Abstract
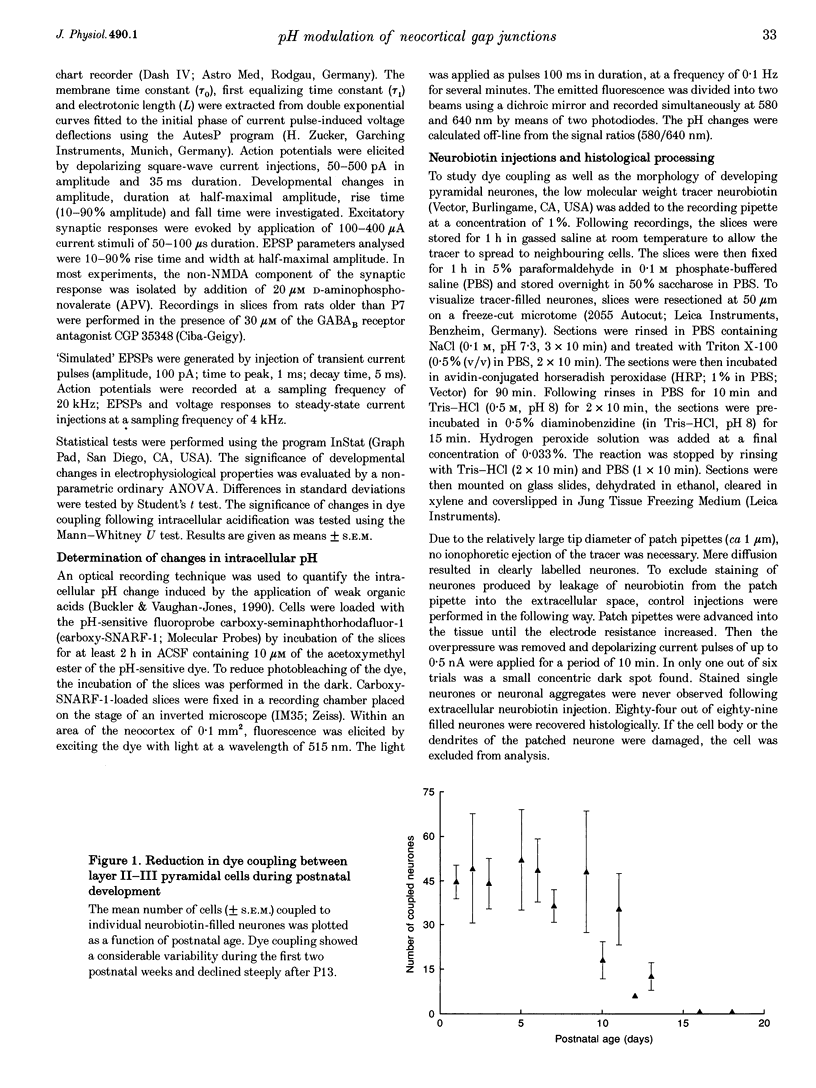
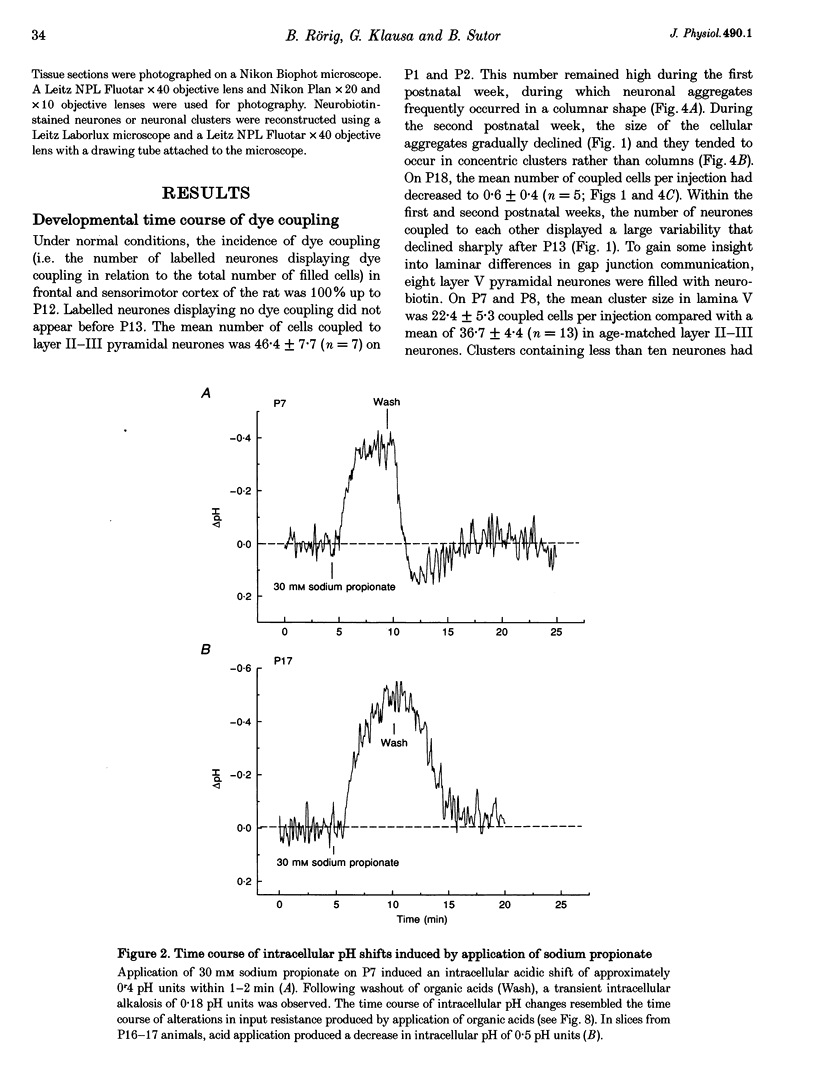
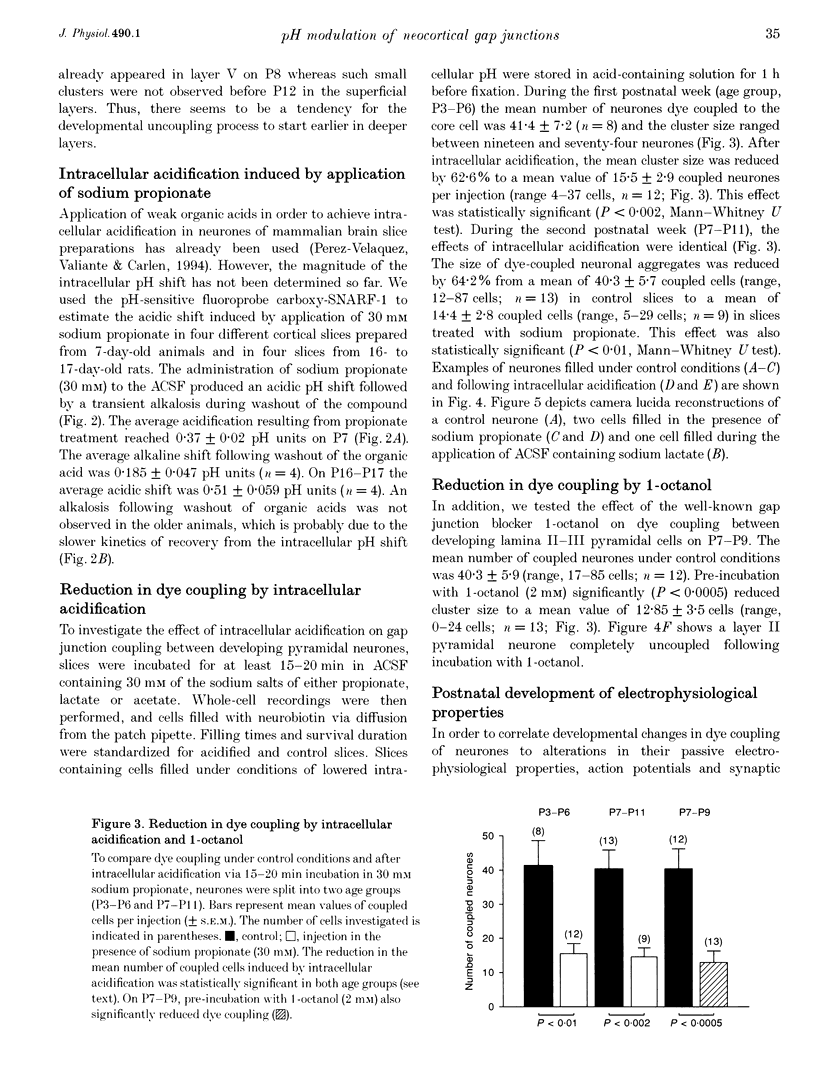
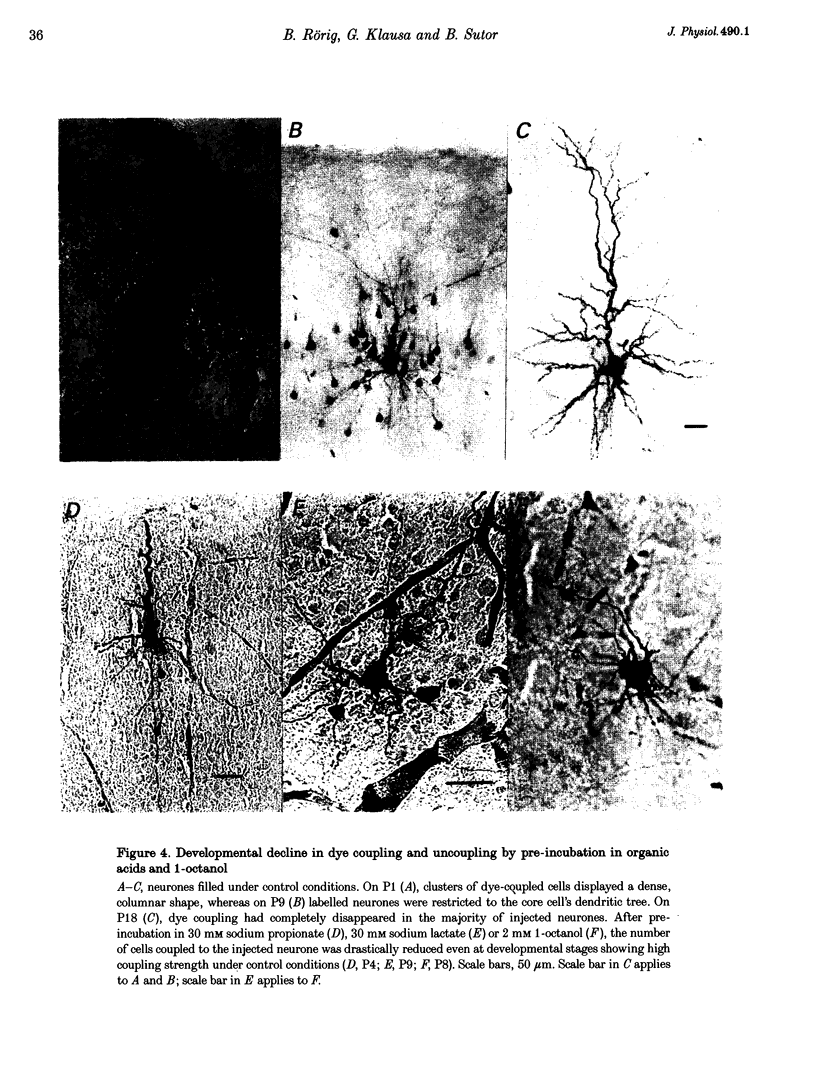
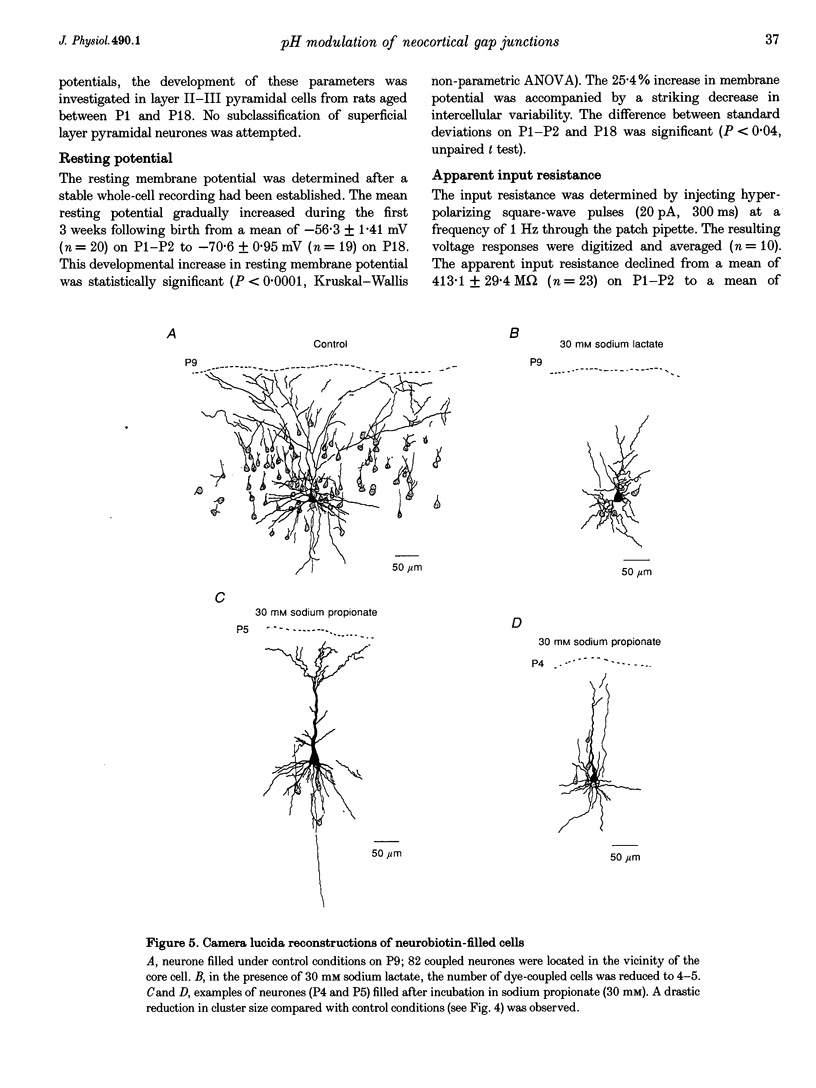
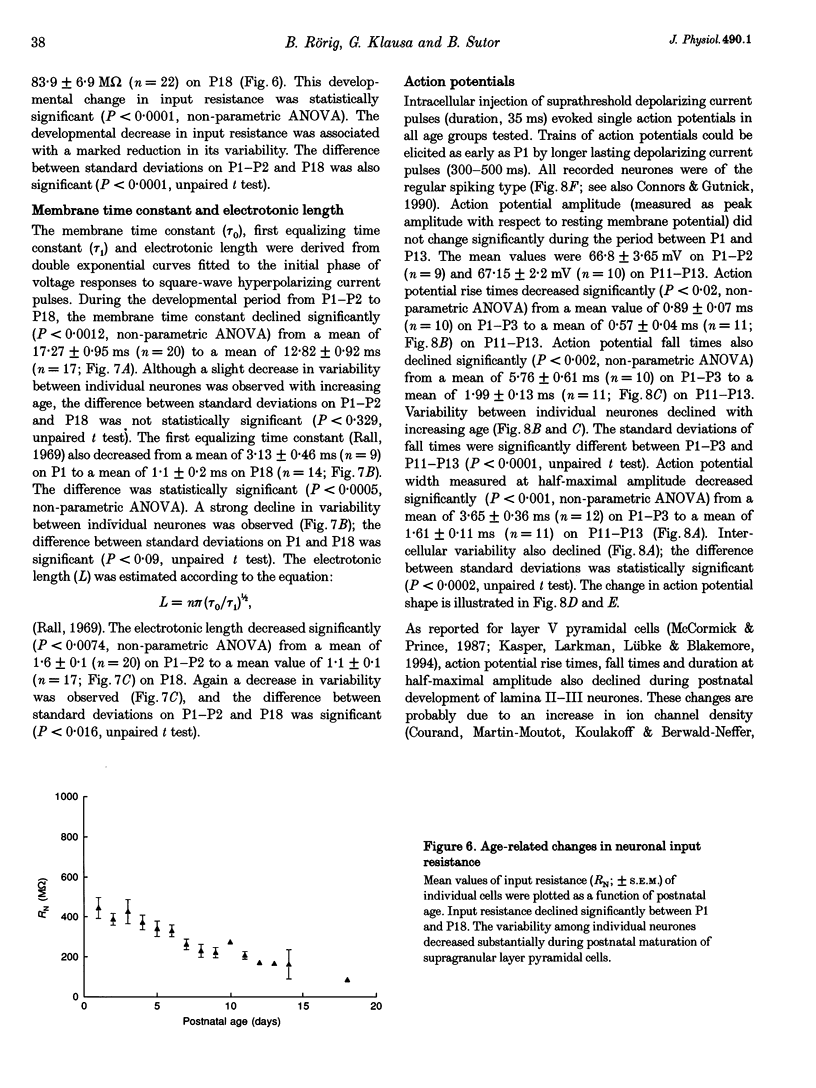
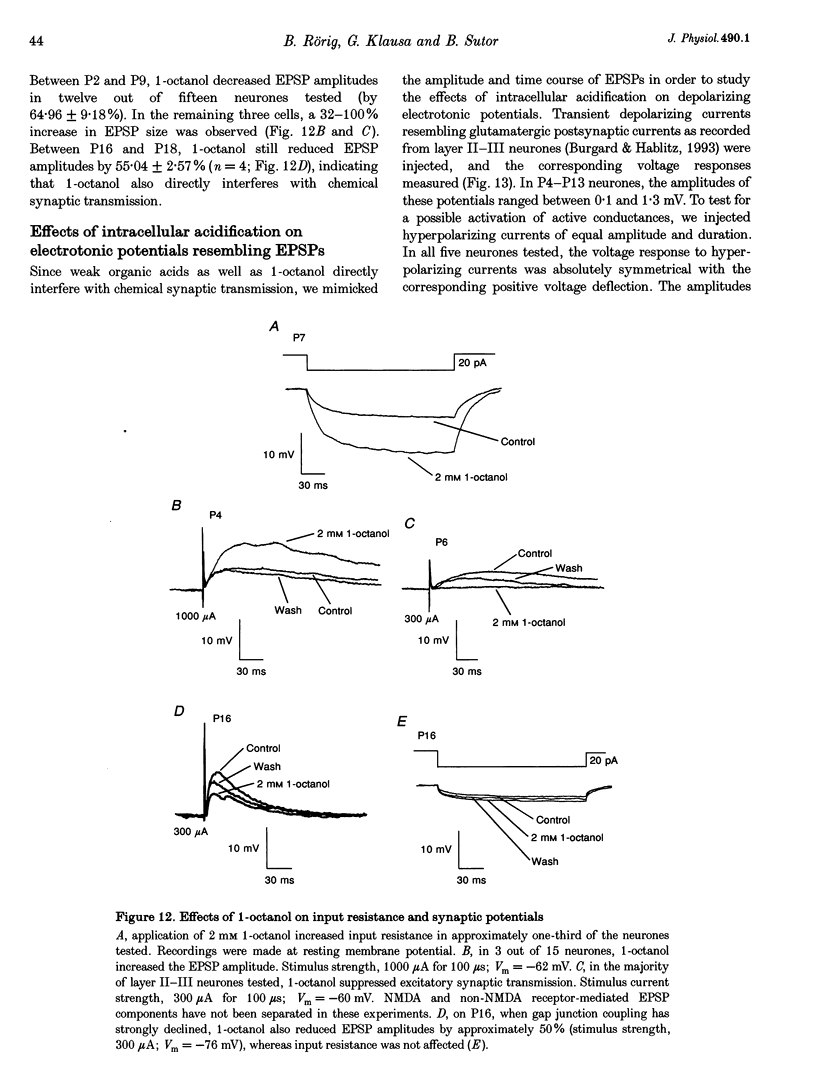
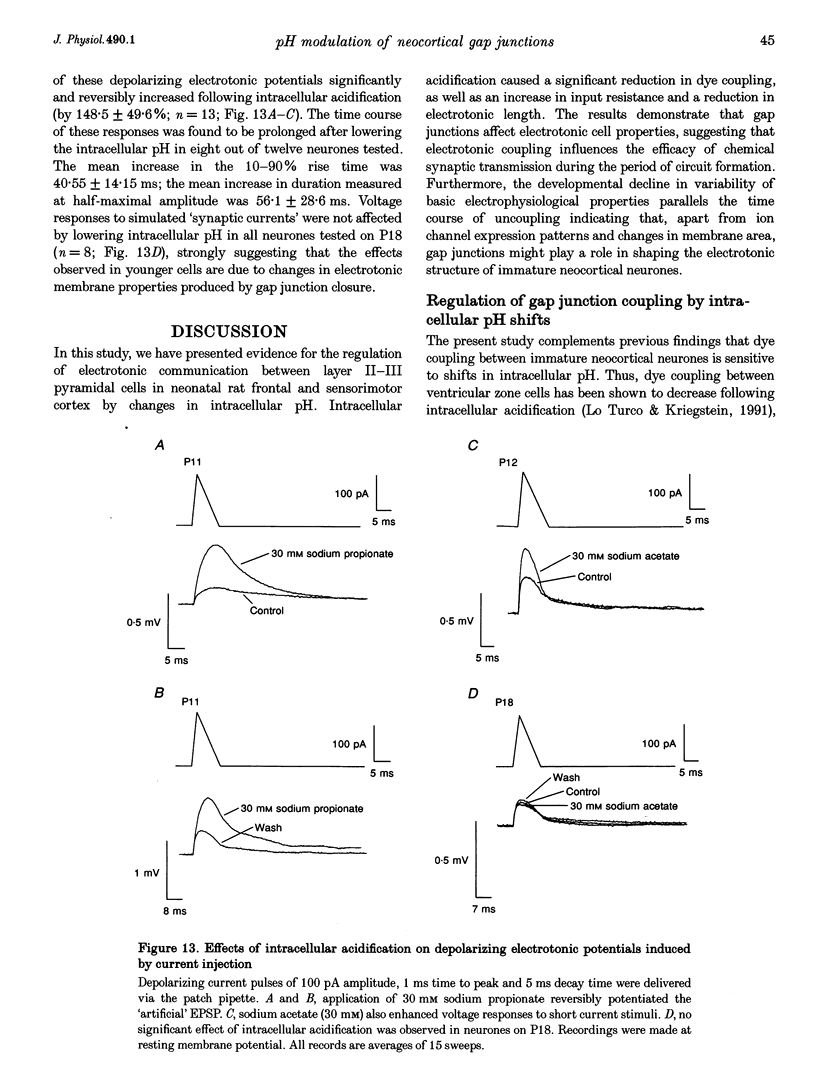
1. Developmental changes in electrophysiological properties of pyramidal neurones correlated with the developmental decline in gap junction-dependent dye coupling were investigated in coronal slices of rat prefrontal and sensorimotor cortex. Effects of intracellular acidification induced by application of weak organic acids on neuronal dye coupling, electrotonic parameters as well as synaptic potentials were examined using the patch clamp technique. Optical monitoring of intracellular pH revealed an acidic shift of 0.4-0.5 pH units following sodium propionate application. 2. Dye coupling between layer II-III neurones was prominent during the first two postnatal weeks. During this period, pre-incubation of slices with 30 mM of the sodium salts of weak organic acids reduced the number of cells coupled to the injected neurones by 64%. 3. Between postnatal days 1 and 18, the mean neuronal input resistance decreased significantly (by 81.0%). Both the membrane time constant (tau 0) and the first equalizing time constant (tau 1) also showed a significant developmental decline of 25.8 and 65.8%, respectively. Electrotonic length decreased by 34.9%. The electrophysiological properties of neurones displayed a pronounced intercellular variability which decreased with on-going development. 4. During the first two postnatal weeks, intracellular acidification led to a mean increase in neuronal input resistance of 55.9% and a mean decreae in electrotonic length of 22.2%. The membrane time constant was reduced by approximately 25% in the majority of neurones tested. Significant electrophysiological effects induced by intracellular acidification were not detected in uncoupled neurones from 18-day-old rats. 5. EPSP width at half-maximal amplitude showed a substantial reduction of approximately 50%, while rise times of the non-NMDA receptor-mediated EPSP components displayed no significant change during development. Both weak organic acids, as well as the gap junction blocker 1-octanol, reduced excitatory synaptic transmission independent of developmental age. 6. We conclude that gap junction permeability is regulated by intracellular pH in developing layer II-III pyramidal cells in the rat neocortex. The prominent correlation between pH-induced reduction in dye coupling and changes in electrophysiological cell properties suggests a significant influence of gap junctions on synaptic integration and information transfer in the immature neocortex.
Full text
PDF

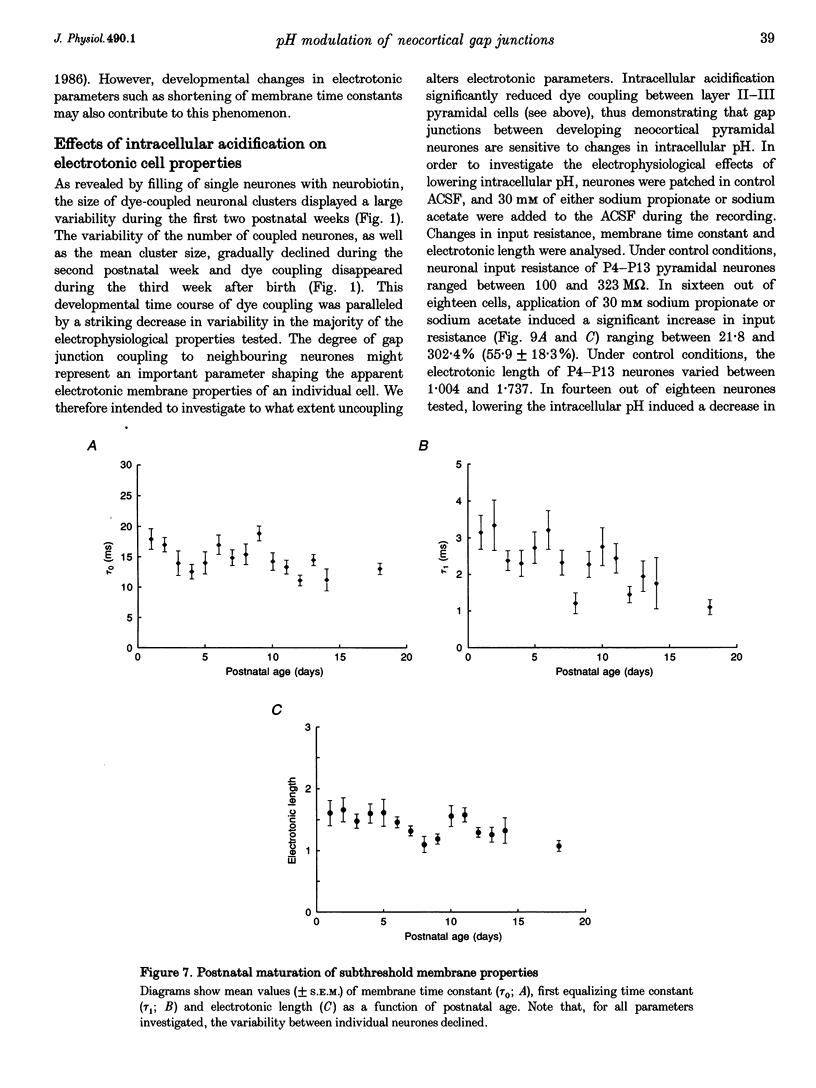




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. C. Gap junctions. Int Rev Cytol. 1993;137C:1–37. [PubMed] [Google Scholar]
- Bigiani A., Roper S. D. Reduction of electrical coupling between Necturus taste receptor cells, a possible role in acid taste. Neurosci Lett. 1994 Aug 1;176(2):212–216. doi: 10.1016/0304-3940(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990 Oct;417(2):234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Burgard E. C., Hablitz J. J. Developmental changes in NMDA and non-NMDA receptor-mediated synaptic potentials in rat neocortex. J Neurophysiol. 1993 Jan;69(1):230–240. doi: 10.1152/jn.1993.69.1.230. [DOI] [PubMed] [Google Scholar]
- Burt J. M., Spray D. C. Single-channel events and gating behavior of the cardiac gap junction channel. Proc Natl Acad Sci U S A. 1988 May;85(10):3431–3434. doi: 10.1073/pnas.85.10.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C., Walsh J. P., Peacock W., Buchwald N. A., Levine M. S. Dye-coupling in human neocortical tissue resected from children with intractable epilepsy. Cereb Cortex. 1993 Mar-Apr;3(2):95–107. doi: 10.1093/cercor/3.2.95. [DOI] [PubMed] [Google Scholar]
- Chesler M., Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992 Oct;15(10):396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Benardo L. S., Prince D. A. Carbon dioxide sensitivity of dye coupling among glia and neurons of the neocortex. J Neurosci. 1984 May;4(5):1324–1330. doi: 10.1523/JNEUROSCI.04-05-01324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Benardo L. S., Prince D. A. Coupling between neurons of the developing rat neocortex. J Neurosci. 1983 Apr;3(4):773–782. doi: 10.1523/JNEUROSCI.03-04-00773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990 Mar;13(3):99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J. Spontaneous ictal-like discharges and sustained potential shifts in the developing rat neocortex. J Neurophysiol. 1987 Nov;58(5):1052–1065. doi: 10.1152/jn.1987.58.5.1052. [DOI] [PubMed] [Google Scholar]
- Johnston M. F., Simon S. A., Ramón F. Interaction of anaesthetics with electrical synapses. Nature. 1980 Jul 31;286(5772):498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- Kasper E. M., Larkman A. U., Lübke J., Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. II. Development of electrophysiological properties. J Comp Neurol. 1994 Jan 22;339(4):475–494. doi: 10.1002/cne.903390403. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Liu S., Taffet S., Stoner L., Delmar M., Vallano M. L., Jalife J. A structural basis for the unequal sensitivity of the major cardiac and liver gap junctions to intracellular acidification: the carboxyl tail length. Biophys J. 1993 May;64(5):1422–1433. doi: 10.1016/S0006-3495(93)81508-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Turco J. J., Kriegstein A. R. Clusters of coupled neuroblasts in embryonic neocortex. Science. 1991 Apr 26;252(5005):563–566. doi: 10.1126/science.1850552. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Dudek F. E. Electrotonic coupling between pyramidal cells: a direct demonstration in rat hippocampal slices. Science. 1981 Aug 14;213(4509):782–785. doi: 10.1126/science.6266013. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Jahnsen H. Uncoupling of CA3 pyramidal neurons by propionate. Brain Res. 1985 Mar 18;330(1):141–145. doi: 10.1016/0006-8993(85)90015-0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J Physiol. 1987 Dec;393:743–762. doi: 10.1113/jphysiol.1987.sp016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienville J. M., Lange G. D., Barker J. L. Reciprocal expression of cell-cell coupling and voltage-dependent Na current during embryogenesis of rat telencephalon. Brain Res Dev Brain Res. 1994 Jan 14;77(1):89–95. doi: 10.1016/0165-3806(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Miyachi E., Kato C., Nakaki T. Arachidonic acid blocks gap junctions between retinal horizontal cells. Neuroreport. 1994 Jan 12;5(4):485–488. doi: 10.1097/00001756-199401120-00029. [DOI] [PubMed] [Google Scholar]
- O'Donnell P., Grace A. A. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. J Neurosci. 1993 Aug;13(8):3456–3471. doi: 10.1523/JNEUROSCI.13-08-03456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paysan J., Bolz J., Mohler H., Fritschy J. M. GABAA receptor alpha 1 subunit, an early marker for area specification in developing rat cerebral cortex. J Comp Neurol. 1994 Dec 1;350(1):133–149. doi: 10.1002/cne.903500110. [DOI] [PubMed] [Google Scholar]
- Peinado A., Yuste R., Katz L. C. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993 Jan;10(1):103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro D. E., Bennett M. V., Zukin R. S. Are Ca(2+)-permeable kainate/AMPA receptors more abundant in immature brain? Neurosci Lett. 1992 Sep 14;144(1-2):65–69. doi: 10.1016/0304-3940(92)90717-l. [DOI] [PubMed] [Google Scholar]
- Perez-Velazquez J. L., Valiante T. A., Carlen P. L. Modulation of gap junctional mechanisms during calcium-free induced field burst activity: a possible role for electrotonic coupling in epileptogenesis. J Neurosci. 1994 Jul;14(7):4308–4317. doi: 10.1523/JNEUROSCI.14-07-04308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A. T., 4th, Teyler T. J. A critical period for long-term potentiation in the developing rat visual cortex. Brain Res. 1988 Jan 26;439(1-2):222–229. doi: 10.1016/0006-8993(88)91478-3. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. P., Thomas R. C. The effects of chloride substitution on intracellular pH in crab muscle. J Physiol. 1981 Mar;312:71–80. doi: 10.1113/jphysiol.1981.sp013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Spruston N., Jaffe D. B., Williams S. H., Johnston D. Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J Neurophysiol. 1993 Aug;70(2):781–802. doi: 10.1152/jn.1993.70.2.781. [DOI] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J., Rucker F., ten Bruggencate G. Spread of epileptiform activity in the immature rat neocortex studied with voltage-sensitive dyes and laser scanning microscopy. J Neurophysiol. 1994 Oct;72(4):1756–1768. doi: 10.1152/jn.1994.72.4.1756. [DOI] [PubMed] [Google Scholar]
- Taylor C. P., Dudek F. E. A physiological test for electrotonic coupling between CA1 pyramidal cells in rat hippocampal slices. Brain Res. 1982 Mar 11;235(2):351–357. doi: 10.1016/0006-8993(82)91013-7. [DOI] [PubMed] [Google Scholar]
- Yuste R., Peinado A., Katz L. C. Neuronal domains in developing neocortex. Science. 1992 Jul 31;257(5070):665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- el Manira A., Cattaert D., Wallén P., DiCaprio R. A., Clarac F. Electrical coupling of mechanoreceptor afferents in the crayfish: a possible mechanism for enhancement of sensory signal transmission. J Neurophysiol. 1993 Jun;69(6):2248–2251. doi: 10.1152/jn.1993.69.6.2248. [DOI] [PubMed] [Google Scholar]