Abstract
Objective
This research aimed to address the critical need for effective prognostic tools in patients with acute coronary syndrome (ACS) and type 2 diabetes mellitus (T2DM) undergoing percutaneous coronary intervention (PCI) by exploring the potential significance of integrating estimated glucose disposal rate (eGDR) and neutrophil-to-lymphocyte ratio (NLR).
Methods
Major adverse cardiovascular and cerebrovascular events (MACCE) were the primary endpoint. Log rank test was conducted to compare the Kaplan–Meier curves across the overall follow-up period, and multivariate Cox regression was used to investigate the association between the eGDR/NLR and MACCE.
Results
One hundred fifty-four patients (9.5%) experienced MACCE including 15 cardiac deaths, 97 nonfatal MI, 120 TVR, and 10 strokes. Patients were distributed into low and high eGDR/NLR groups (lower eGDR [eGDR-L] group, higher eGDR [eGDR-H] group, lower NLR [NLR-L] group, and higher NLR [NLR-H] group) based on the median value of eGDR and NLR, further divided into four groups: eGDR-L + NLR-L, eGDR-H + NLR-L, eGDR-L + NLR-H, and eGDR-H + NLR-H. eGDR-L + NLR-H group exhibited significantly higher risks of MACCE (17.4%), compared to another three groups. An independent correlation between eGDR/NLR and MACCE was demonstrated by Cox regression analysis, establishing if the eGDR and NLR was treated as a continuous or categorical variable. Compared to eGDR-H + NLR-L group, patients in eGDR-L + NLR-H group had the uppermost MACCE risk (HR: 5.201; 95% CI 2.764–7.786; P < 0.001). A linear relationship between eGDR/ NLR and MACCE was showed by restricted cubic spline curves. Incorporating the eGDR and NLR toward the baseline risk model developed the precision of forecasting MACCE (baseline risk model-AUC: 0.611 vs baseline risk model + eGDR + NLR-AUC: 0.695, P < 0.001).
Conclusion
Combining eGDR with NLR can be utilized to forecast long-term MACCE and substantially improve the accuracy of risk stratification in ACS patients with T2DM following PCI.
Keywords: estimated glucose disposal rate, neutrophil-to-lymphocyte ratio, type 2 diabetes mellitus, acute coronary syndrome, percutaneous coronary intervention
Introduction
Atherosclerosis is a persistent inflammatory condition with autoimmune features and serves as the primary underlying cause of cardiovascular diseases (CVD),1 in which inflammatory processes play a crucial role for the atherosclerotic plaque’s instability, atherosclerosis development, and thrombus formation.2 It is important to note that acute coronary syndromes (ACS) are characterized by complex pathological processes involving multiple factors, such as inflammatory, metabolic, and regulatory pathways3 while circulating inflammatory and immune cells, including white blood cells (WBC) and their subtypes such as monocytes, neutrophils, and lymphocytes, serve as indicators of systemic inflammation severity and are crucial contributors to ACS.4 Neutrophils indicate a state of systemic inflammation, while lymphocytes indicate homeostasis of fibrotic hyperplasia and global inflammation. Both respond to inflammation caused by arterial plaque,5 and the neutrophil-to-lymphocyte ratio (NLR) derived from both is a promising and economical inflammatory marker for evaluating the prognosis of various diseases.6 It has been evidenced to be correlated with chronic low-grade inflammation, systemic inflammation, vascular wall lipid accumulation, and atherogenic mechanisms.7 Notably, NLR was correlated with the frequency and type 2 diabetes mellitus (T2DM) occurrence, suggesting that NLR may be an effective and accurate prognostic biomarker for T2DM,8 and in coronary artery diseases (CAD), NLR was elevated in diabetic patients and independently correlated with the prevalence and the scope of severity.9
Among individuals with T2DM, since CVD is the primary cause of mortality, common metabolic markers related to CVD and T2DM, in addition to inflammatory markers, are also of significant importance in assessing CVD risk. The hyperinsulinemic-euglycemic clamp technique is recognized as the definitive method for in vivo valuation of insulin action, precisely determining glucose utilization across the whole body. However, this method is invasive and expensive, limiting its use.10 Another metric is estimated glucose disposal rate (eGDR), initially validated as the metric for assessing insulin resistance (IR) among type 1 diabetes mellitus (T1DM) individuals, built on waist circumference, hypertension, and glycosylated hemoglobin A1c (HbA1c).11,12 Further studies have shown that using BMI instead of waist circumference to determine eGDR can yield similar results.13 Although eGDR was originally developed for individuals with T1DM, research has demonstrated a good correlation between eGDR and the gold standard hyperinsulinemic-euglycemic clamp technique in patients with T2DM,14 and a recent study investigating whether IR assessed by eGDR was associated with cardiorenal risk found that, in the T2DM population, lower eGDR levels were linked to a heightened risk of cardiovascular (but not renal) events.15 Since NLR and eGDR are important predictors of inflammation and metabolism, respectively, their combined use may provide a more comprehensive perspective for assessing CVD prognosis in T2DM patients. Moreover, in pathophysiological mechanisms, IR related to eGDR can activate the protein kinase C and nuclear factor κB pathways, leading to excessive reactive oxygen species production. These pathways triggered inflammatory responses and endothelial damage, ultimately causing CVD.16 As for elevated NLR, it represented the heightened neutrophil activation and/or lymphocyte suppression, so the main pathways might include the neutrophil-associated nicotinamide adenine dinucleotide phosphate oxidase pathway and the T cell receptor signaling pathway, which is crucial for lymphocyte function.17,18 However, no research to date has examined the impact of combining eGDR and NLR on the prognosis of T2DM patients. Therefore, this study seeks to estimate combined predictive values of eGDR and NLR for major adverse cardiovascular and cerebrovascular events (MACCE) in T2DM and ACS patients receiving percutaneous coronary intervention (PCI).
Methods
Study Population
The study is an observational, single-center, retrospective cohort study at Beijing Anzhen Hospital focusing on individuals with T2DM diagnosed with ACS and received elective PCI during the period from January to December 2018. Study inclusion criteria included age ≥18-year-old and diagnosed with ACS and T2DM. The criteria for exclusion were (Figure S1): (1) Missing data about BMI, HbA1c and blood routine examination; (2) body mass index (BMI) with extreme value ≥45kg/m2; (3) Suspected familial hypertriglyceridemia (with triglyceride [TG] level over 500mg/dL); (4) Infectious diseases and active tumors; (5) Severe renal or hepatic dysfunction; (6) Immuno-suppressant or steroid drugs prescriptions; (7) Hematological disorders; (8) Incomplete angiographic, clinical, or laboratory data; (9) Heart failure, cardiogenic shock or coronary artery bypass grafting (CABG) history; (10) Lost to follow-up. 1616 individuals who adhered to the enrollment criteria were incorporated into the current study.
Definitions and Data Collection
Demographic and clinical data, such as blood pressure (systolic blood pressure [SBP] and diastolic blood pressure [DBP]), age, weight, height, and sex, along with family and medical history and current treatments, were retrieved from the healthcare information management system. Following an overnight fast, blood samples were drawn from the veins. Routine hematology (including platelets [PLT], WBC, neutrophils, red blood cells [RBC], and lymphocytes), and biochemical metrics, involving uric acid, high-sensitivity C-reactive protein (hs-CRP), TG, total cholesterol (TC), creatinine, low-density lipoprotein cholesterol (LDL-C), HbA1c, high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), and other metrics, were measured using standardized laboratory procedures at the Beijing Anzhen Hospital’s central laboratory.19
T2DM was defined in terms of the following standards: (1) diabetes diagnosed earlier, currently managed with antidiabetic treatments; (2) classic diabetes symptoms, accompanied by random blood glucose levels of 11.1 mmol/L or higher, FBG levels of 7.0 mmol/L or greater, or 2-hour post-oral glucose tolerance test blood glucose levels of 11.1 mmol/L or above; (3) HbA1c level of 6.5% or higher upon admission.20 BMI was determined: BMI = weight (kg)/[height (m)] 2. In patients with SBP levels of 140 mmHg or greater and/or DBP levels of 90 mmHg or above, or undergoing antihypertensive treatment, it was identified as hypertension. Patients were diagnosed with dyslipidemia if they had fasting TC levels of 5.2 mmol/L or beyond, LDL-C levels of 3.4 mmol/L or above, HDL-C levels below 1.0 mmol/L, TG levels of 1.7 mmol/L or higher, and/or receiving lipid- reducing medications. The estimated glomerular filtration rate (eGFR) was obtained: eGFR [mL/(min × 1.73 m2)] = 186 × serum creatinine (mg/dL)−1.154 × age−0.203 (× 0.742 if female).21 ACS included unstable angina pectoris (UAP), non-ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI), as clarified by relevant guidelines.22 The NLR was determined using the formula with: (plasma neutrophil count, 109/L)/ (plasma lymphocyte count, 109/L). The eGDRBMI (mg/kg/min) was determined following the previously described method13,23 using: eGDRBMI = 19.02 - (0.22 × BMI [kg/m2]) - (3.26 × hypertension) - (0.61 × HbA1c [%]), where hypertension referred to (1 = yes, 0 = no).
Two separate blinded intervention experts interpreted and recorded the characteristics of the CAD after PCI procedures. Any disagreements were resolved by consensus. By one stenosis of 50% or more in the left main (LM), LM disease was identified, and three-vessel disease was defined by one stenosis of 50% or more across all three major coronary arteries: the left circumflex artery, the right coronary artery, and the left anterior descending artery based on coronary angiography. Chronic total occlusion (CTO) was characterized by an absolute blockage with any native coronary artery lasting over 3 months, accompanied by a thrombolysis in myocardial infarction (TIMI) flow grade of 0. Using an online calculator (http://www.syntaxscore.com/), the synergy between PCI with taxus and cardiac surgery (SYNTAX) score, which assesses coronary lesion complexity, was calculated.
Follow-Up and Endpoint
The clinical condition was assessed at 1, 6, and 12 months, and then annually, either through outpatient appointments or telephone interviews. The primary outcome of the follow-up was MACCE, which were termed as a union of cardiac death, nonfatal myocardial infarction (MI), target vessel revascularization (TVR), and nonfatal stroke. The individual components of the MACCE were the secondary observational endpoints. Cardiac death was specified as mortality resulting from cardiac cause. Based on clinical and laboratory parameters, nonfatal MI was diagnosed, following the definition of MI.24 TVR was characterized as any procedure involving the revascularization of any segment of the target vessel repeatedly. Validated by a neurologist through radiological findings, nonfatal stroke was characterized by neurological impairments, whether hemorrhagic or ischemic.25 All events were reviewed by two seasoned, independent experts in cardiology who were blinded to the specifics of this study. Any discrepancies were resolved through consultation with a third expert in cardiology.
Statistical Analysis
As determined by the median eGDR level: lower eGDR (eGDR-L) group (<6.22 mg/kg/min, n = 808) and higher eGDR (eGDR-H) group (≥6.22 mg/kg/min, n = 808), individuals were categorized into two groups. Grounded in the median NLR: lower NLR (NLR-L) group (<2.50, n = 808) and higher NLR (NLR-H) group (≥2.50, n = 808), they were also sorted into two groups. Combining these classifications, patients were further designated as four groups built on eGDR and NLR levels: eGDR-L + NLR-L (n = 418), eGDR-H + NLR-L (n = 390), eGDR-L + NLR-H (n = 390), and eGDR-H + NLR-H (n = 418). Continuous variables were expressed as mean ± standard deviation, whereas categorical variables were summarized in terms of frequencies and percentages. For continuous variables, differences between groups were analyzed using Student’s t-test or Mann–Whitney U-test and for categorical variables differences were assessed with Fisher’s exact test or Chi-square test, as applicable. To assess correlations between eGDR/NLR and cardiovascular risk factors, Spearman’s rank or Pearson correlation test was employed. For measuring the relationship between two continuous variables with normal distributions, Pearson correlation was used, whereas Spearman’s rank correlation was adopted when at least one of the variables were either continuous variables that did not follow a normal distribution or categorical variables. To assess the occurrence rates of adverse outcomes across different groups based on median eGDR or NLR, Kaplan–Meier survival analyses were used. Log rank test was employed for analyzing discrepancies between groups. Sensitivity analyses were performed to examine the impact of baseline MI and stroke on the study outcomes. Patients with baseline MI or stroke were separately analyzed to assess whether their inclusion affected the overall results. The sensitivity analysis confirmed the robustness of our findings. To identify the ideal cutoff value of the eGDR/NLR for estimating the MACCE, receiver-operating characteristic curves (ROC) analyses were conducted.
For the primary endpoint, the predictive value of variables was assessed through both Cox proportional hazards analyses with univariate and multivariate. eGDR and NLR were evaluated in two forms: (1) as a categorical variable including grouping according to their own median and then four combined groups and (2) as a continuous variable. Before applying the models, variance inflation factors (VIF) were used to test for multicollinearity, and all covariates were confirmed to have VIF values below 10. Three models were constructed to determine the forecasting potential of the eGDR and NLR for the MACCE in the multivariate Cox analyses. Confounders were chosen based on their clinical relevance: (1) Model 1: adjusted for sex (male), age, dyslipidemia, previous MI, previous smoking, previous stroke, previous PCI, and family history of CVD. (2) Model 2: Included adjustments within Model 1, further adjusted for TC, TG, LDL-C, HDL-C, FBG, eGFR, creatinine, left ventricular ejection fraction (LVEF) and hs-CRP. (3) Model 3: Composed adjustments in Model 2, further adjusted for complete revascularization, SYNTAX score, number of stents, three-vessel disease, LM disease, and medication use including dual antiplatelet therapy (DAPT), statin, oral antidiabetic agents (OAD), and insulin. Additionally, Model 3 was used to assess the forecasting value of the eGDR and NLR regarding each part of the MACCE. BMI, SBP, DBP, hypertension and HbA1c were not contained within the multivariate analysis since eGDR was calculated built upon these values. Results of the Cox proportional hazards analyses were illustrated as hazard ratios (HR) with 95% confidence intervals (CI). Additionally, several subgroup analyses like LVEF (<50 and ≥50%), age (<65 and ≥65 years), sex (male and female), dyslipidemia, previous smoking, LDL-C (≤1.8 and >1.8 mmol/L), eGFR (<60 and ≥60), type of ACS (UAP, STEMI, and NSTEMI) were performed to determine if the predictive utility of eGDR and NLR remained consistent among patients with varying demographic characteristics and comorbidities.
Including ROC analysis, C-statistics were utilized for assessing how eGDR and NLR enhances the prognostic capacity for the baseline risk model, which incorporated traditional risk factors. To compare the area under the curve (AUC) for each model, DeLong’s test was employed. Additionally, to evaluate how the inclusion of eGDR and NLR upgraded the prediction efficacy of the baseline risk model existed, category-free net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated. A two-tailed P value of below 0.05 was considered statistically significant, and through using SPSS version 22.0, MedCalc version 19.3 and R software version 4.4.0, statistical analyses were performed.
Results
Baseline Characteristics
The entire amount of 1616 consecutive ACS individuals with T2DM (mean age 60.33 ± 9.55 years, 72.2% male) who underwent PCI were incorporated into this study. The following outcomes were recorded: 15 (0.9%) cardiac deaths, 97 (6.0%) nonfatal MI, 120 (7.4%) TVR, 10 (0.6%) strokes, 106 (6.6%) composite outcomes of cardiac deaths and nonfatal MI, and 154 (9.5%) MACCE. Initial patient characteristics categorized following the incidence of MACCE were presented in Table 1. Compared to those in the group without MACCE, those experiencing every component in MACCE tended to have higher level of BMI and had a higher prevalence of accompanying conditions including previous MI, hypertension, and previous PCI. Additionally, patients with a poorer prognosis exhibited higher counts of neutrophils, NLR, hs-CRP, eGDR, FBG, HbA1c, and uric acid along with decreased lymphocytes. Regarding angiographic features, patients classified under MACCE were more frequently diagnosed with three-vessel disease and in-stent restenosis (ISR) disease, while showing lower prevalence of one-vessel disease and complete revascularization. Accordingly, these patients had significantly higher SYNTAX scores.
Table 1.
Baseline Characteristics of Study Patients with and without MACCE
| Variables | Total (n= 1616) | Without MACCE (n= 1462) | With MACCE (n= 154) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 60.33 ± 9.55 | 60.32 ± 9.52 | 60.44 ± 9.87 | 0.879 |
| Sex, n (%) | 1166 (72.2) | 1055 (72.2) | 111 (72.1) | 1.000 |
| BMI, kg/m2 | 26.33 ± 3.27 | 26.26 ± 3.25 | 27.03 ± 3.35 | 0.005 |
| SBP, mmHg | 129.20 ± 17.26 | 129.14 ± 16.94 | 129.87 ± 20.16 | 0.622 |
| DBP, mmHg | 75.52 (10.61) | 74.07 (11.35) | 75.67 (10.53) | 0.079 |
| Previous smoking, n (%) | 190 (11.8) | 172 (11.8) | 18 (11.7) | 1.000 |
| Previous drinking, n (%) | 60 (3.7) | 56 (3.8) | 4 (2.6) | 0.585 |
| Family history of CVD, n (%) | 143 (8.8) | 126 (8.6) | 17 (11.0) | 0.392 |
| Medical histories, n (%) | ||||
| Hypertension | 1064 (65.8) | 941 (64.4) | 123 (79.9) | <0.001 |
| Dyslipidemia | 1126 (69.7) | 1014 (69.4) | 112 (72.7) | 0.439 |
| Previous MI | 235 (14.5) | 195 (13.3) | 40 (26.0) | <0.001 |
| Previous PCI | 465 (28.8) | 406 (27.8) | 59 (38.3) | 0.008 |
| Previous stroke | 66 (4.1) | 61 (4.2) | 5 (3.2) | 0.735 |
| Laboratory results | ||||
| WBC, 109/L | 6.74 [5.74, 7.83] | 6.74 [5.71, 7.82] | 6.84 [5.99, 8.02] | 0.080 |
| RBC, 1012/L | 4.61 [4.29, 4.92] | 4.60 [4.29, 4.92] | 4.62 [4.35, 4.93] | 0.709 |
| PLT, 109/L | 213.00 [180.00, 252.00] | 213.00 [180.00, 252.00] | 219.00 [179.50, 252.25] | 0.595 |
| Neutrophils, 109/L | 4.37 [3.59, 5.36] | 4.34 [3.56, 5.33] | 4.62 [3.91, 5.77] | 0.005 |
| Lymphocyte, 109/L | 1.76 [1.42, 2.12] | 1.78 [1.43, 2.14] | 1.65 [1.34, 2.06] | 0.035 |
| NLR | 2.50 [1.93, 3.28] | 2.47 [1.92, 3.23] | 2.84 [2.17, 3.64] | <0.001 |
| TG, mmol/L | 1.49 [1.05, 2.11] | 1.49 [1.05, 2.11] | 1.56 [1.10, 2.04] | 0.358 |
| TC, mmol/L | 3.89 [3.31, 4.56] | 3.88 [3.30, 4.55] | 4.00 [3.40, 4.70] | 0.162 |
| HDL-C, mmol/L | 1.02 [0.89, 1.19] | 1.03 [0.89, 1.19] | 1.02 [0.89, 1.18] | 0.325 |
| LDL-C, mmol/L | 2.25 [1.79, 2.79] | 2.24 [1.78, 2.78] | 2.35 [1.89, 2.99] | 0.114 |
| LVEF, % | 64.00 [60.00, 67.00] | 64.00 [60.00, 67.00] | 63.00 [59.00, 66.00] | 0.079 |
| eGFR, mL/min/1.73 m2 | 96.86 [87.34, 104.32] | 97.00 [87.96, 104.51] | 95.50 [84.09, 103.32] | 0.148 |
| Creatinine, mmol/L | 68.95 [60.38, 78.03] | 68.70 [60.20, 77.70] | 70.30 [61.62, 80.18] | 0.073 |
| eGDR, mg/kg/min | 6.22 [5.07, 8.08] | 6.32 [5.20, 8.23] | 5.22 [4.37, 6.33] | <0.001 |
| hs-CRP, mg/L | 1.24 [0.54, 3.12] | 1.18 [0.52, 3.00] | 1.70 [0.68, 4.25] | 0.002 |
| FBG, mmol/L | 7.95 [7.00, 9.73] | 7.90 [6.95, 9.65] | 8.13 [7.09, 10.94] | 0.008 |
| Uric acid, μmol/L | 328.70 [270.60, 383.60] | 326.50 [269.80, 381.90] | 338.60 [298.50, 390.15] | 0.015 |
| HbA1c, % | 7.30 [6.40, 8.40] | 7.30 [6.40, 8.30] | 7.90 [7.00, 8.70] | <0.001 |
| Diagnosis on admission, n (%) | ||||
| UAP | 1394 (86.3) | 1259 (86.1) | 135 (87.7) | 0.684 |
| NSTEMI | 116 (7.2) | 109 (7.5) | 7 (4.5) | 0.243 |
| STEMI | 106 (6.6) | 94 (6.4) | 12 (7.8) | 0.632 |
| Medications, n (%) | ||||
| DAPT | 1611 (99.7) | 1458 (99.7) | 153 (99.4) | 0.971 |
| Statin | 1604 (99.3) | 1453 (99.4) | 151 (98.1) | 0.181 |
| β-Blocker | 1108 (68.6) | 997 (68.2) | 111 (72.1) | 0.370 |
| ACEI/ARB | 756 (46.8) | 679 (46.4) | 77 (50.0) | 0.449 |
| CCB | 565 (35.0) | 499 (34.1) | 66 (42.9) | 0.038 |
| Antidiabetic drugs | ||||
| Insulin | 346 (21.4) | 298 (20.4) | 48 (31.2) | 0.003 |
| OAD | 989 (61.2) | 894 (61.1) | 95 (61.7) | 0.965 |
| Angiographic data | ||||
| LM disease, n (%) | 102 (6.3) | 87 (6.0) | 15 (9.7) | 0.096 |
| One-vessel disease, n (%) | 483 (29.9) | 453 (31.0) | 30 (19.5) | 0.004 |
| Two-vessel disease, n (%) | 596 (36.9) | 537 (36.7) | 59 (38.3) | 0.765 |
| Three-vessel disease, n (%) | 537 (33.2) | 472 (32.3) | 65 (42.2) | 0.017 |
| CTO disease, n (%) | 367 (22.7) | 327 (22.4) | 40 (26.1) | 0.340 |
| Diffuse lesion, n (%) | 647 (40.1) | 586 (40.1) | 61 (39.9) | 1.000 |
| Bifurcation lesion, n (%) | 141 (8.7) | 125 (8.6) | 16 (10.5) | 0.521 |
| ISR disease, n (%) | 146 (9.0) | 121 (8.3) | 25 (16.3) | 0.002 |
| SYNTAX score | 12.00 [8.00, 16.00] | 12.00 [8.00, 16.00] | 13.50 [9.00, 18.00] | 0.004 |
| Procedural results | ||||
| Target vessel territory, n (%) | ||||
| LM | 61 (3.8) | 56 (3.8) | 5 (3.2) | 0.889 |
| LAD | 836 (51.7) | 759 (51.9) | 77 (50.0) | 0.713 |
| LCX | 324 (20.0) | 287 (19.6) | 37 (24.0) | 0.234 |
| RCA | 538 (33.3) | 490 (33.5) | 48 (31.2) | 0.619 |
| DES implantation, n (%) | 1561 (96.6) | 1415 (96.8) | 146 (94.8) | 0.291 |
| DCB use, n (%) | 93 (5.8) | 79 (5.4) | 14 (9.1) | 0.092 |
| IABP, n (%) | 16 (1.0) | 14 (1.0) | 2 (1.3) | 1.000 |
| IVUS, n (%) | 13 (0.8) | 10 (0.7) | 3 (1.9) | 0.232 |
| OCT, n (%) | 23 (1.4) | 21 (1.4) | 2 (1.3) | 1.000 |
| Complete revascularization, n (%) | 539 (33.4) | 504 (34.5) | 35 (22.9) | 0.005 |
| Number of stents | 1.00 [1.00, 2.00] | 1.00 [1.00, 2.00] | 1.00 [1.00, 2.00] | 0.100 |
| Diameter of stents, mm | 2.75 [2.50, 3.00] | 2.75 [2.50, 3.00] | 2.75 [2.50, 3.00] | 0.182 |
| Length of stents, mm | 23.00 [18.00, 30.00] | 23.00 [18.00, 30.00] | 22.00 [15.00, 28.00] | 0.050 |
Abbreviations: MACCE, major adverse cardiovascular and cerebrovascular events; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CVD, cardiovascular diseases; MI, myocardial infarction; PCI, percutaneous coronary intervention; WBC, white blood cells; RBC, red blood cells; PLT, platelets; NLR, neutrophil-to-lymphocyte ratio; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; eGDR, estimated glucose disposal rate; hs-CRP, high sensitivity C-reactive protein; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c; UAP, unstable angina pectoris; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST segment elevated myocardial infarction; DAPT, Dual antiplatelet therapy; ACEI, angiotensin converting; enzyme inhibitor, ARB, angiotensin receptor blocker; CCB, calcium calcium entry blockers; OAD, oral antidiabetic drugs; LM, left main; CTO, chronic total occlusion; ISR, in-stent restenosis; SYNTAX, Synergy between PCI with TAXUS and Cardiac Surgery; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; DES, drug-eluting stent; DCB, drug-coated balloon; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasound; OCT, optical coherence tomography.
Compared to eGDR-H + NLR-L group, another three groups showed elevated levels of TG, age, hs-CRP, WBC, FBG, BMI, neutrophils, SBP, TC, creatinine, LDL-C, and uric acid, with lower levels of lymphocytes and eGFR. Additionally, except for eGDR-H + NLR-L, patients in the remaining three groups were more prone to having higher percentage of STEMI, hypertension, LM disease, dyslipidemia, CTO disease, diffuse lesion, and intra-aortic balloon pump use, with lower percentage of one-vessel disease and complete revascularization (Table 2).
Table 2.
Baseline Clinical Characteristics of Patients Stratified by the eGDR and NLR
| Variables | eGDR-L+ NLR-L (n= 418) | eGDR-H + NLR-L (n= 390) | eGDR-L + NLR-H (n= 390) | eGDR -H + NLR -H (n= 418) | P value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 59.65 ± 9.46 | 59.16 ± 9.27 | 62.14 ± 9.35 | 60.41 ± 9.85 | <0.001 |
| Sex, n (%) | 303 (72.5) | 288 (73.8) | 259 (66.4) | 316 (75.6) | 0.024 |
| BMI, kg/m2 | 27.62 ± 3.09 | 25.28 ± 2.89 | 27.51 ± 3.24 | 24.92 ± 2.87 | <0.001 |
| SBP, mmHg | 131.51 ± 17.01 | 125.64 ± 15.96 | 133.48 ± 18.20 | 126.24 ± 16.57 | <0.001 |
| DBP, mmHg | 76.43 ± 10.47 | 74.98 ± 10.26 | 76.29 ± 11.46 | 74.40 ± 10.14 | 0.013 |
| Previous smoking, n (%) | 49 (11.7) | 38 (9.7) | 52 (13.3) | 51 (12.2) | 0.469 |
| Previous drinking, n (%) | 13 (3.1) | 13 (3.3) | 20 (5.1) | 14 (3.3) | 0.404 |
| Family history of CVD, n (%) | 44 (10.5) | 29 (7.4) | 40 (10.3) | 30 (7.2) | 0.185 |
| Medical histories, n (%) | |||||
| Hypertension | 412 (98.6) | 106 (27.2) | 388 (99.5) | 158 (37.8) | <0.001 |
| Dyslipidemia | 321 (76.8) | 248 (63.6) | 286 (73.3) | 271 (64.8) | <0.001 |
| Previous MI | 70 (16.7) | 52 (13.3) | 48 (12.3) | 65 (15.6) | 0.261 |
| Previous PCI | 131 (31.3) | 94 (24.1) | 115 (29.5) | 125 (29.9) | 0.119 |
| Previous stroke | 15 (3.6) | 10 (2.6) | 21 (5.4) | 20 (4.8) | 0.189 |
| Laboratory results | |||||
| WBC, 109/L | 6.46 [5.56, 7.37] | 6.12 [5.28, 7.27] | 7.34 [6.18, 8.45] | 7.14 [6.06, 8.28] | <0.001 |
| RBC, 1012/L | 4.62 [4.35, 4.94] | 4.61 [4.28, 4.91] | 4.60 [4.27, 4.97] | 4.60 [4.26, 4.90] | 0.370 |
| PLT, 109/L | 217.00 [184.00, 252.75] | 207.00 [179.00, 245.00] | 221.00 [181.00, 257.00] | 210.00 [174.25, 250.75] | 0.083 |
| Neutrophils, 109/L | 3.87 [3.23, 4.48] | 3.66 [3.04, 4.38] | 5.20 [4.26, 6.01] | 5.13 [4.24, 5.99] | <0.001 |
| Lymphocyte, 109/L | 2.03 [1.75, 2.41] | 2.00 [1.70, 2.26] | 1.52 [1.25, 1.85] | 1.46 [1.20, 1.78] | <0.001 |
| NLR | 1.94 [1.66, 2.21] | 1.91 [1.61, 2.18] | 3.27 [2.81, 3.91] | 3.29 [2.84, 4.11] | <0.001 |
| TG, mmol/L | 1.73 [1.21, 2.39] | 1.46 [1.00, 2.00] | 1.46 [1.08, 2.10] | 1.34 [0.95, 1.87] | <0.001 |
| TC, mmol/L | 4.04 [3.45, 4.70] | 3.88 [3.34, 4.54] | 3.92 [3.29, 4.48] | 3.76 [3.15, 4.45] | 0.002 |
| HDL-C, mmol/L | 1.02 [0.89, 1.17] | 1.02 [0.90, 1.19] | 1.03 [0.89, 1.17] | 1.03 [0.88, 1.20] | 0.694 |
| LDL-C, mmol/L | 2.35 [1.87, 2.88] | 2.26 [1.80, 2.76] | 2.24 [1.76, 2.80] | 2.15 [1.71, 2.71] | 0.022 |
| LVEF, % | 64.00 [60.00, 66.75] | 65.00 [60.00, 67.00] | 65.00 [60.00, 67.00] | 64.00 [58.00, 67.00] | 0.414 |
| eGFR, mL/min/1.73 m2 | 98.09 [88.58, 105.33] | 98.77 [91.33, 105.89] | 93.68 [82.32, 100.62] | 97.20 [86.09, 104.13] | <0.001 |
| Creatinine, mmol/L | 68.15 [59.80, 78.18] | 66.65 [59.45, 75.88] | 71.25 [60.90, 81.07] | 69.45 [61.40, 77.95] | 0.002 |
| eGDR, mg/kg/min | 5.03 [4.17, 5.67] | 8.26 [7.01, 9.11] | 5.11 [4.37, 5.77] | 7.92 [6.82, 9.11] | <0.001 |
| hs-CRP, mg/L | 1.24 [0.58, 2.85] | 0.95 [0.42, 2.32] | 1.66 [0.68, 4.14] | 1.26 [0.52, 3.86] | <0.001 |
| FBG, mmol/L | 8.01 [6.95, 9.87] | 7.68 [6.69, 8.98] | 8.51 [7.28, 10.94] | 7.72 [6.85, 9.30] | <0.001 |
| Uric acid, μmol/L | 327.75 [277.90, 383.83] | 325.80 [264.10, 384.65] | 332.80 [280.60, 384.00] | 327.60 [265.80, 381.60] | 0.437 |
| HbA1c, % | 7.85 [7.00, 8.80] | 7.00 [6.20, 7.90] | 7.70 [6.80, 8.60] | 6.80 [6.00, 7.60] | <0.001 |
| Diagnosis on admission, n (%) | |||||
| UAP | 372 (89.0) | 353 (90.5) | 322 (82.6) | 347 (83.0) | 0.001 |
| NSTEMI | 29 (6.9) | 20 (5.1) | 38 (9.7) | 29 (6.9) | 0.094 |
| STEMI | 17 (4.1) | 17 (4.4) | 30 (7.7) | 42 (10.0) | 0.001 |
| Medications, n (%) | |||||
| DAPT | 417 (99.8) | 389 (99.7) | 388 (99.5) | 417 (99.8) | 0.875 |
| Statin | 416 (99.5) | 388 (99.5) | 384 (98.5) | 416 (99.5) | 0.219 |
| β-Blocker | 275 (65.8) | 259 (66.4) | 290 (74.4) | 284 (67.9) | 0.037 |
| ACEI/ARB | 249 (59.6) | 100 (25.6) | 247 (63.3) | 160 (38.3) | <0.001 |
| CCB | 201 (48.1) | 58 (14.9) | 208 (53.3) | 98 (23.4) | <0.001 |
| Antidiabetic drugs | |||||
| Insulin | 97 (23.2) | 64 (16.4) | 113 (29.0) | 72 (17.2) | <0.001 |
| OAD | 273 (65.3) | 221 (56.7) | 247 (63.3) | 248 (59.3) | 0.052 |
| Angiographic data | |||||
| LM disease, n (%) | 20 (4.8) | 25 (6.4) | 19 (4.9) | 38 (9.1) | 0.037 |
| One-vessel disease, n (%) | 128 (30.6) | 131 (33.6) | 92 (23.6) | 132 (31.6) | 0.014 |
| Two-vessel disease, n (%) | 152 (36.4) | 147 (37.7) | 150 (38.5) | 147 (35.2) | 0.776 |
| Three-vessel disease, n (%) | 138 (33.0) | 112 (28.7) | 148 (37.9) | 139 (33.3) | 0.058 |
| CTO disease, n (%) | 108 (25.8) | 69 (17.7) | 91 (23.4) | 99 (23.7) | 0.043 |
| Diffuse lesion, n (%) | 165 (39.5) | 141 (36.2) | 179 (46.0) | 162 (38.8) | 0.037 |
| Bifurcation lesion, n (%) | 35 (8.4) | 29 (7.5) | 33 (8.5) | 44 (10.5) | 0.461 |
| ISR disease, n (%) | 41 (9.8) | 28 (7.2) | 35 (9.0) | 42 (10.0) | 0.490 |
| SYNTAX score | 12.00 [8.00, 16.00] | 11.00 [7.00, 16.00] | 12.00 [8.50, 17.00] | 12.00 [8.00, 16.38] | 0.356 |
| Procedural results | |||||
| Target vessel territory, n (%) | |||||
| LM | 16 (3.8) | 15 (3.8) | 9 (2.3) | 21 (5.0) | 0.249 |
| LAD | 211 (50.5) | 208 (53.3) | 198 (50.8) | 219 (52.4) | 0.830 |
| LCX | 86 (20.6) | 72 (18.5) | 86 (22.1) | 80 (19.1) | 0.598 |
| RCA | 142 (34.0) | 131 (33.6) | 127 (32.6) | 138 (33.0) | 0.976 |
| DES implantation, n (%) | 403 (96.4) | 381 (97.7) | 378 (96.9) | 399 (95.5) | 0.354 |
| DCB use, n (%) | 23 (5.5) | 22 (5.6) | 24 (6.2) | 24 (5.7) | 0.982 |
| IABP, n (%) | 3 (0.7) | 1 (0.3) | 3 (0.8) | 9 (2.2) | 0.038 |
| IVUS, n (%) | 4 (1.0) | 2 (0.5) | 2 (0.5) | 5 (1.2) | 0.624 |
| OCT, n (%) | 8 (1.9) | 7 (1.8) | 4 (1.0) | 4 (1.0) | 0.534 |
| Complete revascularization, n (%) | 130 (31.1) | 153 (39.2) | 120 (30.8) | 136 (32.5) | 0.041 |
| Number of stents | 1.00 [1.00, 2.00] | 1.00 [1.00, 2.00] | 1.00 [1.00, 2.00] | 1.00 [1.00, 2.00] | 0.577 |
| Diameter of stents, mm | 2.75 [2.50, 3.00] | 3.00 [2.50, 3.50] | 2.75 [2.50, 3.00] | 2.75 [2.50, 3.00] | 0.007 |
| Length of stents, mm | 22.00 [15.00, 30.00] | 23.00 [18.00, 30.00] | 23.00 [18.00, 30.00] | 23.00 [18.00, 30.00] | 0.429 |
Abbreviations: MACCE, major adverse cardiovascular and cerebrovascular events; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CVD, cardiovascular diseases; MI, myocardial infarction; PCI, percutaneous coronary intervention; WBC, white blood cells; RBC, red blood cells; PLT, platelets; NLR, neutrophil to lymphocyte ratio; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; eGDR, estimated glucose disposal rate; hs-CRP, high sensitivity C-reactive protein; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c; UAP, unstable angina pectoris; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST segment elevated myocardial infarction; DAPT, Dual antiplatelet therapy; ACEI, angiotensin converting; enzyme inhibitor, ARB, angiotensin receptor blocker; CCB, calcium calcium entry blockers; OAD, oral antidiabetic drugs; LM, left main; CTO, chronic total occlusion; ISR, in-stent restenosis; SYNTAX, Synergy between PCI with TAXUS and Cardiac Surgery; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; DES, drug-eluting stent; DCB, drug-coated balloon; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasound; OCT, optical coherence tomography.
Relationship Between Cardiovascular Risk Factors and eGDR/NLR
Pearson correlation or Spearman’s rank analysis was conducted for appraising the relationship between traditional risk factors and eGDR or NLR. eGDR showed negative correlations with sex, LDL-C, FBG, hs-CRP, uric acid, TC, and TG, whereas it was positively interrelated with eGFR. However, NLR showed opposite results including positive correlations with hs-CRP, TG, TC, FBG, LDL-C, and age with opposing correlations to eGFR (Table 3). In addition, there was a negative association between eGDR and NLR with no significant difference.
Table 3.
Correlations Between the eGDR/ NLR and Traditional Cardiovascular Risk Factors
| Variables | eGDR Correlation coefficient |
P value | NLR Correlation coefficient |
P value |
|---|---|---|---|---|
| Age | −0.048 | 0.052 | 0.123 | <0.001 |
| Sex, male | −0.065 | 0.009 | 0.012 | 0.635 |
| FBG | −0.195 | <0.001 | 0.063 | 0.011 |
| TG | −0.174 | <0.001 | 0.117 | <0.001 |
| TC | −0.087 | <0.001 | 0.079 | <0.001 |
| LDL-C | −0.081 | 0.001 | 0.065 | 0.009 |
| Uric acid | −0.059 | 0.024 | 0.005 | 0.854 |
| eGFR | 0.074 | 0.007 | −0.147 | <0.001 |
| hs-CRP | −0.125 | <0.001 | 0.107 | <0.001 |
| eGDR | – | – | −0.031 | 0.211 |
| NLR | −0.031 | 0.211 | – | – |
| LVEF | 0.048 | 0.077 | −0.010 | 0.701 |
| SYXTAX score | −0.032 | 0.196 | 0.048 | 0.053 |
Abbreviations: FBG, fasting blood glucose; NLR, neutrophil-to-lymphocyte ratio; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; eGDR, estimated glucose disposal rate; hs-CRP, high sensitivity C-reactive protein; SYNTAX, Synergy between PCI with TAXUS and Cardiac Surgery.
Clinical Outcomes Across Various Risk Categories Classified by eGDR and NLR
One hundred and fifty-four patients (9.5%) experienced MACCE including 15 (0.9%) cardiac deaths, 97 (6.0%) nonfatal MI, 120 (7.4%) TVR, 10 (0.6%) strokes, and 106 (6.6%) composite outcomes of cardiac deaths and nonfatal MI were recorded. The frequency of adverse events was evaluated among groups divided by median eGDR and NLR. Compared to another three groups, patients in eGDR-L + NLR-H had a significantly increased occurrence of the MACCE (P < 0.001), cardiac death/non-fatal MI (P < 0.001), TVR (P < 0.001), and nonfatal stroke (P = 0.045) (Table 4).
Table 4.
Comparison of Endpoint Events Stratified by the eGDR and NLR
| Variables n (%) |
Total | eGDR-L+ NLR-L (n= 418) | eGDR-H + NLR-L (n= 390) | eGDR-L + NLR-H (n= 390) | eGDR -H + NLR -H (n= 418) | P value |
|---|---|---|---|---|---|---|
| MACCE | 154 (9.5) | 43 (10.3) | 12 (3.1) | 68 (17.4) | 31 (7.4) | <0.001 |
| Cardiac death/ Nonfatal MI | 106 (6.6) | 29 (6.9) | 10 (2.6) | 49 (12.6) | 18 (4.3) | <0.001 |
| TVR | 120 (7.4) | 32 (7.7) | 10 (2.6) | 54 (13.8) | 24 (5.7) | <0.001 |
| Nonfatal stroke | 10 (0.6) | 2 (0.5) | 0 (0.0) | 6 (1.5) | 2 (0.5) | 0.045 |
Abbreviations: eGDR, estimated glucose disposal rate; NLR, neutrophil-to-lymphocyte ratio; eGDR-L, lower eGDR; eGDR-H, higher eGDR; NLR-L, lower NLR; NLR-H, higher NLR; MACCE, major adverse cardiovascular and cerebrovascular events; MI, myocardial infarction; TVR, target vessel revascularization.
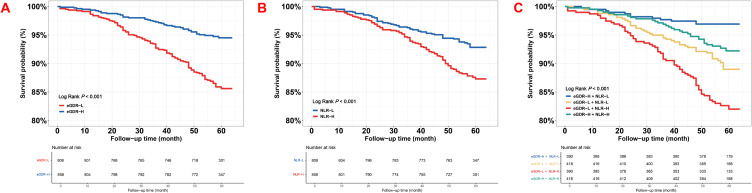
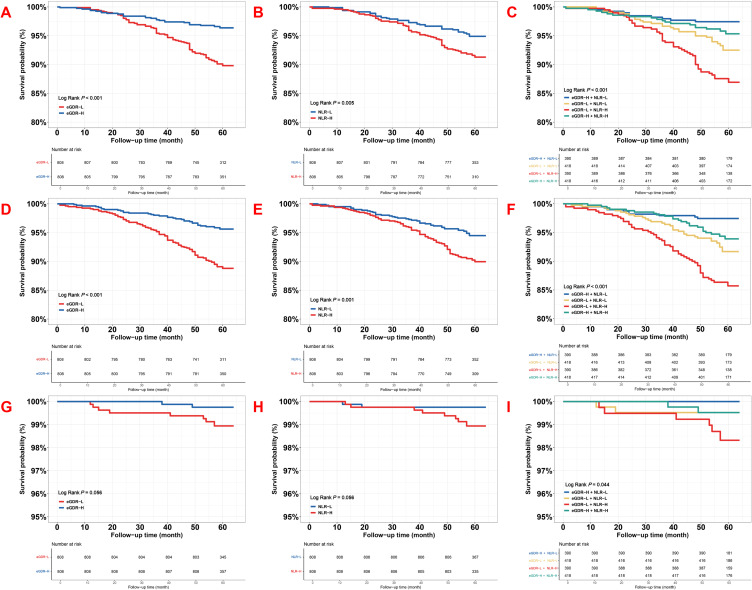
The Kaplan–Meier curves for the emergence of the MACCE with its components, grouped by the median of eGDR and NLR, and the derived four subgroups, were presented in Figures 1 and 2. The curves indicated a significant difference in the MACCE between the eGDR-H and eGDR-L (Log-rank P < 0.001, Figure 1A), NLR-H and NLR-L (Log-rank P < 0.001, Figure 1B), as well as four groups stratified by the medians of eGDR and NLR (Log-rank P < 0.001, Figure 1C), primarily determined by an rising occurrence of cardiac death/non-fatal MI and TVR (both Log-rank P < 0.001, Figure 2A and C, D–F and Log-rank P = 0.005, Figure 2B). Differences in stroke were not statistically significant (Log-rank P = 0.056, Figure 2G and H), but in four groups stratified by the median of eGDR and NLR indicated a significant difference (Figure 2I, Log-rank P = 0.044).
Figure 1.
The event-free survival rate in eGDR, NLR, and combined groups for MACCE (A) Kaplan–Meier curves of eGDR for MACCE; (B) Kaplan–Meier curves of NLR for MACCE; (C) Kaplan–Meier curves of eGDR + NLR for MACCE.
Abbreviations: eGDR, estimated glucose disposal rate; NLR, neutrophil-to-lymphocyte ratio; MACCE, major adverse cardiovascular and cerebrovascular events; eGDR-L, lower eGDR; eGDR-H, higher eGDR; NLR-L, lower NLR; NLR-H, higher NLR.
Figure 2.
The event-free survival rate in eGDR, NLR, and combined groups for the individual adverse events. Adjusted model included: Model 3 in multivariate Cox analyses. (1) Model 1: adjusted for sex (male), age, dyslipidemia, previous MI, previous smoking, previous stroke, previous PCI, and family history of CVD. (2) Model 2: Included adjustments within Model 1, further adjusted for TC, TG, LDL-C, HDL-C, FBG, eGFR, creatinine, LVEF and hs-CRP. (3) Model 3: Composed adjustments in Model 2, further adjusted for complete revascularization, SYNTAX score, number of stents, three-vessel disease, LM disease, and medication use including dual antiplatelet therapy (DAPT), statin, oral antidiabetic agents (OAD), and insulin. (A) Kaplan–Meier curves of eGDR for cardiac death/ nonfatal MI; (B) Kaplan–Meier curves of NLR for cardiac death/ nonfatal MI; (C) Kaplan–Meier curves of eGDR + NLR for cardiac death/ nonfatal MI; (D) Kaplan–Meier curves of eGDR for TVR; (E) Kaplan–Meier curves of NLR for TVR; (F) Kaplan–Meier curves of eGDR + NLR for TVR; (G) Kaplan–Meier curves of eGDR for nonfatal stroke; (H) Kaplan–Meier curves of NLR for nonfatal stroke; (I) Kaplan–Meier curves of eGDR + NLR for nonfatal stroke.
Abbreviations: eGDR, estimated glucose disposal rate; NLR, neutrophil-to-lymphocyte ratio; MACCE, major adverse cardiovascular and cerebrovascular events; eGDR-L, lower eGDR; eGDR-H, higher eGDR; NLR-L, lower NLR; NLR-H, higher NLR; MI, myocardial infarction; TVR, target vessel revascularization.
Cox Proportional Hazards Models Utilized to Assess the Prognostic Significance of eGDR and NLR
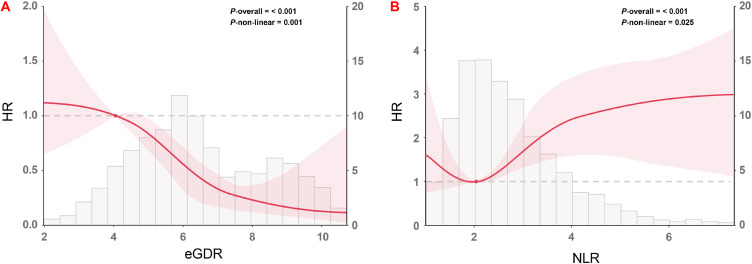
Among the multivariate Cox proportional hazards analysis, three models (Model 1–3) were created to assess the eGDR and NLR prognostic capacity for MACCE, incorporating variables with clinical relevance. After adjusting for interfering variables, as a nominal variable, eGDR-L + NLR-H showed consistently emerged as an independent risk indicator for the MACCE (all P < 0.001), while and lower eGDR and higher NLR also showed significant difference with MACCE, irrespective of whether eGDR and NLR were treated as the nominal or continuous variable (Table 5). Elaborate details about Model 3 were provided in Table S1. As for the RCS curves, also based on Model 3, it showed that NLR was positively correlated with an increased risk of MACCE events in a non-linear manner, while eGDR exhibited a negative trend (eGDR: P for nonlinearity <0.001, Figure 3A; NLR: P for nonlinearity = 0.025, Figure 3B).
Table 5.
Associations of eGDR and NLR with MACCE as Continuous and Categorical Variables in Different Cox Proportional Hazards Models
| Variable | Crude model HR (95% CI) |
P value | Model 1 HR (95% CI) |
P value | Model 2 HR (95% CI) |
P value | Model 3 HR (95% CI) |
P value |
|---|---|---|---|---|---|---|---|---|
| As continuous variables | ||||||||
| eGDR | 0.776 (0.712–0.846) | <0.001 | 0.784 (0.719–0.856) | <0.001 | 0.802 (0.716–0.897) | <0.001 | 0.809 (0.738–0.888) | <0.001 |
| NLR | 1.216 (1.090–1.357) | <0.001 | 1.223 (1.095–1.366) | <0.001 | 1.235 (1.076–1.417) | 0.003 | 1.200 (1.063–1.355) | 0.003 |
| As categorical variables | ||||||||
| eGDR | ||||||||
| Low | 2.701 (1.899–3.840) | <0.001 | 2.647 (1.855–3.778) | <0.001 | 2.651 (1.708–4.114) | <0.001 | 2.329 (1.612–3.363) | <0.001 |
| High | Reference | NA | References | NA | Reference | NA | References | NA |
| NLR | ||||||||
| Low | Reference | NA | References | NA | Reference | NA | References | NA |
| High | 1.852 (1.332–2.576) | <0.001 | 1.898 (1.362–2.645) | <0.001 | 2.207 (1.451–3.357) | <0.001 | 1.801 (1.271–2.552) | 0.001 |
| Combined categories | ||||||||
| eGDR-L + NLR-L | 3.464 (1.827–6.569) | <0.001 | 3.241 (1.704–6.163) | <0.001 | 3.023 (1.583–5.772) | 0.001 | 2.920 (1.525–5.588) | 0.001 |
| eGDR-H + NLR-L | References | NA | References | NA | References | NA | References | NA |
| eGDR-L + NLR-H | 6.122 (3.314–8.310) | <0.001 | 6.205 (3.346–8.508) | <0.001 | 5.569 (2.972–8.432) | <0.001 | 5.201 (2.764–7.786) | <0.001 |
| eGDR-H + NLR-H | 2.458 (1.263–4.787) | 0.008 | 2.401 (1.232–4.680) | 0.010 | 2.396 (1.224–4.690) | 0.011 | 2.327 (1.186–4.566) | 0.014 |
Notes: Adjusted model included: Model 3 in multivariate Cox analyses. (1) Model 1: adjusted for sex (male), age, dyslipidemia, previous MI, previous smoking, previous stroke, previous PCI, and family history of CVD. (2) Model 2: Included adjustments within Model 1, further adjusted for TC, TG, LDL-C, HDL-C, FBG, eGFR, creatinine, LVEF and hs-CRP. (3) Model 3: Composed adjustments in Model 2, further adjusted for complete revascularization, SYNTAX score, number of stents, three-vessel disease, LM disease, and medication use including dual antiplatelet therapy (DAPT), statin, oral antidiabetic agents (OAD), and insulin.
Abbreviations: eGDR, estimated glucose disposal rate; NLR, neutrophil-to-lymphocyte ratio; MACCE, major adverse cardiovascular and cerebrovascular events; HR, hazard ratios; CI, confidence intervals; eGDR-H, higher eGDR; eGDR-L, lower eGDR; NLR-H, higher NLR; NLR-L, lower NLR.
Figure 3.
Restricted cubic spline curves for the association of eGDR and NLR with the risk of MACCE in the adjusted model (A) RCS of eGDR for the risk of MACCE; (B) RCS of NLR for the risk of MACCE. Adjusted model included: Model 3 in multivariate Cox analyses.
Abbreviations: eGDR, estimated glucose disposal rate; NLR, neutrophil-to-lymphocyte ratio; MACCE, major adverse cardiovascular and cerebrovascular events; HR, hazard ratios.
As a nominal variable, the predictive value of the eGDR-L + NLR-H for every component of the MACCE was assessed through Model 3. Results indicated eGDR-L + NLR-H was correlated independently with a boosted risk of cardiac death/non-fatal MI (HR: 4.651, 95% CI 2.302–7.396, P < 0.001) and TVR (HR: 5.151, 95% CI 2.565–8.347, P < 0.001). However, eGDR-L + NLR-H were not predictive of stroke (Table S2).
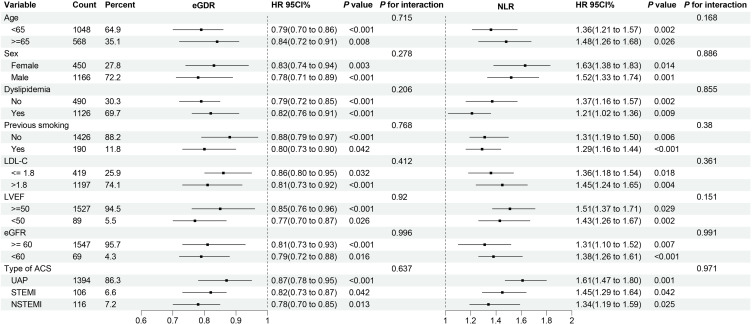
Across various subgroups, risk stratification values of eGDR and NLR for MACCE was further evaluated. The association between eGDR and NLR with MACCE, stratified by age (<65 and ≥65 years), sex (male and female), dyslipidemia, previous smoking, LDL-C (≤1.8 and >1.8 mmol/L), LVEF (<50 and ≥50%), eGFR (<60 and ≥60), type of ACS (UAP, STEMI, and NSTEMI), was illustrated in Figure 4. After adjusting for multiple factors including previous stroke, previous MI, SYNTAX score, previous PCI, three-vessel disease, family history of CVD, FBG, TG, creatinine, TC, hs-CRP, LM disease, LVEF, complete revascularization, number of stents, and medication use including DAPT, statin, OAD, and insulin, there was no interaction between all subgroups.
Figure 4.
Forest plot illustrating the association of the eGDR and NLR with the risk of MACCE stratified by different subgroups. Adjusted model included: Model 3 in multivariate Cox analyses except for subgroups’ variables including age, sex, dyslipidemia, previous smoking, LDL-C, LVEF, eGFR.
Abbreviations: eGDR, estimated glucose disposal rate; NLR, neutrophil-to-lymphocyte ratio; MACCE, major adverse cardiovascular and cerebrovascular events; HR, hazard ratios; CI, confidence intervals; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; ACS, acute coronary syndromes; UAP, unstable angina pectoris; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
Incremental Effect of the eGDR, NLR and eGDR + NLR for Predicting MACCE
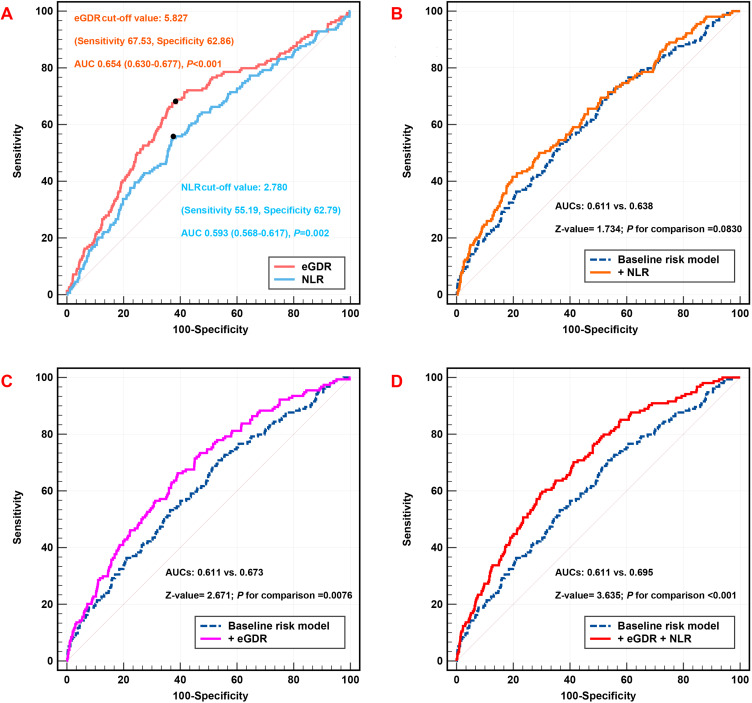
AUC-eGDR and AUC-NLR for predicting MACCE were 0.654 (95% CI: 0.630–0.677, P < 0.001) and 0.593 (95% CI: 0.568–0.617, P = 0.002), respectively, within ROC curve analysis, and for predicting MACCE, the ideal cutoff value of the eGDR and NLR was 5.827 and 2.780, with the sensitivity of 67.53% and a specificity of 62.86% for eGDR and the sensitivity of 55.19% and a specificity of 62.79% for NLR (Figure 5A). Incorporating the eGDR separately or with NLR into the baseline risk model, which included variables such as age, sex (male), dyslipidemia, previous smoking, previous MI, previous stroke, family history of CVD, previous PCI, TC, LDL-C, HDL-C, TG, FBG, creatinine, hs-CRP, eGFR, LVEF, SYNTAX score, complete revascularization, LM disease, number of stents, three-vessel disease, and medication use including DAPT, statin, OAD, and insulin, significantly improved the performance of models. The AUC increased from 0.611 with the model of baseline to 0.638 with the addition NLR (P = 0.0830) (Table 6 and Figure 5B), and the AUC increased from 0.611 with the baseline model to 0.673 with the addition of the eGDR (P = 0.0076) (Table 6 and Figure 5C). It was worth noting that the AUC increased from 0.611 with the baseline model to 0.695 with the addition of the eGDR and NLR (P < 0.001) (Table 6 and Figure 5D).
Figure 5.
C-statistics evaluating incremental effect of eGDR, NLR or eGDR + NLR for MACCE beyond baseline risk model (A) ROC curve analysis of eGDR and NLR for MACCE; (B) baseline risk model vs + NLR; (C) baseline risk model vs + eGDR; (D) baseline risk model vs + NLR + eGDR. Adjusted model included: Model 3 in multivariate Cox analyses.
Abbreviations: eGDR, estimated glucose disposal rate; NLR, neutrophil-to-lymphocyte ratio; MACCE, major adverse cardiovascular and cerebrovascular events; AUC, area under the curve.
Table 6.
C-Statistics for Discrimination Ability of Various Models
| AUC | 95% CI | P value | Z value | P for comparison | |
|---|---|---|---|---|---|
| MACCE | |||||
| Baseline risk model | 0.611 | 0.586–0.634 | <0.001 | Reference | Reference |
| + NLR | 0.638 | 0.614–0.661 | <0.001 | 1.734 | 0.0830 |
| + eGDR | 0.673 | 0.650–0.696 | <0.001 | 2.671 | 0.0076 |
| + eGDR + NLR | 0.695 | 0.672–0.717 | <0.001 | 3.635 | <0.001 |
| Cardiac death/ Nonfatal MI | |||||
| Baseline risk model | 0.610 | 0.586–0.634 | <0.001 | Reference | Reference |
| + NLR | 0.641 | 0.617–0.664 | <0.001 | 1.413 | 0.158 |
| + eGDR | 0.683 | 0.659–0.705 | <0.001 | 2.490 | 0.0128 |
| + eGDR + NLR | 0.698 | 0.675–0.720 | <0.001 | 2.956 | 0.0031 |
| TVR | |||||
| Baseline risk model | 0.637 | 0.613–0.661 | <0.001 | Reference | Reference |
| + NLR | 0.648 | 0.624–0.671 | <0.001 | 1.102 | 0.270 |
| + eGDR | 0.683 | 0.659–0.705 | <0.001 | 1.885 | 0.0594 |
| + eGDR + NLR | 0.683 | 0.660–0.706 | <0.001 | 1.637 | 0.102 |
| Nonfatal stroke | |||||
| Baseline risk model | 0.747 | 0.725–0.768 | <0.001 | Reference | Reference |
| + NLR | 0.828 | 0.809–0.846 | <0.001 | 2.050 | 0.0404 |
| + eGDR | 0.783 | 0.762–0.803 | <0.001 | 0.833 | 0.405 |
| + eGDR + NLR | 0.843 | 0.824–0.860 | <0.001 | 2.242 | 0.025 |
Note: Adjusted model included: Model 3 in multivariate Cox analyses.
Abbreviations: AUC, area under the curve; CI, confidence interval; eGDR, estimated glucose disposal rate; NLR, neutrophil-to-lymphocyte ratio; MI, myocardial infarction; TVR, target vessel revascularization.
Furthermore, eGDR and NLR enhanced reclassification and discrimination for MACCE (NRI: 0.304, P < 0.001; IDI: 0.033, P < 0.001) (Table 7). Similarly, supplementing eGDR and NLR with the baseline model improved prognostic prediction for MACCE components as well, increasing the AUC-cardiac death/nonfatal MI from 0.610 to 0.698 (P = 0.0031) (NRI: 0.336, P < 0.001; IDI: 0.023, P < 0.001) (Table 6 and Table 7, Figure S2); and AUC-nonfatal stroke from 0.747 to 0.843 (P = 0.025) (NRI: 0.552, P < 0.001; IDI: 0.034, P < 0.001) (Table 6 and Table 7, Figure S4). However, in TVR, the addition of eGDR and NLR may not be significant in comparison of AUC (P = 0.102) (NRI: 0.268, P < 0.001; IDI: 0.023, P < 0.001) (Tables 6 and 7, Figure S3).
Table 7.
Category-Free NRI and IDI for the Incremental Predictive Values of Various Models
| NRI Index | 95% CI | P value | IDI Index | 95% CI | P value | |
|---|---|---|---|---|---|---|
| MACCE | ||||||
| Baseline risk model | – | – | Reference | – | – | Reference |
| + NLR | 0.094 | 0.007–0.204 | <0.001 | 0.008 | 0.001–0.019 | <0.001 |
| + eGDR | 0.296 | 0.029–0.341 | <0.001 | 0.025 | 0.002–0.039 | <0.001 |
| + eGDR + NLR | 0.304 | 0.047–0.369 | <0.001 | 0.033 | 0.005–0.064 | <0.001 |
| Cardiac death/ Nonfatal MI | ||||||
| Baseline risk model | – | – | Reference | – | – | Reference |
| + NLR | 0.048 | 0.002–0.147 | <0.001 | 0.001 | 0.001–0.004 | 0.182 |
| + eGDR | 0.277 | 0.052–0.349 | <0.001 | 0.020 | 0.003–0.038 | <0.001 |
| + eGDR + NLR | 0.336 | 0.025–0.351 | <0.001 | 0.023 | 0.002–0.044 | <0.001 |
| TVR | ||||||
| Baseline risk model | – | – | Reference | – | – | Reference |
| + NLR | 0.080 | 0.016–0.172 | <0.001 | 0.003 | 0.001–0.020 | <0.001 |
| + eGDR | 0.266 | 0.012–0.305 | <0.001 | 0.020 | 0.002–0.024 | <0.001 |
| + eGDR + NLR | 0.268 | 0.051–0.357 | <0.001 | 0.023 | 0.002–0.023 | <0.001 |
| Nonfatal stroke | ||||||
| Baseline risk model | – | – | Reference | – | – | Reference |
| + NLR | 0.341 | 0.031–0.605 | <0.001 | 0.008 | 0.001–0.126 | <0.001 |
| + eGDR | 0.406 | 0.029–0.632 | <0.001 | 0.029 | 0.001–0.115 | <0.001 |
| + eGDR + NLR | 0.552 | 0.008–0.656 | <0.001 | 0.034 | 0.002–0.189 | <0.001 |
Note: Adjusted model included: Model 3 in multivariate Cox analyses.
Abbreviations: NRI, net reclassification improvement; IDI, integrated discrimination improvement; CI, confidence interval; eGDR, estimated glucose disposal rate; NLR, neutrophil-to-lymphocyte ratio; MI, myocardial infarction; TVR, target vessel revascularization.
Discussion
Main Findings
This study represents the initial effort to assess the prediction efficacy of eGDR and NLR combination in ACS and T2DM patients receiving PCI. The principal observations are: (1) With lower eGDR and higher NLR, patients showed a positive association with the MACCE. (2) When the eGDR with NLR integrated, the combination improved the prognostic potential of these indicators significantly by raising the risk of MACCE, and compared to those in the eGDR-H + NLR-L group, the eGDR-L + NLR-H group experienced a 5.201-fold higher risk of MACCE. (3) The addition of eGDR separately or with NLR to the established model significantly enhanced its prognostic prediction, discriminatory, and reclassification abilities for predicting MACCE. Integrating eGDR and NLR levels into the model provided valuable prognostic insights, resulting in an AUC of 0.695, which indicated fair predictive accuracy. Insofar as we know, this study is the initial one to verify the prognostic significance of eGDR, and essentially, combining eGDR and NLR is crucial for enhancing risk stratification in ACS and T2DM patients receiving PCI.
eGDR, NLR and CVD
Recently, more and more studies have found that eGDR was highly correlated with CVD and its prognosis. Higher eGDR was correlated with a reduced risk of stroke, CVD, and cardiac events in Chinese population,26 while lower eGDR was related to a heightened risk of CVD, suggesting that eGDR could be used as a therapeutic target and preferred predictor for CVD. In addition, Jin et al found that eGDR was notably negatively connected or linearly correlated with the occurrence of ischemic heart disease.27 Among two studies related to post-PCI by Liu et al, in NSTEMI patients treated with PCI, they revealed that eGDR level was independently linked to a reduced risk of ISR.28 In addition, in non-diabetic NSTEMI patients received PCI, they also observed that low eGDR level was closely correlated with negative prognosis.29 Moreover, in T2DM patients after CABG, Nystrom et al confirmed that eGDR was strongly correlated with the risk of death from all-cause, irrespective of other metabolic and cardiovascular risk factors.30 In terms of stroke, Zabala et al revealed low eGDR was correlated with an elevated risk for stroke and death in T2DM individuals.14 Interestingly, in the community population, Shi et al demonstrated eGDR was correlated with the hazard of left ventricular hypertrophy significantly,31 and in a study with eGDR to evaluate the risk of atrial fibrillation recurrence, Li et al showed that after radiofrequency ablation, reduced eGDR was related to an amplified risk of recurrent atrial fibrillation.32
In addition to eGDR, inflammatory markers also have a significant impact on the prediction and management of CVD. Recently, an increasing number of studies have concentrated on the role of inflammation in the development of CVD, among which NLR, as an important inflammatory biomarker, has received extensive attention. On the one hand, neutrophils are engaged with the various facets of cardiovascular pathophysiology, such as atherosclerosis and thrombosis, ultimately contributing to the development of ACS,33 and lymphocytes in the process of atherosclerosis also play a vital contribution to the inflammatory response. The apoptosis of lymphocytes within atherosclerotic plaques accelerates plaque development, leading to the formation of lipid cores and, ultimately, thrombotic events.34 On the other hand, neutrophils are pivotal in the innate immune system, marking the acute phase of inflammation. Conversely, lymphocytes are central to the adaptive immune system and facilitate the onset of autoimmune inflammation,35 thus, the NLR bridges the innate and adaptive immune systems, serving as a comprehensive marker of inflammation. As an easily available biomarker of inflammation, NLR has been shown in multiple studies to be the strongest predictor of cardiovascular risk in CAD or high-risk CAD patients, independently associated with prognosis,36,37 and independently predicted CVD risk and all-cause mortality.38 Because NLR was closely associated with plaque rupture in critical CAD, tracking NLR could be valuable for assessing risk and managing severe CAD,39 and NLR proved to be an effective indicator of major adverse cardiovascular events (MACE) risk among both STEMI40 and NSTEMI patients.41 The proportion of neutrophils and NLR were increased in ACS patients after PCI,42 and the escalation in NLR upon admission was correlated with absence of reflow and prolonged prognosis.43 Alongside, in patients receiving PCI, various studies have confirmed the high value of NLR in adverse clinical outcomes.44–46 Building on these findings, our research investigated the combined predictive power of eGDR and NLR in MACCE, which suggested that integrating both markers enhanced the accuracy of MACCE stratification compared to using either marker alone. For instance, although eGDR and NLR were not directly associated with the risk of developing stroke, their inclusion in the baseline model significantly enhanced the model’s predictive efficacy. This may suggest that while these biomarkers did not directly cause stroke, they can still provide valuable information regarding overall cardiovascular risk. Specifically, our study found that patients with lower eGDR and higher NLR levels exhibited significantly higher incidences of MACCE. This dual-marker approach could potentially refine therapeutic strategies, enabling more personalized and effective management of ACS and T2DM patients after PCI.
eGDR, NLR and T2DM
eGDR is easy to calculate in clinical practice and may help distinguish high-risk patients who may have subclinical changes that could predict future complications. Previous studies have shown that conventional clinical factors can reliably estimate the degree of IR in T1DM patients, and the use of eGDR has been suggested,11 and its performance was later fully confirmed in larger studies, which also showed that it was able to predict long-term mortality in this population.47,48 Previous studies have focused eGDR on people with T1DM, and the score has been used in several studies to evaluate chronic diabetes complications in the T2DM population and community populations. In a Chinese population with T2DM, Meng et al identified that lower eGDR was linked to an increased risk of developing diabetic retinopathy independently.49 In addition, Penno et al confirmed that eGDR was independent of confounding factors such as diabetic kidney disease in predicting all-cause mortality among T2DM individuals.50 Besides, Zhang et al emphasized the importance of IR represented by eGDR in the improvement of diabetic peripheral neuropathy in T2DM patients.51 What’s more, Peng et al found that decreased eGDR was a predictor of renal function deterioration in T2DM patients.52 What’s more, Sun et al showed that eGDR was a distinct predictor of death in the senior population, and assessed by eGDR, IR was strongly linked to increased long-term mortality from all causes among older adults.53
Like eGDR, NLR has shown significant value in cardiovascular risk assessment in T2DM patients. Most previous research focused on the relationship between NLR and diabetic complications and poor prognosis. Recently, the association between NLR and poor prognosis of CVD in T2DM patients has gradually increased. In prediabetes and diabetes, NLR was not only significantly elevated,54 but elevated NLR was closely linked to a higher risk of death from CVD.55 In patients with T2DM, NLR, independent of CRP, was correlated with a greater risk of CVD and mortality. NLR was associated with CVD even when CRP was low, suggesting that NLR was a risk marker for CVD in addition to CRP.56 In Chinese adults with diabetes, after adjusting for potential confounders, Wan et al emphasized higher NLR was associated with an increasing prevalence of CVD.57
It is important to note that IR is a key factor contributing to the occurrence of cardiovascular issues58 as a primary mechanism uniting all features of metabolic syndrome, such as hyperglycemia, dyslipidemia, central obesity, and hypertension, representing the chief risk factors for cardiovascular issues.59 In the study that first reported the correlation between NLR and different levels of glucose tolerance and IR, Shiny et al validated that NLR could be used as a complementary forecasting indicator for macro and microvascular complications with impaired glucose tolerance.60 Similarly, Chen et al pointed out that NLR was correlated with coronary microvascular dysfunction (CMD) in T2DM patients, and the prevalence of CMD may increase with the decrease of NLR level.61 Other studies have shown that NLR may be related to metabolic syndrome. Hashemi et al found that NLR, as a marker of low-grade inflammation, was positively correlated with central obesity. In their study, the incidence, severity and control of diabetes were related to NLR.62 In addition, Adane et al identified that higher NLR values in T2DM patients were associated with increased HbA1c, and pointed out that in T2DM patients, NLR could also be used as an indicator of blood glucose control in addition to HbA1c.63 At the same time, eGDR is an IR marker similar in accuracy to the hyperinsulinemic-euglycemic clamp test and is appropriate for clinical use.64 In summary, the significance of combining NLR and eGDR was that NLR reflected the inflammatory state, while eGDR reflected insulin resistance, both of which played a major element in the emergence of diabetes and its complications. As an easily measured inflammatory marker, NLR can reveal inflammatory processes, closely tied to the appearance of cardiovascular events. eGDR, on the other hand, provided a reliable indicator to assess IR, one of the major drivers of CVD risk in people with T2DM. More importantly, NLR may have a close relationship with T2DM and IR, which indicated that these two indicators may interact in the pathophysiological process. Compared to previous research, this study focused on diabetic individuals with increased risk of ACS undergoing PCI, with a mean period of follow-up of 58 months, of which findings demonstrated that eGDR separately or with NLR integrated, whether analyzed as a continuous or categorical variable, was correlated with a growing MACCE risk. The association remained significant following adjustments for conventional cardiovascular risk factors, laboratory parameters, clinical presentations, medication, and CAD severity. These results underscored the integration of eGDR and NLR as a robust independent indicator for MACCE in diabetic ACS patients after PCI.
Therefore, eGDR united with NLR can provide a more comprehensive assessment to more accurately predict and manage adverse events after PCI in patients with ACS and T2DM. This comprehensive assessment method can not only identify high-risk patients but also provide an important basis for the formulation of personalized treatment plans. By integrating indicators of inflammation and IR, clinicians can better understand a patient’s overall health and develop more effective treatments and interventions accordingly. For example, in patients with eGDR-L + NRL-H, more aggressive anti-inflammatory therapy and IR improvement strategies may be needed to mitigate the likelihood of cardiovascular events. In addition, the joint assessment approach may have potential applications in the management of other CVD. With further research and validation, the combination of NLR and eGDR was expected to become a standard tool for CVD risk assessment, thereby improving patient outcomes and enhancing clinical outcomes. To sum up, the fusion of NLR and eGDR was not only of great significance in theory but also showed its great potential in practice, which is worthy of further promotion and application in clinic.
Study Limitations
This study faced several limitations. To begin with, being an observational, single-center, and retrospective analysis, it could not definitively establish causation between eGDR, NLR, and MACCE, and there was a possibility of selection bias and unaccounted confounding variables. Second, since eGDR and NLR were assessed only at one time point, their variations over time were unknown, potentially introducing bias. Future studies should prioritize long-term surveillance and evaluation of these biomarkers to confirm their predictive value. Additionally, variations in patient treatments, such as the use of medications that influenced IR or inflammatory responses, may have impacted the study results. Although adjustments were made for some treatment factors, the specifics of medication types, dosages, and their variability were not fully accounted for, which might have skewed the findings. Furthermore, the study did not include comparisons with other relevant biomarkers, like insulin levels, which limited the comprehensive understanding of eGDR and NLR’s predictive capabilities. Regardless of these limitations, the study first provided critical understanding of the potential clinical significance of combining eGDR and NLR for predicting MACCE in ACS with T2DM patients following PCI.
Conclusions
The present study was the first to demonstrate that lower eGDR and higher NLR were independent predictors of MACCE among individuals with ACS and T2DM undergoing PCI. Merging eGDR and NLR provided complementary effects in predicting these outcomes, significantly enhancing predictive accuracy when included in risk assessment models. These findings suggested that incorporating eGDR and NLR into risk stratification could improve the identification and management of vulnerability among ACS and T2DM patients receiving PCI.
Acknowledgments
We thank all our colleagues at the department of Cardiology, Beijing Anzhen Hospital, Capital Medical University. Meanwhile, XXF is currently a medical doctoral candidate co-trained by Beijing Anzhen Hospital, Capital Medical University, and the University of California, Los Angeles (UCLA), in the United States, so we want to thank Prof. Aldons J. Lusis from UCLA for providing the learning opportunity for XXF.
Funding Statement
XXF was supported by the grant from China Scholarship Council (CSC) (Grant No. 202308110233). QYG was supported by the grant from Beijing Hospitals Authority Youth Programme (Grant No. QML20210601) and National Natural Science Foundation of China (Grant No. 82300368). YJZ was supported by National Key Research and Development Program of China (Grant No. 2022YFC3602500), Beijing Municipal Administration of Hospitals’ Mission plan (Grant No. SML20180601), Capital’s Funds for Health Improvement and Research (Grant No. CFH 2020-2-2063), National Natural Science Foundation of China (Grant No. 82070293), and Beijing Municipal Natural Science Foundation (Grant No. 7202041).
Abbreviations
CVD, cardiovascular diseases; ACS, acute coronary syndromes; WBC, white blood cells; NLR, neutrophil-to-lymphocyte ratio; T2DM, type 2 diabetes mellitus; CAD, coronary artery diseases; eGDR, estimated glucose disposal rate; IR, insulin resistance; T1DM, type 1 diabetes mellitus; HbA1c, glycosylated hemoglobin A1c; MACCE, major adverse cardiovascular and cerebrovascular events; PCI, percutaneous coronary intervention; BMI, body mass index; TG, triglyceride; CABG, coronary artery bypass grafting; SBP, systolic blood pressure; DBP, diastolic blood pressure; PLT, platelets; RBC, red blood cells; hs-CRP, high-sensitivity C-reactive protein; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FBG, fasting blood glucose; eGFR, estimated glomerular filtration rate; UAP, unstable angina pectoris; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; LM, left main; CTO, chronic total occlusion; TIMI, thrombolysis in myocardial infarction; SYNTAX, synergy between PCI with taxus and cardiac surgery; MI, myocardial infarction; TVR, target vessel revascularization; eGDR-L, lower eGDR; eGDR-H, higher eGDR; NLR-L, lower NLR; NLR-H, higher NLR; ROC, receiver-operating characteristic curves; VIF, variance inflation factors; LVEF, left ventricular ejection fraction; DAPT, dual antiplatelet therapy; OAD, oral antidiabetic agents; HR, hazard ratios; CI, confidence intervals; AUC, area under the curve; NRI, net reclassification improvement; IDI, integrated discrimination improvement; ISR, in-stent restenosis; MACE, major adverse cardiovascular events; MINOCA, myocardial infarction with non-obstructive coronary arteries; CMD, coronary microvascular dysfunction.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study protocol strictly adhered to the Declaration of Helsinki. This study was approved by the Ethics Review Board of the Beijing Anzhen Hospital, and all laboratory tests were conducted in accordance with the approved protocol. All patients signed an informed consent form.
Author Contributions
Drs. Zhou and Guo contributed equally to this work and are joint last authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17(7):387–401. doi: 10.1038/s41569-020-0352-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124(2):315–327. doi: 10.1161/CIRCRESAHA.118.313591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox KAA, Metra M, Morais J, Atar D. The myth of ‘stable’ coronary artery disease. Nat Rev Cardiol. 2020;17(1):9–21. doi: 10.1038/s41569-019-0233-y [DOI] [PubMed] [Google Scholar]
- 4.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643. doi: 10.1016/j.jacc.2005.02.054 [DOI] [PubMed] [Google Scholar]
- 5.Zhao L, Xu T, Li Y, et al. Variability in blood lipids affects the neutrophil to lymphocyte ratio in patients undergoing elective percutaneous coronary intervention: a retrospective study. Lipids Health Dis. 2020;19(1):124. doi: 10.1186/s12944-020-01304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahajan M, Prasad MK, Ashok C, et al. The Correlation of the Neutrophil-to-Lymphocyte Ratio With Microvascular Complications in Patients With Diabetes Mellitus. Cureus. 2023;15(9):e44601. doi: 10.7759/cureus.44601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suárez-Cuenca JA, Ruíz-Hernández AS, Mendoza-Castañeda AA, et al. Neutrophil-to-lymphocyte ratio and its relation with pro-inflammatory mediators, visceral adiposity and carotid intima-media thickness in population with obesity. Eur J Clin Invest. 2019;49(5):e13085. doi: 10.1111/eci.13085 [DOI] [PubMed] [Google Scholar]
- 8.Guo X, Zhang S, Zhang Q, et al. Neutrophil: lymphocyte ratio is positively related to type 2 diabetes in a large-scale adult population: a Tianjin Chronic Low-Grade Systemic Inflammation and Health cohort study. Eur J Endocrinol. 2015;173(2):217–225. doi: 10.1530/EJE-15-0176 [DOI] [PubMed] [Google Scholar]
- 9.Verdoia M, Schaffer A, Barbieri L, et al. Impact of diabetes on neutrophil-to-lymphocyte ratio and its relationship to coronary artery disease. Diabetes Metab. 2015;41(4):304–311. doi: 10.1016/j.diabet.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11:158. doi: 10.1186/1471-2288-11-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49(4):626–632. doi: 10.2337/diabetes.49.4.626 [DOI] [PubMed] [Google Scholar]
- 12.Kietsiriroje N, Pearson S, Campbell M, Ariëns RAS, Ajjan RA. Double diabetes: a distinct high-risk group? Diabetes Obes Metab. 2019;21(12):2609–2618. doi: 10.1111/dom.13848 [DOI] [PubMed] [Google Scholar]
- 13.Nyström T, Holzmann MJ, Eliasson B, Svensson AM, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20(3):556–563. doi: 10.1111/dom.13110 [DOI] [PubMed] [Google Scholar]
- 14.Zabala A, Darsalia V, Lind M, et al. Estimated glucose disposal rate and risk of stroke and mortality in type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2021;20(1):202. doi: 10.1186/s12933-021-01394-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebert T, Anker SD, Ruilope LM, et al. Outcomes With Finerenone in Patients With Chronic Kidney Disease and Type 2 Diabetes by Baseline Insulin Resistance. Diabetes Care. 2024;47(3):362–370. doi: 10.2337/dc23-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Wang X, Chen J, et al. Household air pollution, adherence to a healthy lifestyle, and risk of cardiometabolic multimorbidity: results from the China health and retirement longitudinal study. Sci Total Environ. 2023;855:158896. doi: 10.1016/j.scitotenv.2022.158896 [DOI] [PubMed] [Google Scholar]
- 17.Amara N, Cooper MP, Voronkova MA, et al. Selective activation of PFKL suppresses the phagocytic oxidative burst. Cell. 2021;184(17):4480–4494.e15. doi: 10.1016/j.cell.2021.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Cai J, Lin B, et al. GPR34-mediated sensing of lysophosphatidylserine released by apoptotic neutrophils activates type 3 innate lymphoid cells to mediate tissue repair. Immunity. 2021;54(6):1123–1136.e8. doi: 10.1016/j.immuni.2021.05.007 [DOI] [PubMed] [Google Scholar]
- 19.Feng X, Guo Q, Zhou S, et al. Could remnant-like particle cholesterol become a risk factor in diabetic menopausal women with coronary artery disease? A cross-sectional study of single academic center in China. Lipids Health Dis. 2020;19(1):44. doi: 10.1186/s12944-020-01224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association Professional Practice Committee. Classification and Diagnosis of Diabetes: standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. doi: 10.2337/dc22-S002 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Feng X, Yang J, et al. The relation between atherogenic index of plasma and cardiovascular outcomes in prediabetic individuals with unstable angina pectoris. BMC Endocr Disord. 2023;23(1):187. doi: 10.1186/s12902-023-01443-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrne RA, Rossello X, Coughlan JJ, et al. ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38):3720–3826. doi: 10.1093/eurheartj/ehad191 [DOI] [PubMed] [Google Scholar]
- 23.Helliwell R, Warnes H, Kietsiriroje N, et al. Body mass index, estimated glucose disposal rate and vascular complications in type 1 diabetes: beyond glycated haemoglobin. Diabet Med. 2021;38(5):e14529. doi: 10.1111/dme.14529 [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–2567. doi: 10.1093/eurheartj/ehs184 [DOI] [PubMed] [Google Scholar]
- 25.Guo Q, Feng X, Zhang B, et al. Influence of the Triglyceride-Glucose Index on Adverse Cardiovascular and Cerebrovascular Events in Prediabetic Patients With Acute Coronary Syndrome. Front Endocrinol (Lausanne). 2022;13:843072. doi: 10.3389/fendo.2022.843072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren X, Jiang M, Han L, Zheng X. Estimated glucose disposal rate and risk of cardiovascular disease: evidence from the China Health and Retirement Longitudinal Study. BMC Geriatr. 2022;22(1):968. doi: 10.1186/s12877-022-03689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xuan J, Juan D, Yuyu N, Anjing J. Impact of estimated glucose disposal rate for identifying prevalent ischemic heart disease: findings from a cross-sectional study. BMC Cardiovasc Disord. 2022;22(1):378. doi: 10.1186/s12872-022-02817-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Zhao Q, Zhao Z, et al. Correlation between estimated glucose disposal rate and in-stent restenosis following percutaneous coronary intervention in individuals with non-ST-segment elevation acute coronary syndrome. Front Endocrinol (Lausanne). 2022;13:1033354. doi: 10.3389/fendo.2022.1033354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Liu X, Ma X, et al. Predictive worth of estimated glucose disposal rate: evaluation in patients with non-ST-segment elevation acute coronary syndrome and non-diabetic patients after percutaneous coronary intervention. Diabetol Metab Syndr. 2022;14(1):145. doi: 10.1186/s13098-022-00915-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyström T, Holzmann MJ, Eliasson B, Svensson AM, Kuhl J, Sartipy U. Estimated glucose disposal rate and long-term survival in type 2 diabetes after coronary artery bypass grafting. Heart Vessels. 2017;32(3):269–278. doi: 10.1007/s00380-016-0875-1 [DOI] [PubMed] [Google Scholar]
- 31.Shi W, Qin M, Wu S, Xu K, Zheng Q, Liu X. Value of estimated glucose disposal rate to detect prevalent left ventricular hypertrophy: implications from a general population. Postgrad Med. 2023;135(1):58–66. doi: 10.1080/00325481.2022.2131153 [DOI] [PubMed] [Google Scholar]
- 32.Li X, Zhou Z, Xia Z, et al. Association between estimated glucose disposal rate and atrial fibrillation recurrence in patients undergoing radiofrequency catheter ablation: a retrospective study. Eur J Med Res. 2024;29(1):325. doi: 10.1186/s40001-024-01911-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu LF, Gu J, Wang SB, et al. Combination of D-dimer level and neutrophil to lymphocyte ratio predicts long-term clinical outcomes in acute coronary syndrome after percutaneous coronary intervention. Cardiol J. 2023;30(4):576–586. doi: 10.5603/CJ.a2021.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoneman VE, Bennett MR. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci (Lond). 2004;107(4):343–354. doi: 10.1042/CS20040086 [DOI] [PubMed] [Google Scholar]
- 35.García-Escobar A, Vera-Vera S, Tébar-Márquez D, et al. Neutrophil-to-lymphocyte ratio an inflammatory biomarker, and prognostic marker in heart failure, cardiovascular disease and chronic inflammatory diseases: new insights for a potential predictor of anti-cytokine therapy responsiveness. Microvasc Res. 2023;150:104598. doi: 10.1016/j.mvr.2023.104598 [DOI] [PubMed] [Google Scholar]
- 36.Cho KH, Jeong MH, Ahmed K, et al. Value of early risk stratification using hemoglobin level and neutrophil-to-lymphocyte ratio in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011;107(6):849–856. doi: 10.1016/j.amjcard.2010.10.067 [DOI] [PubMed] [Google Scholar]
- 37.Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225(2):456–460. doi: 10.1016/j.atherosclerosis.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 38.Adamstein NH, MacFadyen JG, Rose LM, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42(9):896–903. doi: 10.1093/eurheartj/ehaa1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang J, Zeng H, Zhuo Y, et al. Association of Neutrophil to Lymphocyte Ratio With Plaque Rupture in Acute Coronary Syndrome Patients With Only Intermediate Coronary Artery Lesions Assessed by Optical Coherence Tomography. Front Cardiovasc Med. 2022;9:770760. doi: 10.3389/fcvm.2022.770760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruc M, Kubica J, Banach M, et al. Diagnostic and prognostic performance of the neutrophil-to-lymphocyte ratio in acute coronary syndromes: a meta-analysis of 90 studies including 45 990 patients. Kardiol Pol. 2024;82(3):276–284. doi: 10.33963/v.phj.99554 [DOI] [PubMed] [Google Scholar]
- 41.Fan Z, Li Y, Ji H, Jian X. Prognostic utility of the combination of monocyte-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with NSTEMI after primary percutaneous coronary intervention: a retrospective cohort study. BMJ Open. 2018;8(10):e023459. doi: 10.1136/bmjopen-2018-023459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheng J, Liu N, He F, Cheng C, Shen S, Sun Y. Changes in the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios before and after percutaneous coronary intervention and their impact on the prognosis of patients with acute coronary syndrome. Clinics (Sao Paulo). 2021;76:e2580. doi: 10.6061/clinics/2021/e2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sen N, Afsar B, Ozcan F, et al. The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long term adverse outcome in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis. 2013;228(1):203–210. doi: 10.1016/j.atherosclerosis.2013.02.017 [DOI] [PubMed] [Google Scholar]
- 44.Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97(7):993–996. doi: 10.1016/j.amjcard.2005.10.034 [DOI] [PubMed] [Google Scholar]
- 45.Shahsanaei F, Abbaszadeh S, Behrooj S, Rahimi Petrudi N, Ramezani B. The value of neutrophil-to-lymphocyte ratio in predicting severity of coronary involvement and long-term outcome of percutaneous coronary intervention in patients with acute coronary syndrome: a systematic review and meta-analysis. Egypt Heart J. 2024;76(1):39. doi: 10.1186/s43044-024-00469-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S, Diao J, Qi C, et al. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. BMC Cardiovasc Disord. 2018;18(1):75. doi: 10.1186/s12872-018-0812-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care. 2013;36(8):2280–2285. doi: 10.2337/dc12-1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garofolo M, Gualdani E, Scarale MG, et al. Insulin Resistance and Risk of Major Vascular Events and All-Cause Mortality in Type 1 Diabetes: a 10-Year Follow-up Study. Diabetes Care. 2020;43(10):e139–e141. doi: 10.2337/dc20-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng C, Xing Y, Huo L, Ma H. Relationship Between Estimated Glucose Disposal Rate and Type 2 Diabetic Retinopathy. Diabetes Metab Syndr Obes. 2023;16:807–818. doi: 10.2147/DMSO.S395818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penno G, Solini A, Orsi E, et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: a prospective cohort study. BMC Med. 2021;19(1):66. doi: 10.1186/s12916-021-01936-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Sun W, Zhang Q, et al. Estimated glucose disposal rate predicts the risk of diabetic peripheral neuropathy in type 2 diabetes: a 5-year follow-up study. J Diabetes. 2024;16(5):e13482. doi: 10.1111/1753-0407.13482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng J, Li A, Yin L, Yang Q, Pan J, Yi B. Estimated Glucose Disposal Rate Predicts Renal Progression in Type 2 Diabetes Mellitus: a Retrospective Cohort Study. J Endocr Soc. 2023;7(7):bvad069. doi: 10.1210/jendso/bvad069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun J, Wang N, Li S, et al. Estimated glucose disposal rate and risk of arterial stiffness and long-term all-cause mortality: a 10-year prospective study. J Epidemiol Community Health. 2023;78(3):jech–2023–220664. doi: 10.1136/jech-2023-220664 [DOI] [PubMed] [Google Scholar]
- 54.Mertoglu C, Gunay M. Neutrophil-Lymphocyte ratio and Platelet-Lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr. 2017;11:S127–S131. doi: 10.1016/j.dsx.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 55.Chen G, Che L, Lai M, et al. Association of neutrophil-lymphocyte ratio with all-cause and cardiovascular mortality in US adults with diabetes and prediabetes: a prospective cohort study. BMC Endocr Disord. 2024;24(1):64. doi: 10.1186/s12902-024-01592-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoes LLF, Riksen NP, Geleijnse JM, et al. Relationship of neutrophil-to-lymphocyte ratio, in addition to C-reactive protein, with cardiovascular events in patients with type 2 diabetes. Diabet Res Clin Pract. doi: 10.1016/j.diabres.2024.111727 [DOI] [PubMed] [Google Scholar]
- 57.Wan H, Wang Y, Fang S, et al. Associations between the Neutrophil-to-Lymphocyte Ratio and Diabetic Complications in Adults with Diabetes: a Cross-Sectional Study. J Diabetes Res. 2020;2020:6219545. doi: 10.1155/2020/6219545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. doi: 10.1038/nrendo.2014.29 [DOI] [PubMed] [Google Scholar]
- 59.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 60.Shiny A, Bibin YS, Shanthirani CS, et al. Association of neutrophil-lymphocyte ratio with glucose intolerance: an indicator of systemic inflammation in patients with type 2 diabetes. Diabetes Technol Ther. 2014;16(8):524–530. doi: 10.1089/dia.2013.0264 [DOI] [PubMed] [Google Scholar]
- 61.Chen Y, Chai Q, Wang Q, et al. Neutrophil-to-lymphocyte ratio is associated with coronary microvascular dysfunction in type 2 diabetes mellitus patients. Diabet Res Clin Pract. 2021;178:108983. doi: 10.1016/j.diabres.2021.108983 [DOI] [PubMed] [Google Scholar]
- 62.Hashemi Moghanjoughi P, Neshat S, Rezaei A, Heshmat-Ghahdarijani K. Is the Neutrophil-to-Lymphocyte Ratio an Exceptional Indicator for Metabolic Syndrome Disease and Outcomes? Endocr Pract. 2022;28(3):342–348. doi: 10.1016/j.eprac.2021.11.083 [DOI] [PubMed] [Google Scholar]
- 63.Adane T, Melku M, Worku YB, et al. The Association between Neutrophil-to-Lymphocyte Ratio and Glycemic Control in Type 2 Diabetes Mellitus: a Systematic Review and Meta-Analysis. J Diabetes Res. 2023;2023:3117396. doi: 10.1155/2023/3117396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Komosinska-Vassev K, Gala O, Olczyk K, Jura-Półtorak A, Olczyk P. The Usefulness of Diagnostic Panels Based on Circulating Adipocytokines/Regulatory Peptides, Renal Function Tests, Insulin Resistance Indicators and Lipid-Carbohydrate Metabolism Parameters in Diagnosis and Prognosis of Type 2 Diabetes Mellitus with Obesity. Biomolecules. 2020;10(9):1304. doi: 10.3390/biom10091304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.