Abstract
Objective
The employment of the no-touch technique in harvesting the great saphenous vein (GSV) for coronary artery bypass grafting has been associated with a significant improvement in clinical patency rates. Despite these advantages, such grafts may predispose patients to complications in the lower limbs. This study endeavors to evaluate the incidence of complications in the lower extremities by deploying an enhanced protocol for the no-touch harvesting technique.
Methods
The historical control group in this study included patients who underwent coronary artery bypass grafting (CABG) with the no-touch technique for GSV harvesting at our institution from August 2018 to April 2020, in compliance with ethical standards. The intervention group consisted of patients who received CABG and were subjected to an optimized no-touch technique for GSV harvesting from May 2020 to June 2022. Technical modifications were applied to reduce lower limb complications, including limited use of electrocautery, minimization of extravascular tissue preservation, relaxation of postoperative elastic compression bandages, and elevation of the lower extremities. These measures aimed to decrease the incidence of postoperative lower limb complications, such as pain, numbness, edema, exudation, and delayed healing. The occurrences of postoperative complications were meticulously documented, compared, and analyzed between the two groups.
Results
The adoption of the optimized no-touch technique resulted in a significant decrease in the incidence of postoperative lower extremity incisional complications among patients subjected to off-pump CABG (p < 0.05).
Conclusion
The results of this study substantiate that the application of an optimized no-touch technique to harvest the GSV significantly diminishes the incidence of postoperative lower limb complications in patients receiving CABG. These results highlight the importance of adopting and integrating this optimized technique into clinical protocols, emphasizing its critical role in advancing patient care outcomes.
Keywords: historical controlled study, lower limb complications, no-touch great saphenous vein, off-pump coronary artery bypass grafting, propensity score matching
Introduction
Coronary artery disease (CAD) poses a substantial threat to public health, being linked with high rates of morbidity and mortality. Coronary artery bypass grafting (CABG) has been established as an effective treatment for CAD, with its application extending to over one million patients globally on an annual basis.1 Currently, the great saphenous vein (GSV) is the most commonly utilized conduit for grafting, favored for its superficial anatomical location, straightforward accessibility, and low propensity for spasm, accounting for 80% of all graft procedures.2 Nevertheless, the mid- and long-term patency rates of saphenous vein grafts (SVGs) are less than ideal. Goldman et al reported a noteworthy 10-year patency rate of 85% for the left internal mammary artery, in stark contrast to a considerably lower 61% for venous grafts.3 Introduced by Souza in 1996,4 the no-touch technique for GSV harvesting has been shown to result in enhanced patency rates, as evidenced by multiple national and international studies.5–12 Despite its benefits, the no-touch technique is associated with certain disadvantages, notably lower extremity incision complications including pain, numbness, edema, and exudation. These complications can lead to prolonged hospital stays and elevated healthcare costs for patients.
To address these challenges, we developed an optimized version of the no-touch technique, which includes measures such as minimizing the use of electrocautery and reducing the retention of extravascular tissue. Our study, adhering to ethical standards, employs a retrospective historical control design to evaluate the effectiveness of these technical advancements. The historical control group comprises patients who received CABG with the original no-touch technique for GSV harvesting between August 2018 and April 2020. In contrast, the intervention group includes individuals who underwent CABG with the optimized no-touch technique from May 2020 to June 2022. This single-center study aims to assess the incidence of lower extremity incision complications before and after the introduction of the optimized no-touch technique and to explore its effects on postoperative incision healing.
Methods
Trial Design
A single-center, retrospective, consecutive, single-blind, historically controlled study was conducted.
Inclusion Criteria: (1) Patients aged 18 years and older undergoing coronary artery bypass surgery. (2) Patients requiring the harvesting of the GSV for coronary artery grafting purposes. (3) Patients who met the clinical criteria and had been advised to undergo coronary artery bypass grafting surgery based on medical indications and decisions. (4) Those undergoing GSV harvesting surgery utilizing the no-touch technique. (5) Individuals capable of understanding the study’s requirements and consenting to participate before undergoing surgery, demonstrated by signing an informed consent form.
Exclusion Criteria: (1) A history of lower limb arterial diseases, including arterial embolism, ischemia, or significant venous varicose veins. (2) The presence of chronic lower limb ulcers or other severe skin lesions on the lower limb. (3) Severe lower limb neurological disorders, such as peripheral neuropathy or neuropathic pain syndromes. (4) Evidence of lower limb deep vein thrombosis or thromboembolic conditions. (5) A history of allergic reactions to local anesthetics or the anesthetic drugs used in surgery. (6) The presence of systemic diseases that severely affect cardiac, hepatic, or renal functions, or severe respiratory system disorders, making the patient unsuitable for surgical procedures. (7) Patients who may not be available for follow-up, including those intending to receive further treatment at other facilities or those unwilling to participate in follow-up procedures post-surgery.
The study received approval from the Hospital Ethics Committee (Approval No. 2016–827), and informed consent was obtained from all participating patients, as evidenced by their signatures on consent forms.
Study Participants
In this clinical trial, 458 consecutive patients undergoing their initial, off-pump coronary artery bypass grafting (OPCABG) were examined. These procedures took place at the Department of Cardiovascular Surgery, the Second Hospital of Hebei Medical University, from August 2018 to June 2022. In every procedure, the left internal mammary artery was utilized for grafting the anterior descending branch, and the no-touch technique was employed for harvesting the GSV as the conduit for other target vessels. The GSV, with a diameter between 1.5 and 2.5 mm, was harvested from the bilateral leg area. The historical control group consisted of 177 patients who underwent surgery from August 2018 to April 2020, while the intervention group included 281 patients operated on from May 2020 to June 2022. To facilitate the comparison of postoperative complication rates, preoperative data from the entire cohort of 458 patients were analyzed using propensity score matching (PSM) to identify comparable cases. This preoperative data collection was performed through patient history interviews and relevant preoperative diagnostic evaluations.
Surgical Strategies
The GSV was harvested using the no-touch technique, consistently applied by the same senior physicians treating all study participants. Within the historical control group, the conventional method of no-touch GSV harvesting was utilized.13 Preoperatively, vascular Doppler imaging was employed to map the GSV’s path and to identify any collateral branches. The harvesting process involved both legs. Following the administration of anesthesia, an incision was made at the ankle and extended upward along a predetermined line, employing tissue scissors to preserve the vein’s tunica adventitia and surrounding adipose tissue. The electric cautery’s coagulation settings were regulated between 20 and 30 Joules, facilitating the release of subcutaneous tissue adjacent to the vein using scissors. The retained tissue margins on either side of the vein were maintained at an approximate width of 0.5–0.8 cm, with meticulous efforts to conserve the small vasa vasorum around the vein as much as possible.
After the vein was fully liberated, collateral branches were ligated and dissected to reduce the duration of the GSV dissection. Following hemostasis at the incision site, the closure of the leg incisions was achieved through a two-layer continuous suture technique: the subcutaneous tissue was closed using 2–0 VICRYL thread, and the intradermal layer was sutured with 4–0 synthetic absorbable surgical thread. In areas with thicker tissue, a three-layer continuous suture was employed to ensure no dead space remained. The application of pressure through elastic bandages was advised for a period of 48 to 72 hours post-operation to aid in healing and reduce swelling.
In the intervention group, several modifications were applied to the conventional no-touch technique for harvesting the GSV, aimed at enhancing outcomes and reducing complications. These modifications included:
1) Limited use of the electric cautery, only employing electrocoagulation in instances of significant bleeding to minimize thermal damage.
2) Reduction in the retention of extravascular tissue while preserving the tunica adventitia of the GSV and maintaining an SVG diameter of approximately 0.4–0.6 cm, in adherence to the no-touch principle, aiming to conserve as much lower limb vascular tissue as possible (illustrated in Figures 1–3).
Figure 1.
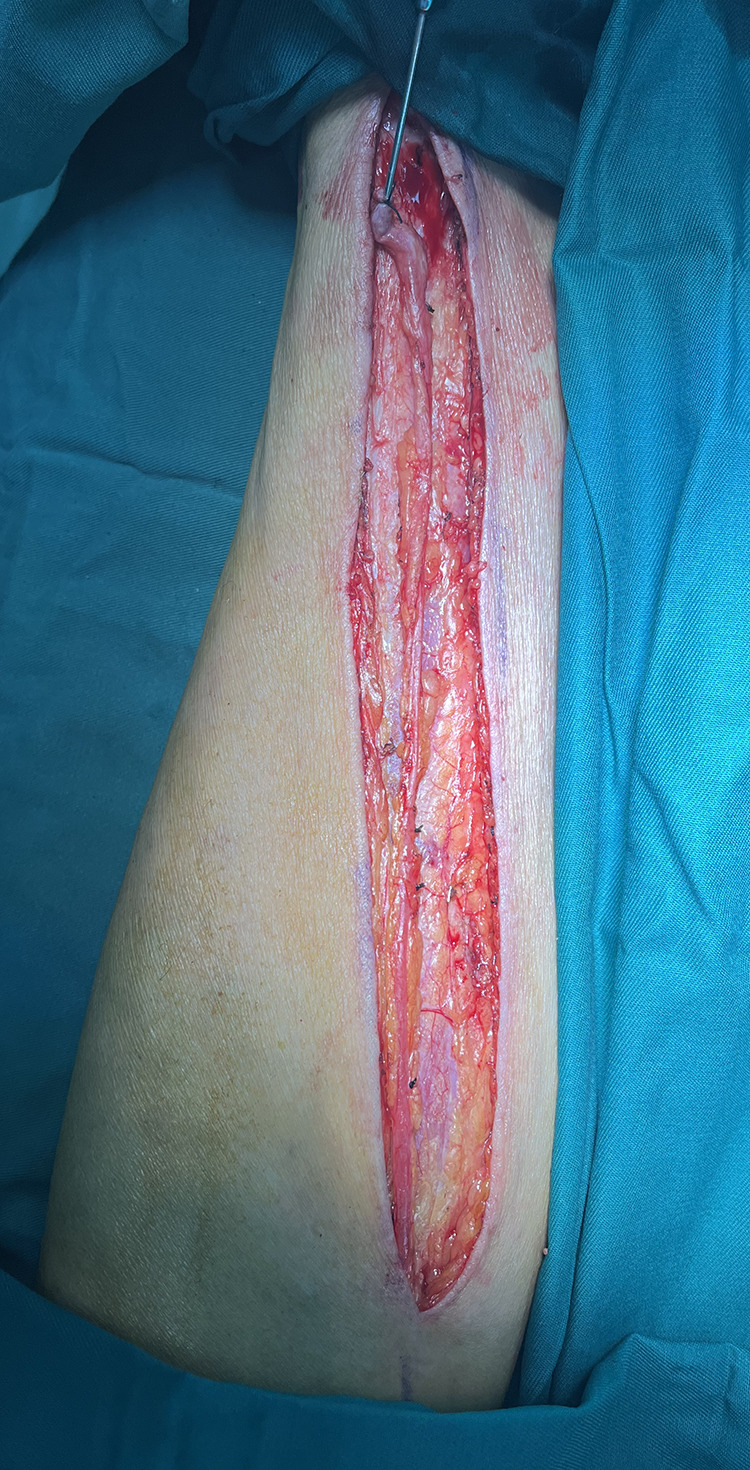
Wound after saphenous vein graft harvesting.
Figure 2.
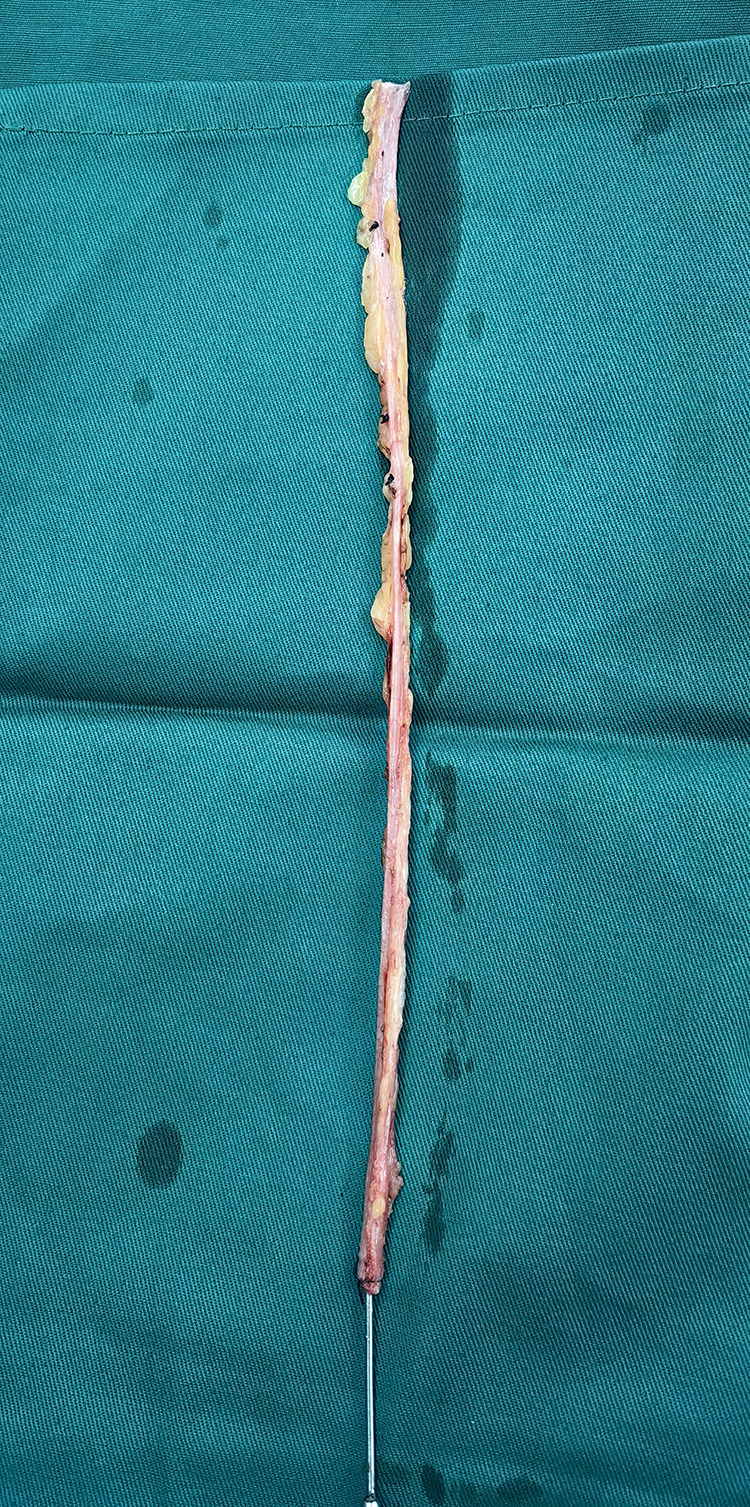
Great saphenous vein.
Figure 3.
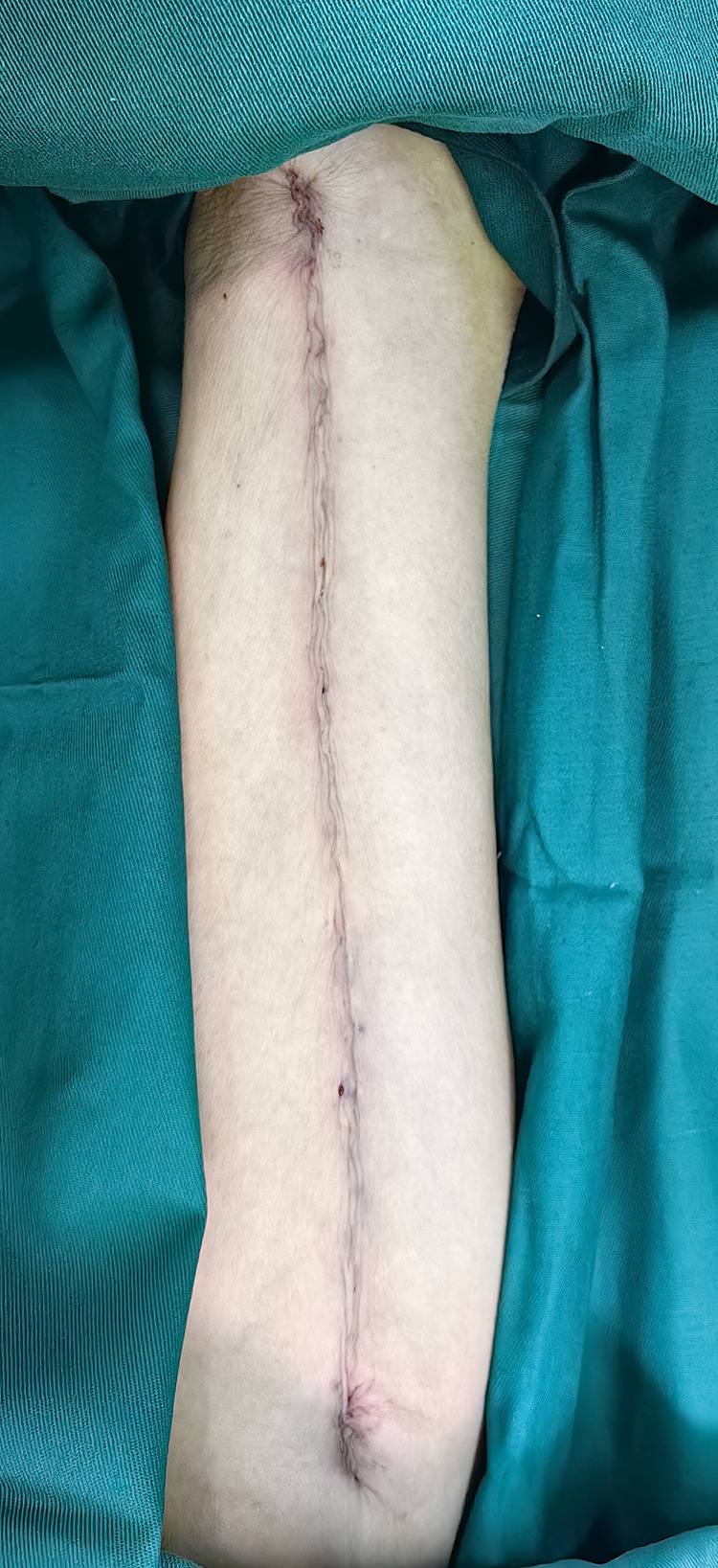
Suturing effect.
3) Careful avoidance of damage to the peripheral nerves associated with the GSV.
4) Closure of the vascular bed using a continuous 2–0 VICRYL thread following GSV harvesting, to ensure proper healing.
5) Application of a postoperative gradient compression bandage, adjusted to a tightness that allows the insertion of a finger underneath, to prevent excessive pressure.
6) Encouragement of early ambulation within one month after surgery and recommending that the lower limbs be elevated by 30 degrees in the supine position, compared to 15 degrees in the control group, to facilitate recovery.
Following the transfer of patients from the cardiac intensive care unit (ICU) to the general ward, the elastic compression bandages applied to the lower limbs were removed, and patients were positioned supine. To facilitate elevation of the lower limbs, a leg elevation cushion inclined at 30 degrees was employed until the patient’s discharge. Under the supervision of the attending nurse, the lower limbs of the patients were elevated for 3 hours in both the morning and afternoon, and for an additional 4 hours in the evening, to promote circulation and reduce swelling.14,15
Postoperative care for all participants included changing the incision dressings every three days, accompanied by ongoing monitoring of incision healing up to the point of discharge. Dual antiplatelet therapy commenced on the day following CABG surgery and was maintained for at least 12 months thereafter. The prescription of statins, antihypertensive medications, hypoglycemic agents, β-blockers, and nitrates was guided by the recommendations of the American College of Cardiology/American Heart Association, ensuring adherence to established clinical guidelines for postoperative management.
Evaluation of Clinical Outcomes
The evaluation of healing progress from the lower extremity incision was systematically documented at 5–7 days and 1 month after surgery. The assessment parameters included pain evaluation utilizing a visual analog scale. A score of 0 indicated no noticeable pain, 1–2 represented tolerable pain, and a score of 3 or higher signified a need for analgesic intervention for pain management. Additionally, the presence of numbness, specifically around the incision site and in the lower limbs, along with other sensory disturbances, was thoroughly assessed. The manifestation of edema or exudation was noted in the patients, characterized by edema observed in the lower extremities, localized edema surrounding the surgical incision, and persistent exudation from the incision site. Notably, subcrustal healing included the development of wide, dark brown, indurated crusts, indicative of epidermal necrosis at the surgical site, observed more than two weeks post-operation. Infection was defined as the failure of proper integration between the epidermis or subcutaneous tissue in a meticulously sutured wound, leading to the formation of a barrier separating adjacent tissues. This necessitated debridement and subsequent resuturing, usually following a period exceeding two weeks post-surgery.
Statistical Analysis
SPSS 26.0 software and the R 4.1.2 statistical package were used for data analysis. Initially, descriptive statistics such as mean ± standard deviation were employed to characterize measurement data, with group comparisons facilitated through ANOVA. Count data were described using frequency and percentage, and comparisons were conducted using the chi-squared test. Rank data were assessed using the Wilcoxon Rank Sum test. In the subsequent stage, variables including gender, age, BMI, hypertension, diabetes, smoking status, history of stroke, and chronic obstructive pulmonary disease (COPD) were utilized for one-to-one PSM to mitigate baseline bias between the two groups. Following PSM, statistical analyses similar to the initial stage were performed.
Results
Baseline Data
A total of 458 participants were enrolled in the study, comprising 177 individuals who underwent GSVs harvesting via the conventional no-touch technique and 281 via the optimized no-touch technique. Initial analysis revealed no significant differences between the groups regarding potential confounders, including gender, age, BMI, hypertension, diabetes, smoking, stroke, and history of COPD. However, to enhance comparability between the groups, PSM was employed. Standardized mean differences for all covariates between the groups were determined to be significantly less than 0.1 post-matching, indicating successful covariate equalization. Table 1 displays baseline characteristics both before and after matching.
Table 1.
Preoperative Patient Characteristics
| Before PS Matched | After PS Matched | |||||||
|---|---|---|---|---|---|---|---|---|
| Historical Control Group | Technical Improvement Group | P -value | SMD | Historical Control Group | Technical Improvement Group | P -value | SMD | |
| N | 177 | 281 | 143 | 143 | ||||
| Age | 62.10±7.72 | 62.98±7.56 | 0.147 | −0.114 | 62.89±7.19 | 62.80±7.90 | 0.757 | 0.012 |
| BMI | 26.17±3.02 | 25.89±2.87 | 0.329 | 0.092 | 26.13±3.11 | 25.98±2.83 | 0.707 | 0.047 |
| Female Sex | 46 (26.0) | 59 (21.0) | 0.216 | 0.114 | 32 (22.4) | 32 (22.4) | >0.999 | 0 |
| Hypertension | 117 (66.1) | 181 (64.4) | 0.712 | 0.036 | 95 (66.4) | 97 (67.8) | 0.801 | −0.03 |
| Diabetes | 59 (33.3) | 96 (34.2) | 0.855 | −0.018 | 47 (32.9) | 51 (35.7) | 0.618 | −0.059 |
| Smoking | 44 (24.9) | 77 (27.4) | 0.548 | −0.059 | 34 (23.8) | 33 (23.1) | 0.889 | 0.016 |
| Stroke | 16 (9.0) | 23 (8.2) | 0.75 | 0.03 | 13 (9.1) | 10 (7.0) | 0.514 | 0.073 |
| COPD | 8 (4.5) | 7 (2.5) | 0.235 | 0.098 | 2 (1.4) | 3 (2.1) | >0.999 | −0.034 |
Abbreviations: PS, propensity score; BMI, Body Mass Index; COPD, chronic obstructive pulmonary disease.
Clinical Outcomes
Complications related to the lower extremity incision were evaluated at two time points: days 5–7 and 1 month following the surgery. Pain scores and the incidence of numbness, edema, exudation, subcrustal healing, infection, or dehiscence were compared pre- and post-matching process, The results showed that the clinical effect of the technical improvement group was better than that of the historical control group. (Table 2).
Table 2.
Complications of Lower Limb Incision Before and After PS Matching
| Observations | Before PS Matched | After PS Matched | ||||
|---|---|---|---|---|---|---|
| Historical Control Group (n=177) | Technical Improvement Group (n=281) | P -value | Historical Control Group (n=143) | Technical Improvement Group (n=143) | P -value | |
| Pain (score) | 0 (1.0) | 0 (0) | P<0.001 | 0 (1.0) | 0 (0) | P<0.001 |
| No pain | 113 | 233 | P<0.001 | 90 | 123 | P<0.001 |
| Mild pain | 49 | 42 | 42 | 18 | ||
| Moderate or higher pain | 15 | 6 | 11 | 2 | ||
| Skin numbness | 63 (35.6%) | 22 (7.8%) | P<0.001 | 52 (36.4%) | 11 (7.7%) | P<0.001 |
| Persistent exudation | 21 (11.9%) | 38 (13.5%) | 0.606 | 14 (9.8%) | 21 (14.7%) | 0.207 |
| Edema | 42 (23.7%) | 44 (15.7%) | 0.031 | 28 (19.6%) | 23 (16.1%) | 0.440 |
| Subeschar healing | 9 (5.1%) | 1 (0.4%) | 0.001 | 7 (4.9%) | 1 (0.7%) | 0.066 |
| Infection or rupture | 7 (4.0%) | 2 (0.7%) | 0.031 | 3 (2.1%) | 1 (0.7%) | 0.622 |
Abbreviation: PS, propensity score.
Discussion
In this study, PSM was employed to assess the incidence of complications in lower extremity incisions among patients undergoing OPCABG before and after the implementation of the optimized no-touch technique. Our findings affirm that the adoption of the optimized no-touch technique confers notable advantages in reducing both incisional pain and skin numbness.
Currently, SVGs are predominantly harvested via an open incision during CABG surgery. The conventional approach to obtaining GSV bridging vessels involves the excision of perivenous tissues and the application of pressurized dilatation to the venous lumen to alleviate vasospasm. However, this procedure incurs endothelial damage attributable to mechanical trauma during surgical manipulation and dilatation maneuvers. Endothelial injury constitutes a principal determinant of platelet aggregation and thrombotic events. Additionally, endothelial damage and the removal of the tunica adventitia layer precipitate diminished levels of vascular nitric oxide, thereby predisposing the patient to vein spasms.16 The application of manual compression and dilation exacerbates spasm, resulting in further damage to the tunica intima and tunica media layers.17,18 Consequently, this sequence of events initiates the activation, migration, and proliferation of vascular smooth muscle cells, culminating in the formation of new venous endothelium.19,20
Souza et al elucidated that the no-touch technique involves several key practices, including the avoidance of direct contact with the vascular wall, abstention from instrumental manipulation of the vein, and prevention of venous spasm by preserving the tunica adventitia and surrounding tissues.21 This technique circumvents the use of pressurized dilatation to mitigate venous spasm, thereby safeguarding the structural integrity of the venous endothelial layer and the smooth muscle layer of the tunica media against disruption, as corroborated by electron microscopic observation.22 Research suggests that perivascular tissues possess the ability to secrete numerous vasodilatory factors, including adipose-derived relaxing factor, nitric oxide, leptin, adiponectin, prostaglandins, and hydrogen sulfide. In addition to their vasodilatory properties, these paracrine factors can impact the structure of the vessel wall by influencing cell migration, proliferation, and apoptosis.23 Moreover, intact perivascular tissue may serve as a natural external scaffold, potentially mitigating the risk of angulation and tortuosity in graft-bridged vessels.
The no-touch technique offers enhanced protection to venous vessels compared to the conventional method. This is accomplished by gently harvesting the GSV in a tapered manner. In contrast to the conventional vein harvesting method utilized in CABG, no-touch techniques have substantially diminished the risk of vein graft occlusion, ultimately improving patient outcomes.10 Theoretically, this approach may lead to increased trauma to the lower extremity, sparking controversy regarding its impact on the postoperative healing of lower extremity incisions.12 Souza et al were the pioneers in investigating the no-touch technique, with their initial study revealing a postoperative incisional complication incidence of 11.1%, albeit with a limited sample size of 45 participants.24 Similarly, the Chinese Heart Center has documented varied incidences of poor incision healing, ranging from 4.3% to 15.4%.13,25–27 Within the historical control group, comprising 177 patients, the incidence of infection or dehiscence stood at 9.1%. Among these cases, 5.1% experienced healing beneath the scab, with some electing for surgical intervention, while those not requiring surgery underwent a minimum of 2 months of incision recovery. Another study revealed a heightened incidence of postoperative vein site infections one month following surgery in patients utilizing the no-touch technique (23.3% vs 9.5%, p < 0.01).11 Moreover, 4.0% of patients developed wound infection or dehiscence, necessitating surgical debridement and suturing.
Previous studies have identified several risk factors that impact the healing of lower extremity incisions.10,28,29 These factors include female gender, hypertension, diabetes, obesity, peripheral vascular disease, excessive use of a high-frequency electric knife, and perioperative application of an intra-aortic balloon pump.
Considering the mentioned risk factors and clinical practices, we hypothesize that the no-touch technique may have several potential impacts on exacerbating poor healing of lower limb incisions:
1) Preservation of the tunica adventitia of the graft vein and its surrounding tissues by the no-touch technique may lead to the absence or damage of adipose tissue in the lower limb, affecting cutaneous nerves, capillaries, and lymphatics. This not only influences tissue blood supply but also increases incisional tension, thereby impacting the healing process.
2) Relying on electrocautery to free blood vessels and minimize bleeding from bridging vessels in the no-touch technique may result in excessive tissue damage. To mitigate this, technical improvements were proposed, including reducing the retention of adipose tissue outside the GSV while preserving its tunica adventitia to minimize incision depth. Additionally, efforts were made to minimize irritation and damage to the saphenous nerve surrounding the GSV.
3) In our center, the previous no-touch technique utilized a low-frequency electrocautery device, which has been identified as a risk factor for fat liquefaction. In response, measures were implemented to reduce electrocautery use unless significant bleeding occurred, thereby eliminating electro-coagulation when unnecessary.
4) Suturing technique is considered pivotal. Therefore, we employed absorbable sutures for continuous suturing of the vascular bed with a spacing of 5–8 mm. Attention was focused on eliminating dead space, and intradermal continuous suturing of the epidermis and dermis skin was performed with 4–0 absorbable sutures.
5) The lower limb incision was bandaged with gradient pressure using an elastic bandage for 48–72 hours, ensuring appropriate tightness. After this period, the bandage and gauze were removed, and the area was sterilized using iodophor every 2 days if no significant exudation was observed. Additionally, lower limbs were elevated 30 degrees while the patients were lying in bed to alleviate lower limb edema. The study results indicate that these enhancements significantly contribute to reducing incisional pain and skin numbness.
Study Limitations
The inherent limitations of this study primarily stem from its retrospective design. Firstly, incomplete data collection is a frequent issue in retrospective studies, potentially affecting the comprehensive assessment of postoperative complications. Critical clinical data might be missed or incompletely recorded during the review process. Secondly, despite employing various statistical methods to mitigate selection bias, retrospective studies remain susceptible to recall and reporting biases, which can compromise the accuracy of the research findings. In addition, because retrospective studies cannot strictly control all variables like prospective randomized controlled trials, there may be unmeasured confounding factors, and the influence of these factors on the research results is difficult to completely eliminate. Although the sample size of this study is relatively large, for some rare complications, the sample size is still insufficient, which may affect the universality and statistical power of the conclusion. Finally, due to the lack of long-term follow-up data, this study mainly focused on the incidence of complications in the short term after surgery and could not comprehensively evaluate the impact of the improved technique on the long-term postoperative effect. Therefore, although this study provides some preliminary evidence for the improved no-touch technique, its conclusions still need to be further verified and improved in future prospective studies.
Conclusion
In this retrospective study, we analyzed data obtained from patients who underwent CABG and underwent saphenous vein harvesting using the no-touch technique at our institution from August 2018 to June 2022. During this timeframe, we introduced technical enhancements, notably reducing the use of electrocautery, minimizing excessive retention of extravascular tissue, and adjusting the tightness of postoperative compression bandages, beginning in May 2020. We compared outcomes following these enhancements with those observed prior to May 2020. PSM analysis revealed a significant reduction in the occurrence of postoperative complications associated with lower extremity incisions, such as incisional pain and skin numbness, among patients undergoing OPCABG. These findings tentatively suggest that the adoption of these strategies is beneficial and considered to be useful in clinical practice. However, all possible confounding factors were not considered in this study, so future prospective studies should consider more comprehensive factors to verify the current results.
Funding Statement
1. Hebei Provincial Department of Finance Government Funding for Public hospital reform and high-quality development demonstration project (No.36); 2. Hebei Provincial Department of Finance Government Funding for Specialty Capacity Building and Specialty Leader Train (No. 303-2022-27-07).
Data Sharing Statement
Date will be made available on request from the corresponding author (Zi-Ying Chen) on reasonable request.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by Ethics Committee of the Second Hospital of Hebei Medical University (No. 2016-827). Written informed consent was obtained from all participants.
Disclosure
The authors declare no conflict of interest.
References
- 1.Taggart DP. How I deploy arterial grafts. Ann Cardiothorac Surg. 2018;7(5):690–697. doi: 10.21037/acs.2018.09.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klima U, Elsebay AA, Gantri MR, Bangardt J, Miller G, Emery RW. Computerized tomographic angiography in patients having eSVS Mesh(R) supported coronary saphenous vein grafts: intermediate term results. J Cardiothorac Surg. 2014;9(1):138. doi: 10.1186/1749-8090-9-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a department of veterans affairs cooperative study. J Am Coll Cardiol. 2004;44(11):2149–2156. doi: 10.1016/j.jacc.2004.08.064 [DOI] [PubMed] [Google Scholar]
- 4.Souza D. A new no-touch preparation technique. Technical notes. Scand J Thorac Cardiovasc Surg. 1996;30(1):41–44. doi: 10.3109/14017439609107239 [DOI] [PubMed] [Google Scholar]
- 5.Samano N, Geijer H, Liden M, Fremes S, Bodin L, Souza D. The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: a randomized trial. J Thorac Cardiovasc Surg. 2015;150(4):880–888. doi: 10.1016/j.jtcvs.2015.07.027 [DOI] [PubMed] [Google Scholar]
- 6.Kim YH, Oh HC, Choi JW, Hwang HY, Kim KB. No-touch saphenous vein harvesting may improve further the patency of saphenous vein composite grafts: early outcomes and 1-year angiographic results. Ann Thorac Surg. 2017;103(5):1489–1497. doi: 10.1016/j.athoracsur.2016.09.024 [DOI] [PubMed] [Google Scholar]
- 7.Dreifaldt M, Mannion JD, Bodin L, Olsson H, Zagozdzon L, Souza D. The no-touch saphenous vein as the preferred second conduit for coronary artery bypass grafting. Ann Thorac Surg. 2013;96(1):105–111. doi: 10.1016/j.athoracsur.2013.01.102 [DOI] [PubMed] [Google Scholar]
- 8.Pettersen Ø, Haram PM, Winnerkvist A, et al. Pedicled vein grafts in coronary surgery: perioperative data from a randomized trial. Ann Thorac Surg. 2017;104(4):1313–1317. doi: 10.1016/j.athoracsur.2017.03.076 [DOI] [PubMed] [Google Scholar]
- 9.Zhao TY, Bu JQ, Gu JJ, Liu Y, Zhang WL, Chen ZY. The short-term patency rate of a saphenous vein bridge using the no-touch technique in off-pump coronary artery bypass grafting in vein harvesting. Int J Gen Med. 2021;14:2281–2288. doi: 10.2147/IJGM.S311249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian M, Wang X, Sun H, et al. No-touch versus conventional vein harvesting techniques at 12 months after coronary artery bypass grafting surgery: multicenter randomized, controlled trial. Circulation. 2021;144(14):1120–1129. doi: 10.1161/CIRCULATIONAHA.121.055525 [DOI] [PubMed] [Google Scholar]
- 11.Deb S, Singh SK, de Souza D, et al. SUPERIOR SVG: no touch saphenous harvesting to improve patency following coronary bypass grafting (a multi-Centre randomized control trial, NCT01047449). J Cardiothorac Surg. 2019;14(1):85. doi: 10.1186/s13019-019-0887-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedetto U, Angelini GD. Saphenous vein graft harvesting and patency: still an unanswered question. J Thorac Cardiovasc Surg. 2016;152(5):1462–1463. doi: 10.1016/j.jtcvs.2016.07.045 [DOI] [PubMed] [Google Scholar]
- 13.Yu L. Clinical observation of lower limb complications after obtaining vein graft by No-Touch technique. J Hebei Med Univ. 2020;41(05):528–531. [Google Scholar]
- 14.Aihua W, Xijing Z, Yang GAO. Observation and nursing care of postoperative complications in elderly patients with great saphenous vein after coronary artery bypass transplantation[J]. Chinese J Pract Nursing. 2016;32(4):268–270. doi: 10.3760/cma.j.issn.1672-7088.2016.04.008 [DOI] [Google Scholar]
- 15.Juan H. Study on different elevation angles of lower limbs during leg vein graft harvesting for coronary artery bypass grafting [J]. J Nurs Sci. 2022;37(14):23–26+52. [Google Scholar]
- 16.Parang P, Arora R. Coronary vein graft disease: pathogenesis and prevention. Can J Cardiol. 2009;25(2):e57–e62. doi: 10.1016/s0828-282x(09)70486-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza DS, Arbeus M, Botelho Pinheiro B, Filbey D. The no-touch technique of harvesting the saphenous vein for coronary artery bypass grafting surgery. Multimed Man Cardiothorac Surg. 2009;2009(731):mmcts.2008. doi: 10.1510/mmcts.2008.003624 [DOI] [PubMed] [Google Scholar]
- 18.Angelini GD, Passani SL, Breckenridge IM, Newby AC. Nature and pressure dependence of damage induced by distension of human saphenous vein coronary artery bypass grafts. Cardiovasc Res. 1987;21(12):902–907. doi: 10.1093/cvr/21.12.902 [DOI] [PubMed] [Google Scholar]
- 19.Khaleel MS, Dorheim TA, Duryee MJ, et al. High-pressure distention of the saphenous vein during preparation results in increased markers of inflammation: a potential mechanism for graft failure. Ann Thorac Surg. 2012;93(2):552–558. doi: 10.1016/j.athoracsur.2011.10.035 [DOI] [PubMed] [Google Scholar]
- 20.Nolte A, Secker S, Walker T, et al. Veins are no arteries: even moderate arterial pressure induces significant adhesion molecule expression of vein grafts in an ex vivo circulation model. J Cardiovasc Surg. 2011;52(2):251–259. [PubMed] [Google Scholar]
- 21.Samano N, Souza D, Dashwood MR. Saphenous veins in coronary artery bypass grafting need external support. Asian Cardiovasc Thorac Ann. 2021;29(5):457–467. doi: 10.1177/0218492320980936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Alfonso MS, Gil-Ortega M, Aranguez I, et al. Role of PVAT in coronary atherosclerosis and vein graft patency: friend or foe? Br J Pharmacol. 2017;174(20):3561–3572. doi: 10.1111/bph.13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonsen U, Boedtkjer E. New roles of factors from perivascular tissue in regulation of vascular tone. Acta Physiol. 2016;216(2):159–162. doi: 10.1111/apha.12620 [DOI] [PubMed] [Google Scholar]
- 24.Souza DS, Dashwood MR, Tsui JC, et al. Improved patency in vein grafts harvested with surrounding tissue: results of a randomized study using three harvesting techniques. Ann Thorac Surg. 2002;73(4):1189–1195. doi: 10.1016/s0003-4975(02)03425-2 [DOI] [PubMed] [Google Scholar]
- 25.Jinyuan L, Dong C, Yawei D, et al. Clinical effect analysis of non-contact great saphenous vein harvesting technique in coronary artery bypass grafting. J Clin Surg. 2023;31(04):340–343. [Google Scholar]
- 26.Haisu X, Lixiang H, Zhi L, et al. Comparison of the perioperative period between the ”no contact” method and the conventional method in the harvesting of great saphenous vein in coronary artery bypass grafting. J Nanjing Med Univers. 2022;42(11):1583–1587. [Google Scholar]
- 27.Hui A, Liu S, Ziying C, et al. Application of No-touch harvesting of great saphenous vein grafts in CABG and observation of short-term and mid-term graft patency rate. J Hebei Med Univ. 2020;41(01):24–28. [Google Scholar]
- 28.Allen KB, Heimansohn DA, Robison RJ, et al. Risk factors for leg wound complications following endoscopic versus traditional saphenous vein harvesting. Heart Surg Forum. 2000;3(4):325–330. [PubMed] [Google Scholar]
- 29.Paletta CE, Huang DB, Fiore AC, Swartz MT, Rilloraza FL, Gardner JE. Major leg wound complications after saphenous vein harvest for coronary revascularization. Ann Thorac Surg. 2000;70(2):492–497. doi: 10.1016/s0003-4975(00)01414-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Date will be made available on request from the corresponding author (Zi-Ying Chen) on reasonable request.


