48.7 (29.3) | 35.4 (24.4) | < 0.0001* | |
| Dyspnea | 30.7 (31.6) | 18.3 (24.0) | < 0.0001* | |
| Insomnia | 38.2 (33.5) | 24.9 (30.3) | 0.0001* | |
| Pain | 38.2 (31.8) | 26.4 (27.3) | 0.0002* | |
| Constipation | 33.8 (35.5) | 25.4 (29.4) | 0.0133 | |
| Nausea/vomiting | 12.6 (14.4) | 10.8 (13.0) | 0.2106 | |
| Depressionb (PHQ-9 total score) | No (0–4)/mild (5–9)/moderate (10–14)/moderately severe (15–19)/severe (20–27) | 46 (43.8%)/62 (56.4%)/61 (72.5%)/39 (79.6%)/26 (89.7%) | 59 (56.2%)/48 (43.6%)/22 (26.5%)/10 (20.4%)/3 (10.3%) | < 0.0001* |
| Patients’ perceived life expectancyd | Similar to those with same age/several years or more/several months or more/within a few months/not sure | 14 (21.2%)/72 (67.9%)/24 (75.0%)/19 (100%)/109 (69.4%) | 52 (78.8%)/34 (32.1%)/8 (25.0%)/0 (0%)/48 (30.6%) | < 0.0001* |
| 1-year survival | Alive at 1-year follow-up/expired/censored | 119 (55.0%)/114 (70.8%)/6 (75.0%) | 94 (44.1%)/47 (29.2%)/2 (25.0%) | 0.0097 |
| End-of-life care (Place of death) | Tertiary or secondary hospital/nursing hospital/hospice/home/unspecified location | 40 (60.6%)/1 (50%)/17 (94.4%)/4 (57.1%)/52 (76.5%) | 26 (39.4%)/1 (50%)/1 (5.6%)/3 (42.9%)/16 (23.5%) | 0.0358 |
*P < 0.001.
HR, hazard ratio; CI, confidence interval; GI, gastrointestinal; ECOG-PS, Eastern Cooperative Oncology Group performance status; EORTC QLQ-C15-PAL, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 Palliative scores; USD, United States dollar; PHQ-9, Patient Health Questionnaire-9.
aMissing, n = 1; bmissing, n = 4; cmissing, n = 5; dmissing, n = 2.
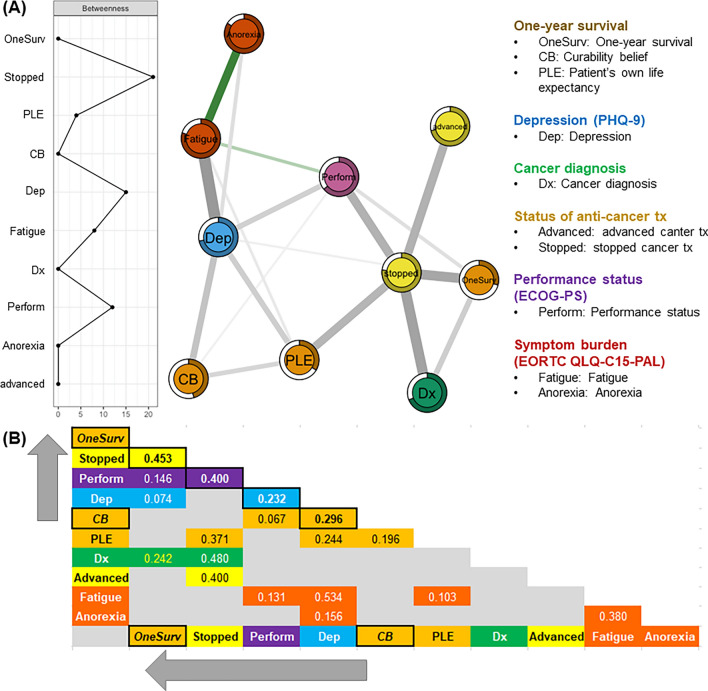
Fig. 2.
Mixed graphical model. Conditional joint probability distributions or regularized partial correlations of 1-year survival (Y/N), curability belief (“will be cured” or “may be cured if treatment is successful”: Y/N), patient’s perceived life expectancy (≥ years/ ≤ months), depression (PHQ-9; Y/N), cancer diagnosis (gastrointestinal-hepato-biliary-pancreatic/others), cancer treatment status [ongoing advanced anti-cancer therapy (Y/N), discontinued anti-cancer therapy (Y/N)], performance status (ECOG-PS; 0/1/2/3/4), and symptom burdens of fatigue and appetite loss (EORTC QLQ-C15-PAL) in patients with advanced cancer.
Depressive symptoms at baseline were measured using the Patient Health Questionnaire-9 (PHQ-9)29,30, a nine-item tool where total scores indicate depression severity: 0–4 (none), 5–9 (mild), 10–19 (moderate), and 20–27 (severe)29. In this study, comorbid depression was defined as a baseline PHQ-9 score of ≥ 1031.
Measures: patient survival at 1-year follow-up and end-of-life care metrics
Patient survival at 1 year from study enrollment and baseline measures were assessed through information collected from participants, families, and physicians. Follow-up was conducted by research assistants at 3, 6, and 12 months in inpatient or outpatient settings. For patients who did not survive the 1-year follow-up, end-of-life data, including place of death, was obtained from caregivers. If participants could not be reached, physicians were consulted to confirm survival status. Those unreachable were categorized as “unable to contact.”
Statistical analyses: survival analyses
Demographic, socioeconomic, physical health, and mental health parameters were compared between the subgroups with curability beliefs (n = 239) and without curability beliefs (n = 143) using t-test for continuous variables and the chi-square test for categorical variables (Table 1). Univariate Cox proportional hazards regression analyses were conducted to examine the associations between 1-year survival of patients with advanced cancer and factors such as demographics, socioeconomic status, primary tumor site, number of metastatic sites, type of cancer treatment, performance status, symptom burden, and depression (Table 2). Furthermore, multivariate Cox proportional hazards regression models of 1-year survival were used to examine associations between 1-year survival and curability beliefs, with adjustment for demographic and clinical variables that posed significant risks to 1-year survival, as identified in the univariate analyses (Fig. 1). Post hoc multivariate Cox proportional hazards regression models were utilized to compare the association between survival and curability beliefs in subgroups without (PHQ-9 total score < 10) and with (PHQ-9 total score ≥ 10) comorbid depression at baseline, after adjustment for demographic and clinical variables associated with significant risks to 1-year survival in the univariate analyses (Fig. 1). Kaplan–Meier survival curves for the curability beliefs-by-depression interaction in patients with advanced cancer (Fig. 1) were generated using the MatSurv function32 implemented in MATLAB software version R2022a (http://www.mathworks.com). Fitting of the Cox proportional hazards regression models, along with calculation of hazard ratios (HRs) and 95% confidence intervals (CIs), was performed using the coxph function included in the R package survival (https://cran.r-project.org/web/packages/survival/index.html). P-values < 0.05 were considered statistically significant.
Table 2.
Univariate Cox regression analyses: 1-year survival, sociodemographic factors, and clinical characteristics at baseline.
| Variable | Category | Crude HR (95% CI) |
P-value |
|---|---|---|---|
| Age (years) | < 65 | 1 | 0.38 |
| ≥ 65 | 0.87 (0.63–1.19) | ||
| Sex | Female | 1 | 0.047 |
| Male | 1.38 (1.00–1.91) | ||
| Monthly income (USD) | ≥ 3,000 | 1 | 0.91 |
| < 3,000 | 1.02 (0.72–1.45) | ||
| Educational achievement | ≥ High school | 1 | 0.73 |
| < High school | 1.06 (0.77–1.46) | ||
| Place of residence | Metropolitan area | 1 | 0.004 |
| Rural/Suburban | 1.60 (1.16–2.22) | ||
| Marital status | Married | 1 | 1 |
| Unmarried | 1.00 (0.69–1.45) | ||
| Religious practice | Yes | 1 | 0.13 |
| No | 0.78 (0.57–1.08) | ||
| Primary tumor site | Breast/lung/hematologic | 1 | < 0.0001* |
| Stomach/colon/liver/biliary-pancreatic | 2.31 (1.67–3.20) | ||
| Number of metastatic sites | < 3 | 1 | 0.20 |
| ≥ 3 | 1.55 (0.79–3.04) | ||
| Cancer treatment | Standard chemotherapy ongoing | 1 | < 0.0001* |
| Standard chemotherapy suspended/advanced cancer treatment (including immunotherapy and clinical trials)/all anti-cancer treatment stopped | 3.03 (2.03–4.50) | ||
| Performance status (ECOG-PS) | 0 (Fully active and can carry out all pre-disease activities without restriction)/1 (Restricted in physically strenuous activities, but remains ambulatory and able to carry out work of a light or sedentary nature)/2 (ambulatory and capable of all self-care, but unable to carry out any work activities; “up and about” > 50% of waking hours) | 1 | < 0.0001* |
| 3 (capable of only limited self-care; confined to bed or chair > 50% of waking hours)/4 (completely disabled; cannot carry out any self-care; totally confined to bed or chair) | 3.03 (2.21–4.15) | ||
| Symptom burden (EORTC QLQ-C15-PAL) | Fatigue (≥ 33.3 compared to < 33.3; median) | 1.94 (1.41–2.67) | 0.0003* |
| Appetite loss (≥ 33.3 compared to < 33.3; median) | 2.01 (1.46–2.78) | 0.0001* | |
| Dyspnea (≥ 33.3 compared to < 33.3; median) | 1.67 (1.22–2.27) | 0.0014 | |
| Insomnia (≥ 33.3 compared to < 33.3; median) | 1.54 (1.13–2.12) | 0.0095 | |
| Pain (≥ 33.3 compared to < 33.3; median) | 1.68 (1.23–2.29) | 0.0011 | |
| Depression (PHQ-9 total score) | No (0–4)/mild (5–9) | 1 | < 0.0001* |
| Moderate (10–14)/moderately severe (15–19)/severe (20–27) | 2.51 (1.83–3.43) |
*P < 0.001.
HR, hazard ratio; CI, confidence interval; GI, gastrointestinal; ECOG-PS, Eastern Cooperative Oncology Group performance status; EORTC QLQ-C15-PAL, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 Palliative scores; USD, United States dollar; PHQ-9, Patient Health Questionnaire-9.
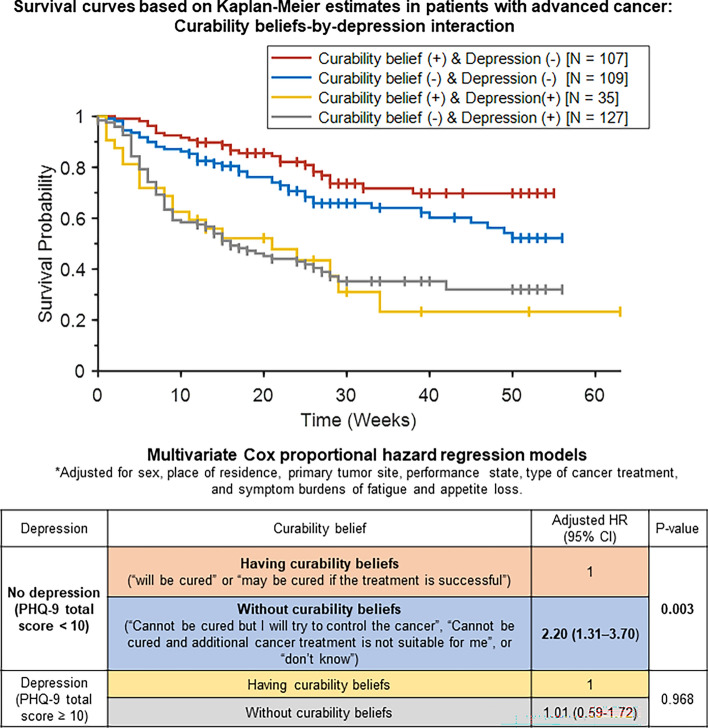
Fig. 1.
Interaction between curability beliefs and depression in survival probability. Upper panel shows survival probability plotted using Kaplan–Meier estimation. Lower panel displays the adjusted hazard ratio of not surviving 1 year, calculated through multivariate Cox proportional hazards regression. The model is adjusted for sex, place of residence, primary tumor site, performance status (ECOG-PS), cancer treatment type, and symptom burdens (EORTC QLQ-C15-PAL) of fatigue and appetite loss in patients diagnosed with advanced cancer. CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group-Performance Status; HR, hazard ratio; PHQ-9, Patient Health Questionnaire-9.
Statistical analyses: MGM
To investigate associations among key clinical variables, we employed a MGM, focusing on 1-year survival (yes/no), curability belief (with/without), patient’s life expectancy (≥ years/ ≤ months), depression (PHQ-9 score ≥ 10/ < 10), primary tumor site (gastrointestinal-hepato-biliary-pancreatic/others), ongoing advanced anti-cancer treatment (including immunotherapy and clinical trials; yes/no), discontinued anti-cancer therapy (yes/no), performance status (ECOG-PS; 0–4), and symptom burdens of fatigue and appetite loss (EORTC QLQ-C15-PAL scores) in advanced cancer patients (Fig. 2). The MGM involved ten categorical, ordinal, and continuous variables33, where each variable was represented as a “node” and edges between nodes indicated undirected conditional dependencies or regularized partial correlations34–36. Two variables (i.e., nodes) were considered independent if they were not connected when conditioned on other variables37. To address potential spurious associations, we applied the least absolute shrinkage and selection operator (LASSO) method, which adjusted edge weights, setting smaller weights to zero, thereby reducing unnecessary connections38. For the MGM network derivation, we optimized edge weights through LASSO regularization (controlled by parameter λ) using a pairwise interaction model (k = 2) and the extended Bayesian information criterion (γ = 0)36. The strength of each association was represented by the thickness of the edges, with thicker edges signifying stronger associations39. To identify key nodes in the MGM network, we calculated betweenness centrality, which indicated the proportion of shortest paths passing through a given node, highlighting its importance in the network40. Additionally, “node predictability values” (displayed as pie charts41) provided insight into how well a node’s value can be predicted by the other connected nodes, comparable to R2 in regression analysis36,41,42. The MGM network was generated using the R package mgm43 and visualized through the Fruchterman-Reingold algorithm in the qgraph package39.
Results
Sociodemographic and clinical features in patients with/without curability belief
Of the 393 eligible patients with advanced cancer, 382 participated in this study. Eight patients were excluded due to health conditions (e.g., feeling unwell) or inconvenience of the study, and three were excluded due to ECOG-PS grade 5 (death). Table 1 presents the demographics, socioeconomic status, and physical and psychological clinical features of patients with and without curability belief. Compared to those without curability beliefs (N = 239), patients with curability beliefs (N = 143) were more likely to have better physical performance (indicated by a ECOG-PS grade of 0–2), exhibit fewer cancer-related symptoms (as shown by EORTC QLQ-C15-PAL sub-scores), receive either standard chemotherapy or advanced anti-cancer treatments (e.g., immunotherapy or participation in clinical trials), report no or mild depressive symptoms (defined as PHQ-9 total score < 10), and perceive their life expectancy as similar to that of a healthy person of the same age or more than a few years (all P < 0.001). Moreover, patients with curability belief had a higher 1-year survival rate (99/143; 69.2%) than those without curability belief (119/239; 49.8%) (P = 0.01). End-of-life care location (place of death) in patients who did not survive the 1-year follow-up (N = 161) also differed: patients with curability belief more often were located in nursing hospitals or at home, whereas those without curability belief were more frequently located in hospices or tertiary/secondary hospitals (P = 0.036). Conversely, educational achievement, monthly household income, marital status, religion, primary tumor site, and number of metastatic sites were similar between these two subgroups (all P > 0.05).
Univariate Cox regression: sociodemographic and clinical features and 1-year survival
In the univariate Cox regression survival model for patients with stage IV advanced cancer (Table 2), a higher risk of not surviving the 1-year follow-up was associated with reduced performance status or inability to perform self-care [ECOG-PS grade 3–4; unadjusted hazard ratio (HR) = 3.03], anti-cancer treatment status of suspended standard chemotherapy, ongoing advanced anti-cancer treatment, or cessation of all anti-cancer treatments (HR = 3.03), comorbid depression (PHQ-9 total score ≥ 10; HR = 2.51), primary tumor site in the stomach, colon, liver, or pancreatobiliary tract (HR = 2.31), and higher cancer-related symptom burden [EORTC QLQ-C15-PAL sub-scores; appetite loss (HR = 2.01) and fatigue (HR = 1.94)] (all P < 0.001). Consequently, performance status, types of anti-cancer treatment, primary tumor site, symptom burdens of fatigue and appetite loss, as well as male sex (HR = 1.38; P = 0.047) and residential area in rural/suburban regions (HR = 1.60; P = 0.004), were included as covariates in the multivariate Cox proportional hazards regression model for 1-year survival (Fig. 1).
Multivariate Cox regression: interactions among curability belief, depression, and 1-year survival
A multivariate Cox proportional hazards regression model (Fig. 1) was used to evaluate the adjusted hazard ratio (aHR) of curability belief, depression, and their interaction for not surviving to the 1-year follow-up in patients with stage IV advanced cancer. The model was adjusted for significant sociodemographic and physical health factors identified in the univariate Cox regression models (Table 2). After adjusting for sex, place of residence, primary tumor site, performance status, cancer treatment type, and symptom burdens of fatigue and appetite loss, curability beliefs (P = 0.003), depression (P = 0.003), and their interaction (P = 0.040) showed statistically significant associations with 1-year survival in advanced cancer patients. Post hoc multivariate Cox proportional hazards regression analysis (Table within Fig. 1) demonstrated that: (1) in the subgroup without depression (N = 216), patients without curability belief had a 120% higher risk of not surviving to the 1-year follow-up [aHR (95% confidence interval (CI)) = 2.20 (1.31–3.70)] than those with curability belief; (2) in the subgroup with depression (PHQ-9 total score ≥ 10; N = 162), the risk of not surviving to the 1-year follow-up was similar between patients with and without curability belief [aHR (95% CI) = 1.01 (0.59–1.72)].
MGM
The MGM was used to estimate the regularized partial correlations among 1-year survival, curability belief, patient’s life expectancy, depression, primary tumor site, anti-cancer treatment type, performance status, and symptom burdens in advanced cancer patients. This model allowed for the identification of conditional dependencies, highlighting the relationships between variables in the network (Fig. 2).The node predictability values, indicating how well each node’s value could be inferred from its connected nodes, were 0.68, 0.50, and 0.70 for curability belief, depression, and 1-year survival, respectively. Curability Belief was associated with depression (r = 0.30), patient’s perceived life expectancy (r = 0.20), and performance status (r = 0.07). Depression was associated with symptom burdens of fatigue (r = 0.53) and anorexia (r = 0.16), curability belief, patient’s life expectancy (r = 0.24), performance status (r = 0.23), and 1-year survival (r = 0.07). One-year survival was associated with suspended or stopped anti-cancer treatment (r = 0.45), primary tumor site (r = 0.24), performance status (r = 0.15), and depression (r = 0.07). Performance status, depression, and stopped anti-cancer treatment emerged as the three nodes with the highest betweenness centrality, frequently forming shortcuts between different nodes and connecting curability belief with 1-year survival. These central nodes facilitated indirect pathways between variables, highlighting their role as intermediaries within the network (Fig. 2A, leftward graph).
Discussion
Curability belief lowers the risk of 1-year mortality by 45.5% in the absence of depression
The current study demonstrated that curability belief (“my cancer will be cured” or “may be cured if treatment is successful”) at baseline lowered the risk of 1-year mortality by 45.5% after adjustments for performance status, cancer-related fatigue and appetite loss, type of cancer treatment, primary tumor site, residential area, and sex, compared to those without curability belief (Fig. 1). Our results were in agreement with those of previous studies, which demonstrated positive effects of curability beliefs or hope on the survival of advanced cancer patients14,44. Prognostic awareness is important for the estimation of one’s life expectancy and making decisions regarding further cancer treatment45. Moreover, patient beliefs about outcomes affect their behavior, including health-related behavior and treatment adherence18–20. The current findings also support the results of other studies, which showed that comorbid depression is associated with significantly lower survival in patients with advanced cancer46. Alleviation of depressive symptoms through palliative care47 and manual-based psychotherapeutic intervention48 may reduce the negative impact of comorbid depression on survival in these patients. Intriguingly, a recent phase II trial demonstrated that intranasal racemic ketamine, administered three times over a 1-week period, reduced depressive symptoms by ≥ 50% in 70% of patients with advanced cancer and comorbid depression (n = 20)49. Therefore, clinicians should screen for depressive symptoms in patients with advanced cancer by asking, “How often have you experienced diminished interest or pleasure in doing things or felt down, depressed, or hopeless over the past 2 weeks?” or by using self-administered questionnaires. Patients who develop comorbid depressive symptoms should be provided with psychiatric consultation to ensure timely care for depressive symptoms and related functional impairment in daily life. Once the comorbid depressive symptoms improve, motivational interviewing or discussions between patients and clinicians can be used to cultivate meaning in life, regardless of its remaining duration, based on curability beliefs and values in life, among other factors.
Healthcare utilization and end-of-life metrics varied with curability belief
In this study, patients with stage IV advanced cancer who exhibited curability belief were more likely to undergo either standard chemotherapy or advanced anti-cancer therapy at baseline, whereas those without curability belief were more likely to have either suspended or stopped anti-cancer therapy (P < 0.001; Table 1). Although some studies have reported no association between curability belief and the preference for chemotherapy50, others have found a relationship between curability belief and a willingness to compromise quality of life to improve survival through chemotherapy51. The present study demonstrated regularized partial correlations of curability belief with depression and patients’ perceived life expectancy using MGM (Fig. 2), suggesting that depressive symptoms could lead to feelings of helplessness and hopelessness in advanced cancer patients52. End-of-life care location (i.e., place of death) among patients who did not survive to the 1-year follow-up (N = 161) varied; those with a curability belief more often were located in nursing hospitals or at home, whereas those without curability belief more often were located in hospices or tertiary/secondary hospitals (P = 0.036; Table 1). On the one hand, our findings align with previous studies showing a preference among advanced cancer patients without curability belief for hospice enrollment50. On the other hand, this finding suggests greater physical and emotional distress and worse quality of life among these patients53.
Associations of performance status with depression and anti-cancer treatment status mediate the relationship between curability belief and 1-year survival in patients without depression
Upon further exploration of the relationship between curability belief and 1-year survival in patients with stage IV advanced cancer using MGM, we demonstrated that regularized partial correlations of performance status with depression and discontinued anti-cancer treatment may mediate the association between curability belief and 1-year survival (Fig. 2). Importantly, physical functioning in daily life, as determined by the ECOG-PS, is a reliable predictor of survival in advanced cancer patients54,55. Even prior to receiving a cancer diagnosis, limited physical performance may be correlated with heightened depressive symptoms in cancer patients56. In addition, patients with cancer and limited physical function have a higher rate of emergency department visits and higher costs of inpatient care57.
Immunotherapy, alone or in combination with chemotherapy, can improve overall survival in patients with advanced-stage solid cancers58. However, patients with advanced cancer and poor performance status (ECOG-PS grade 3–4) may not benefit from palliative chemotherapy in terms of survival extension59. Physical activity programs tailored according to physical ability and cancer type can improve physical function60. For instance, coaching programs utilizing wearable devices for tracking physical activity and motivational interviews combined with a multidisciplinary approach enhance the physical functioning of cancer patients61. In summary, measures to improve physical functioning in daily life are a crucial component in the care of advanced cancer patients.
Limitations
This study had some limitations. First, it did not encompass all components of prognostication, such as prognostic predictions, disclosures, awareness, and patient acceptance of outcomes62. Second, we did not evaluate potential changes in prognostic awareness or curability beliefs. However, previous studies have demonstrated consistency in prognostic awareness among cancer patients over the course of the illness2,3. Third, comorbid depressive symptoms were evaluated using the self-administered PHQ-9, although the comorbid depression subgroup underwent additional clinical assessment by a psychiatrist for confirming the presence of depression.
Conclusions
To the best of our knowledge, this was the first study to examine the potential interactions of curability belief, comorbid depression, and 1-year survival in patients with stage IV advanced cancer who had an oncologist-estimated survival of > 6 months. Advanced cancer patients should be screened for depressive symptoms in the outpatient or inpatient setting. For patients exhibiting depressive symptoms, timely psychiatric consultation is required. Once comorbid depressive symptoms improve, motivational interviews or discussions between patients and clinicians can cultivate meaning in life, regardless of its remaining duration, based on curability beliefs and values in life, among other factors. Additionally, physical functioning in daily life should be improved to enable self-care. Further longitudinal trajectory modeling of depression, performance status, and healthcare utilization, with covariates of primary tumor site and curability belief, is warranted.
Acknowledgements
We thank Jihye Lee and Jiyeon Choo for their contributions to patient recruitment and data collection.
Author contributions
YHY had full access to the data used in this study and is responsible for its integrity and accuracy. JYY and JYJ contributed equally to the work, including its conceptualization, formal analysis, methodology, validation, and original draft preparation. BK, NRL, JHK, YJK, HJS, KHJ, SJK, HR, SHY, and EKK were involved in data curation. All authors have read and approved the final manuscript.
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HC15C1391), and by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (grant No. 2023R1A2C1006783). The views expressed herein do not necessarily represent those of the funders.
Data availability
Data and analytical methods used in this study are available from the corresponding author, Prof. Young Ho Yun (lawyun08@gmail.com), upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with the 1975 Declaration of Helsinki and its 2013 amendment. The study protocol was approved by the Institutional Review Board of Seoul National University College of Medicine and Hospital (approval no.: 1602-142-745). Informed consent was obtained from all participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Je-Yeon Yun and Ju Youn Jung.
References
- 1.Applebaum, A. J. et al. Conceptualizing prognostic awareness in advanced cancer: a systematic review. J. Health Psychol.19, 1103–1119. 10.1177/1359105313484782 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loučka, M. et al. Prognostic awareness in advanced cancer patients and their caregivers: A longitudinal cohort study. Psychooncology30, 1449–1456. 10.1002/pon.5704 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Agarwal, R. et al. Accuracy of curability expectations in patients with gastrointestinal cancers. Cancer Med.12, 20–29. 10.1002/cam4.4947 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C. H., Kuo, S. C. & Tang, S. T. Current status of accurate prognostic awareness in advanced/terminally ill cancer patients: Systematic review and meta-regression analysis. Palliat. Med.31, 406–418. 10.1177/0269216316663976 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa, T. et al. Prognostic awareness and discussions of incurability in patients with pretreated non-small cell lung cancer and caregivers: a prospective cohort study. Oncologist27, 982–990. 10.1093/oncolo/oyac178 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami, P. Impact of advance care planning and end-of-life conversations on patients with cancer: An integrative review of literature. J. Nurs. Scholarsh.55, 272–290. 10.1111/jnu.12804 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Nipp, R. D. et al. Coping and prognostic awareness in patients with advanced cancer. J. Clin. Oncol.35, 2551–2557. 10.1200/jco.2016.71.3404 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin, E. J. & Widera, E. Prognostication in serious illness. Med. Clin. N. Am.104, 391–403. 10.1016/j.mcna.2019.12.002 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Vlckova, K., Polakova, K., Tuckova, A., Houska, A. & Loucka, M. Association between prognostic awareness and quality of life in patients with advanced cancer. Qual. Life Res.31, 2367–2374. 10.1007/s11136-022-03097-z (2022). [DOI] [PubMed] [Google Scholar]
- 10.Madhu, S. et al. Analysis of short-term versus long-term readmission-free survival after metastatic spine tumor surgery. World Neurosurg.158, e946–e955. 10.1016/j.wneu.2021.11.119 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Taber, J. M., Stacey, C. L. & Sheehan, D. K. Understanding hospice patients’ beliefs about their life expectancy: a qualitative interview study. Am. J. Hosp. Palliat. Care38, 238–245. 10.1177/1049909120948486 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Nierop-van Baalen, C., Grypdonck, M., van Hecke, A. & Verhaeghe, S. Associated factors of hope in cancer patients during treatment: A systematic literature review. J. Adv. Nurs.76, 1520–1537. 10.1111/jan.14344 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Snyder, C. R. Hope theory: Rainbows in the mind. Psychol. Inquiry13, 249–275. 10.1207/S15327965PLI1304_01 (2002). [Google Scholar]
- 14.Corn, B. W., Feldman, D. B., Hull, J. G., O’Rourke, M. A. & Bakitas, M. A. Dispositional hope as a potential outcome parameter among patients with advanced malignancy: An analysis of the ENABLE database. Cancer128, 401–409. 10.1002/cncr.33907 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everson, S. A. et al. Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Psychosom. Med.58, 113–121. 10.1097/00006842-199603000-00003 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Ozdemir, S. et al. Advanced cancer patients’ prognostic awareness and its association with anxiety, depression and spiritual well-being: a multi-country study in Asia. Clin. Oncol. (R. Coll. Radiol.)34, 368–375. 10.1016/j.clon.2021.11.041 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Kang, E. et al. Impact of family caregivers’ awareness of the prognosis on their quality of life/depression and those of patients with advanced cancer: a prospective cohort study. Support Care Cancer29, 397–407. 10.1007/s00520-020-05489-8 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Ong, A. D., Mroczek, D. K. & Riffin, C. The health significance of positive emotions in adulthood and later life. Soc. Personal. Psychol. Compass.5, 538–551. 10.1111/j.1751-9004.2011.00370.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chew, B. H., Shariff-Ghazali, S. & Fernandez, A. Psychological aspects of diabetes care: Effecting behavioral change in patients. World J. Diabetes5, 796–808. 10.4239/wjd.v5.i6.796 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botter, L., Gerritsen, D. L. & Oude Voshaar, R. C. Schema therapy in the nursing home setting: a case study of a cognitively impaired patient. Clin. Case Stud.21, 552–570. 10.1177/15346501221091790 (2022). [Google Scholar]
- 21.Zucker, A., Tsai, C. J., Loscalzo, J., Calves, P. & Kao, J. The NEAT predictive model for survival in patients with advanced cancer. Cancer Res. Treat.50, 1433–1443. 10.4143/crt.2017.223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang, E. et al. The impacts of prognostic awareness on mood and quality of life among patients with advanced cancer. Am. J. Hosp. Palliat. Care37, 904–912. 10.1177/1049909120905789 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Oken, M. M. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol.5, 649–655 (1982). [PubMed] [Google Scholar]
- 24.Shin, D. W. et al. Cross-cultural application of the Korean version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 15-Palliative Care. J. Pain Symptom Manag.41, 478–484. 10.1016/j.jpainsymman.2010.05.009 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Yun, Y. H. et al. Validation of the Korean version of the EORTC QLQ-C30. Qual. Life Res.13, 863–868. 10.1023/b:Qure.0000021692.81214.70 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Groenvold, M. et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur. J. Cancer42, 55–64. 10.1016/j.ejca.2005.06.022 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Koyama, N. et al. The role of EORTC QLQ-C15-PAL scores and inflammatory biomarkers in predicting survival in terminally ill patients with cancer. BMC Cancer21, 304. 10.1186/s12885-021-08049-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein, A. S., Prigerson, H. G., O’Reilly, E. M. & Maciejewski, P. K. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J. Clin. Oncol.34, 2398–2403. 10.1200/jco.2015.63.6696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park, S. J., Choi, H. R., Choi, J. H., Kim, K. W. & Hong, J. P. Reliability and Validity of the Korean Version of the Patient Health Questionnaire-9 [PHQ-9]. Anxiety Mood6, 119–124 (2010). [Google Scholar]
- 30.Spitzer, R. L., Kroenke, K. & Williams, J. B. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA282, 1737–1744. 10.1001/jama.282.18.1737 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Ganz, P. A. et al. Screening for depression in younger breast cancer survivors: outcomes from use of the 9-item Patient Health Questionnaire. JNCI Cancer Spectr.10.1093/jncics/pkab017 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creed, J. H., Gerke, T. A. & Berglund, A. E. MatSurv: Survival analysis and visualization in MATLAB. J. Open Source Softw.5, 1830 (2020). [Google Scholar]
- 33.Haslbeck, J. M. B. & Waldorp, L. J. How well do network models predict observations? On the importance of predictability in network models. Behav. Res. Methods50, 853–861. 10.3758/s13428-017-0910-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu, Y. K. Commentary: Is structural equation modelling a step forward for epidemiologists?. Int. J. Epidemiol.38, 549–551. 10.1093/ije/dyn346 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Epskamp, S. & Fried, E. I. A tutorial on regularized partial correlation networks. Psychol. Methods23, 617–634. 10.1037/met0000167 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Fried, E. I. et al. Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates. Psychol. Med.50, 2682–2690. 10.1017/s0033291719002770 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Epskamp, S., Borsboom, D. & Fried, E. I. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods50, 195–212. 10.3758/s13428-017-0862-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B (Methodol.)58, 267–288 (1996). [Google Scholar]
- 39.Epskamp, S., Cramer, A. O. J., Waldorp, L. J., Schmittmann, V. D. & Borsboom, D. qgraph: network visualizations of relationships in psychometric data. J. Stat. Softw.48, 1–18. 10.18637/jss.v048.i04 (2012). [Google Scholar]
- 40.Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage52, 1059–1069. 10.1016/j.neuroimage.2009.10.003 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Zavlis, O. et al. How does the COVID-19 pandemic impact on population mental health? A network analysis of COVID influences on depression, anxiety and traumatic stress in the UK population. Psychol. Med.10.1017/s0033291721000635 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haslbeck, J. M. B. & Fried, E. I. How predictable are symptoms in psychopathological networks? A reanalysis of 18 published datasets. Psychol. Med.47, 2767–2776. 10.1017/s0033291717001258 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Haslbeck, J. M. B. & Waldorp, L. J. mgm: estimating time-varying mixed graphical models in high-dimensional data. J. Stat. Softw.93, 1–46. 10.18637/jss.v093.i08 (2020). [Google Scholar]
- 44.Solano, J. P., da Silva, A. G., Soares, I. A., Ashmawi, H. A. & Vieira, J. E. Resilience and hope during advanced disease: a pilot study with metastatic colorectal cancer patients. BMC Palliat. Care15, 70. 10.1186/s12904-016-0139-y (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkelstein, E. A. et al. Hope, bias and survival expectations of advanced cancer patients: A cross-sectional study. Psychooncology30, 780–788. 10.1002/pon.5675 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Andersen, B. L. et al. Psychological symptom trajectories and non-small cell lung cancer survival: a joint model analysis. Psychosom. Med.84, 215–223. 10.1097/psy.0000000000001027 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prescott, A. T. et al. The role of a palliative care intervention in moderating the relationship between depression and survival among individuals with advanced cancer. Health Psychol.36, 1140–1146. 10.1037/hea0000544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodin, G. et al. Managing cancer and living meaningfully (CALM): a randomized controlled trial of a psychological intervention for patients with advanced cancer. J. Clin. Oncol.36, 2422–2432. 10.1200/jco.2017.77.1097 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenblat, J. D. et al. A phase II, open-label clinical trial of intranasal ketamine for depression in patients with cancer receiving palliative care (INKeD-PC study). Cancers10.3390/cancers15020400 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mack, J. W. et al. Patient beliefs that chemotherapy may be curative and care received at the end of life among patients with metastatic lung and colorectal cancer. Cancer121, 1891–1897. 10.1002/cncr.29250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loh, K. P. et al. Willingness to bear adversity and beliefs about the curability of advanced cancer in older adults. Cancer125, 2506–2513. 10.1002/cncr.32074 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bovero, A. et al. Exploring demoralization in end-of-life cancer patients: prevalence, latent dimensions, and associations with other psychosocial variables. Palliat. Support Care17, 596–603. 10.1017/s1478951519000191 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Wright, A. A. et al. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J. Clin. Oncol.28, 4457–4464. 10.1200/jco.2009.26.3863 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sehgal, K. et al. Association of performance status with survival in patients with advanced non-small cell lung cancer treated with pembrolizumab monotherapy. JAMA Netw. Open4, e2037120. 10.1001/jamanetworkopen.2020.37120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fanotto, V. et al. Impact of age, ECOG PS and type of treatment on progression-free survival and overall survival in second-line therapy for advanced gastric cancer: analysis on 709 cases. Ann. Oncol.26, vi91. 10.1093/annonc/mdv344.06 (2015). [Google Scholar]
- 56.Miwata, K. et al. Performance status is a risk factor for depression before the diagnosis of lung cancer patients. Intern. Med.58, 915–920. 10.2169/internalmedicine.1812-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chavan, P. P., Kedia, S. K. & Yu, X. Impact of physical and functional limitations on health care utilization in older cancer survivors: a medicare current beneficiary survey. J. Aging Health32, 987–997. 10.1177/0898264319872309 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Punchhi, G., Hussein, A. & Kulkarni, S. Real-world survival outcomes of immunotherapy for advanced non-small cell lung cancer: A single-center retrospective review. Thorac. Cancer15, 394–401. 10.1111/1759-7714.15205 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasconcellos, V. F., Bonadio, R. R. C. C., Avanco, G., Negrão, M. V. & Riechelmann, R. Palliative chemotherapy for patient with advanced tumor and poor performance status: Are oncologists’ hopes of benefit justified?. Ann. Oncol.29, viii553. 10.1093/annonc/mdy295.017 (2018). [Google Scholar]
- 60.Rock, C. L. et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin.62, 243–274. 10.3322/caac.21142 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Wood, W. A. et al. Piloting HealthScore: Feasibility and acceptability of a clinically integrated health coaching program for people living with cancer. Cancer Med.12, 8804–8814. 10.1002/cam4.5625 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hui, D., Mo, L. & Paiva, C. E. The importance of prognostication: impact of prognostic predictions, disclosures, awareness, and acceptance on patient outcomes. Curr. Treat Options Oncol.22, 12. 10.1007/s11864-020-00810-3 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and analytical methods used in this study are available from the corresponding author, Prof. Young Ho Yun (lawyun08@gmail.com), upon reasonable request.