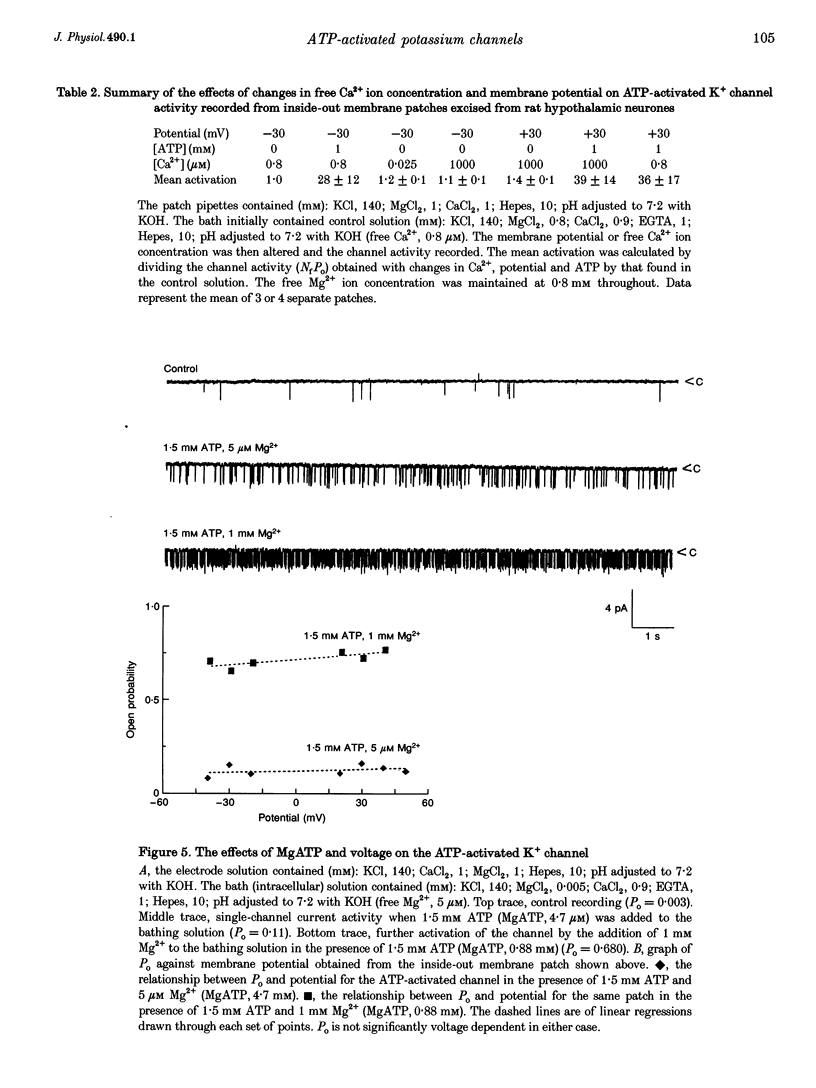
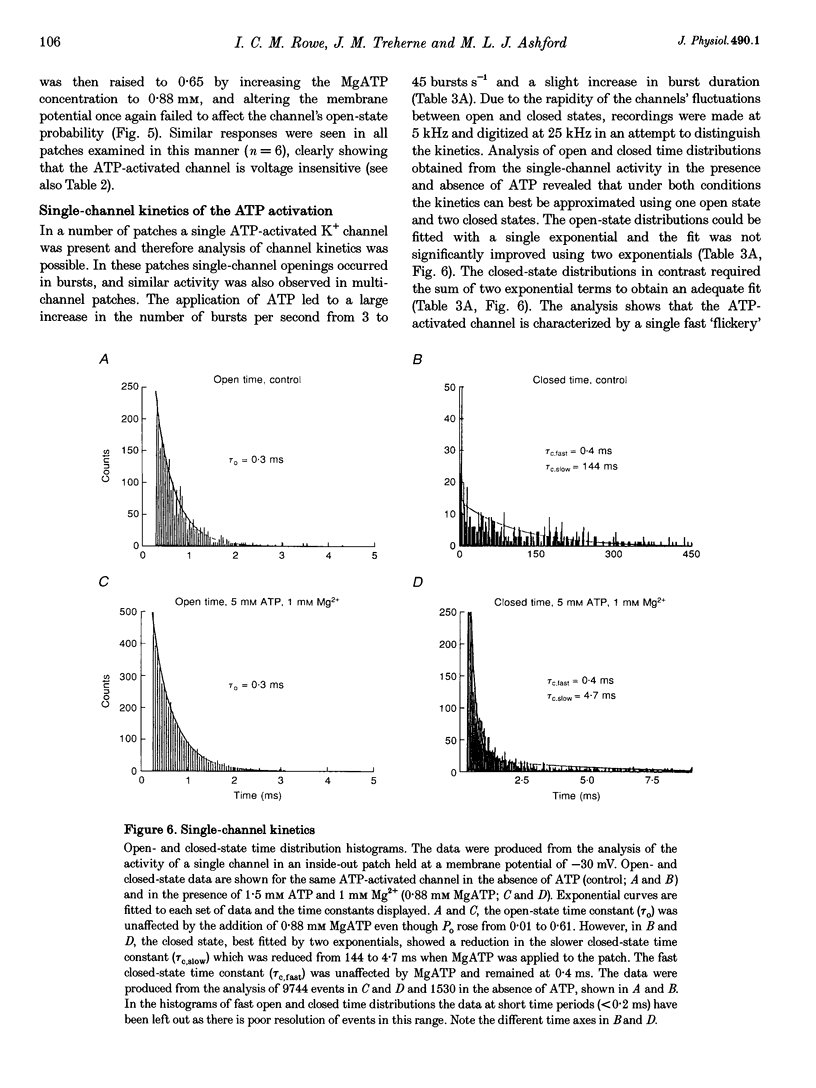
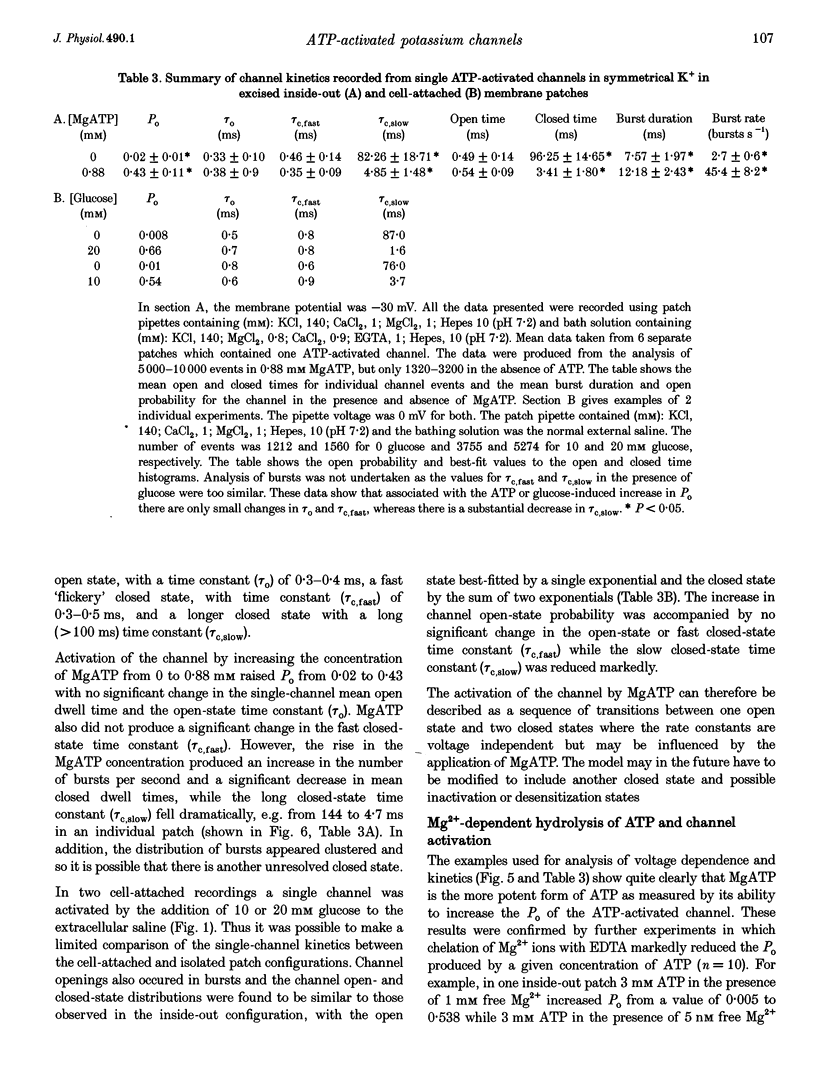
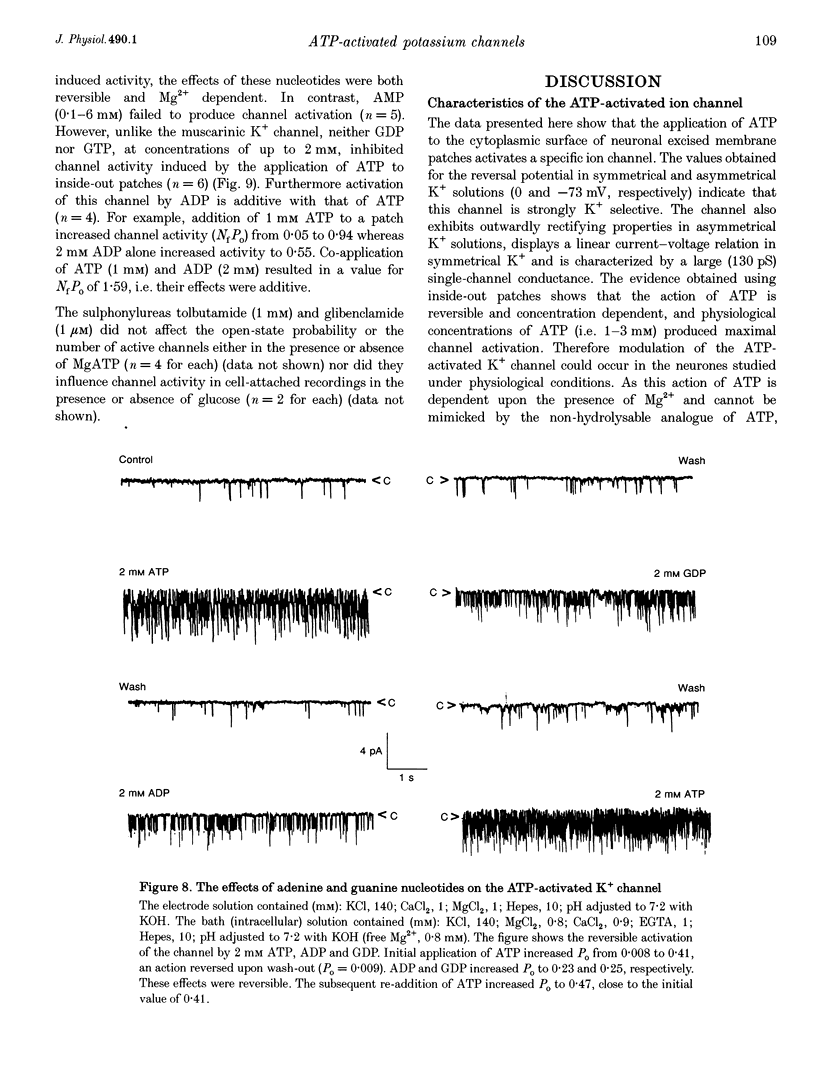
Abstract
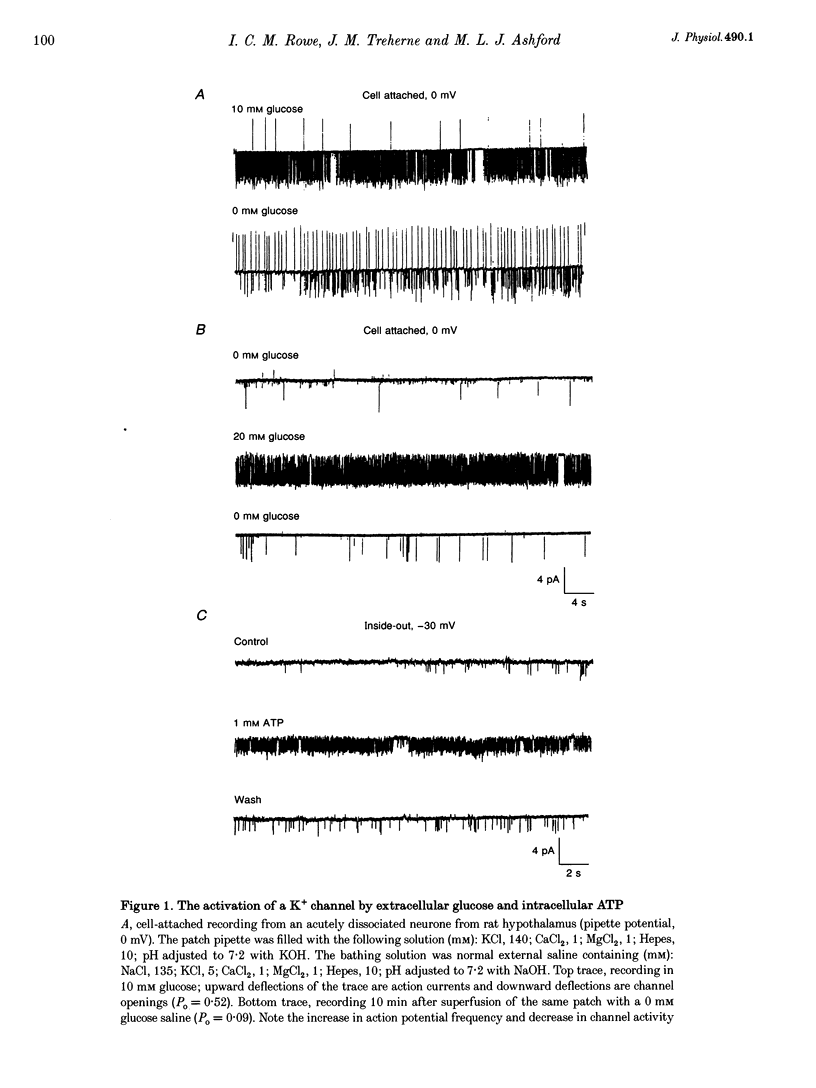
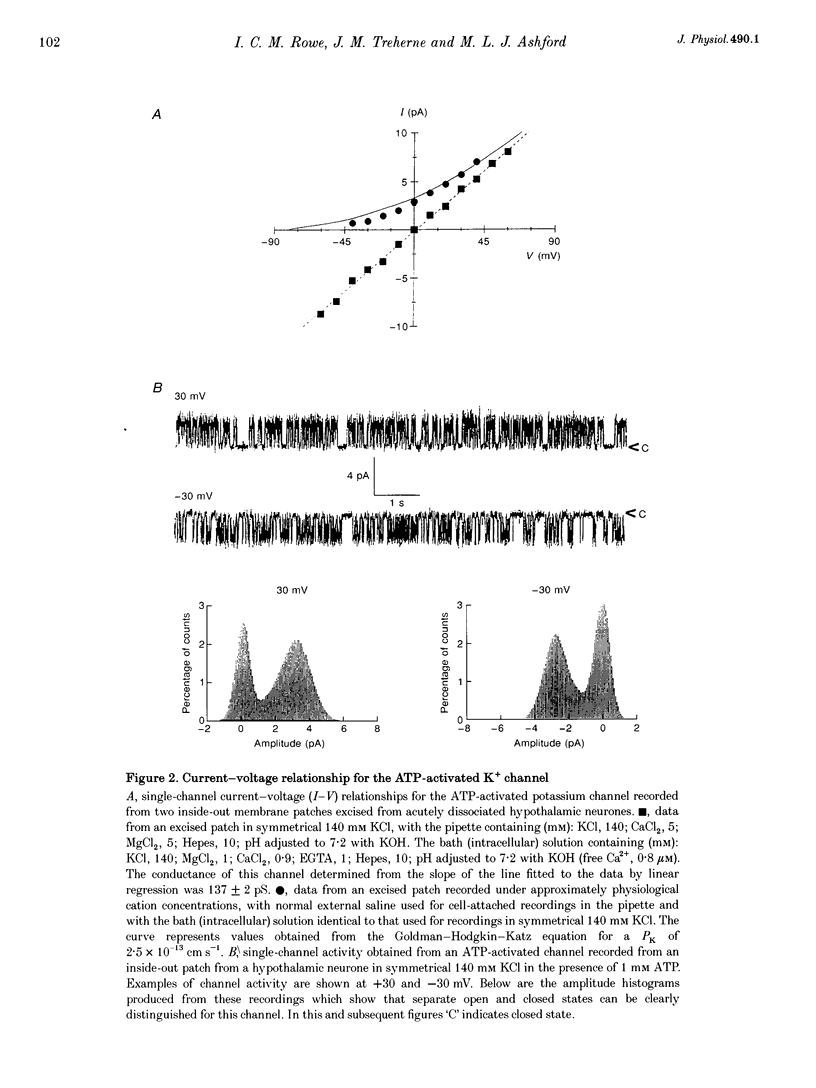
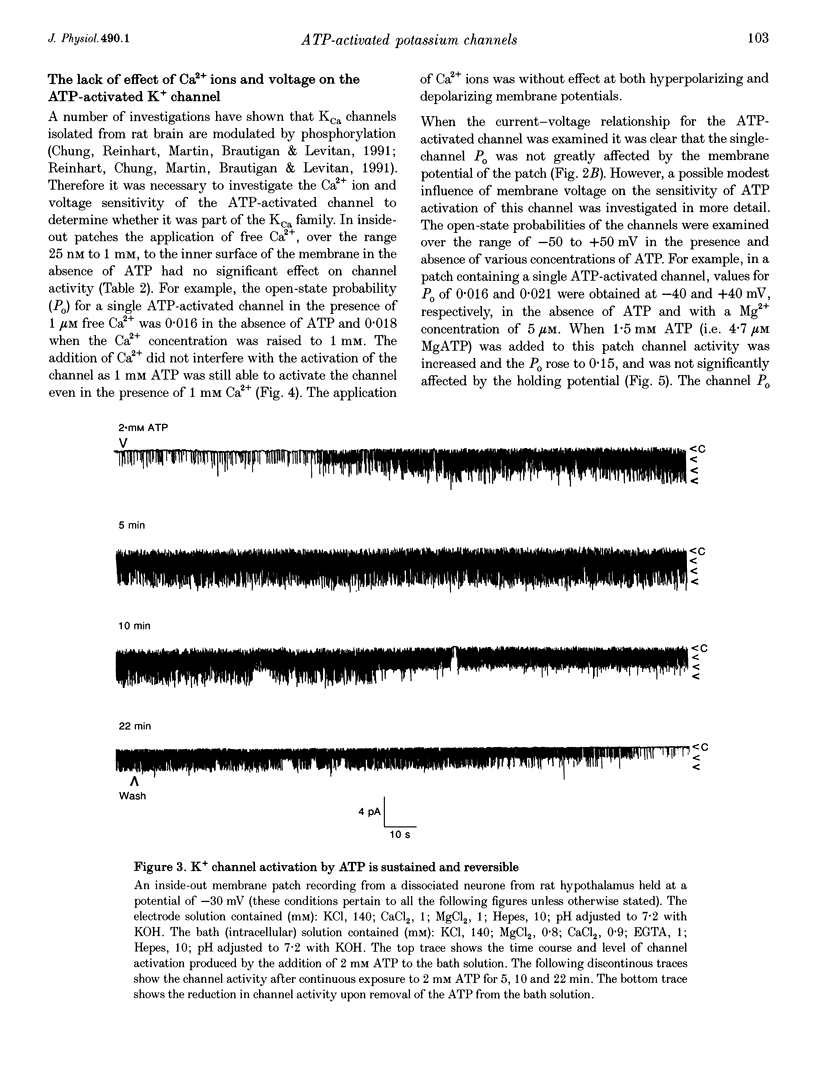
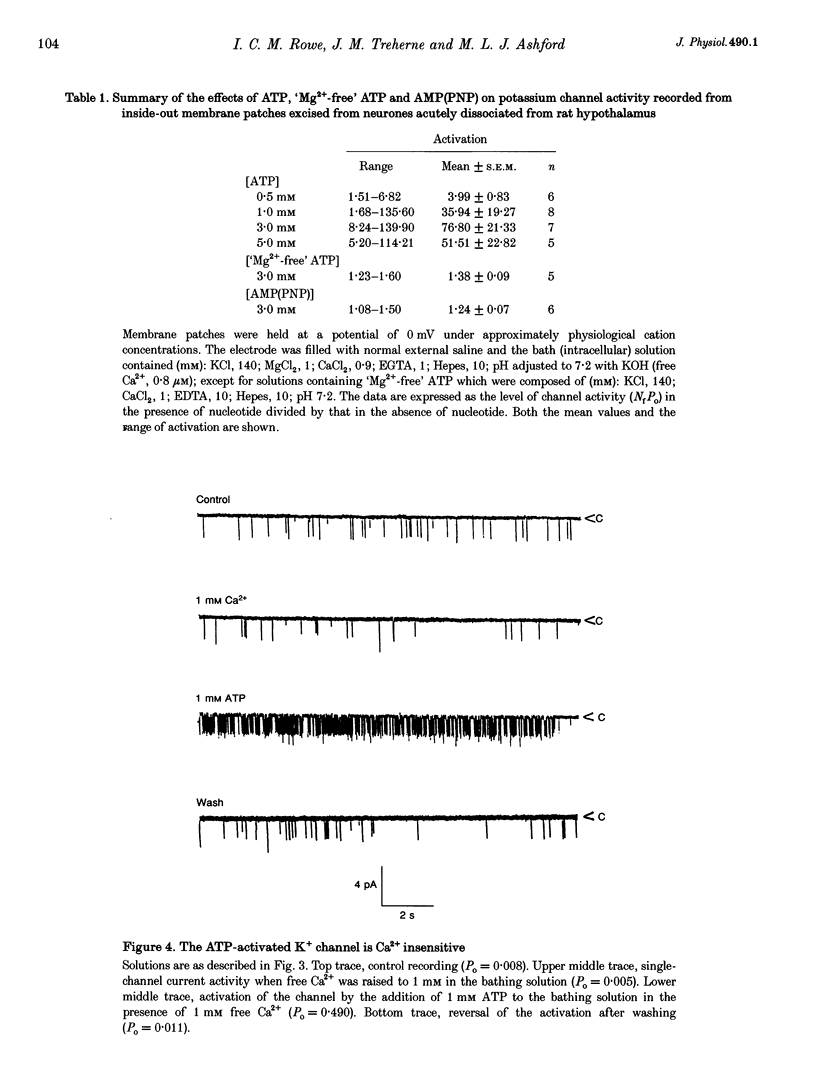
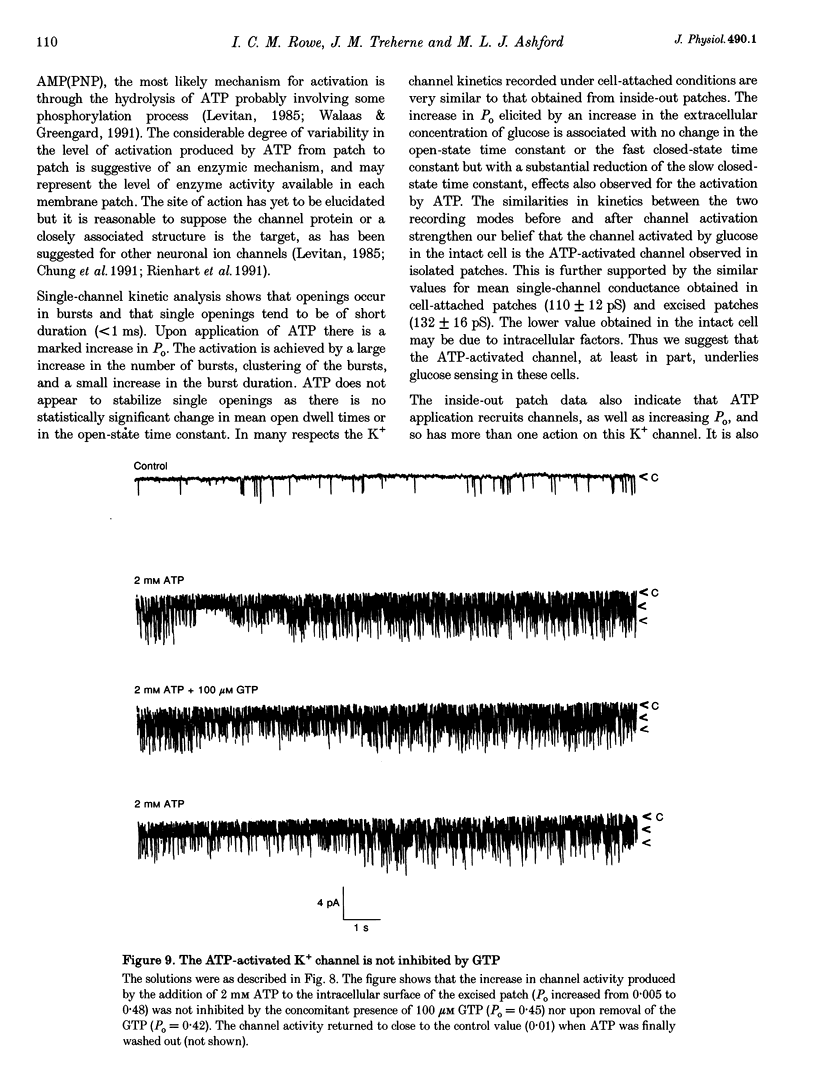
1. Cell-attached recordings from isolated glucose-sensitive hypothalamic neurones show that on removal of extracellular glucose there is an increased action current frequency concomitant with decreased single-channel activity. Conversely activation of single K+ channels was observed when extracellular glucose was increased. Isolation of membrane patches into the inside-out configuration following cell-attached recording demonstrated the presence of an ATP-activated K+ channel. 2. The ATP-activated K+ channel was characterized by a mean single-channel conductance of 132 pS in symmetrical 140 mM KCl solutions. Single-channel open-state probability (Po) was not calcium dependent, and the presence of calcium did not prevent activation of the channel by ATP. 3. Activation of the channel by ATP was concentration dependent and the Po of the ATP-activated channel was unaffected by membrane voltage, regardless of the degree of activation elicited by ATP. 4. Open and closed time histograms were constructed from inside-out and cell-attached recordings and were consistent with a single open and two closed states. Channel openings were grouped in bursts. Application of ATP, in isolated patches, and glucose, in cell-attached patches, increased the burst duration and number of bursts per second and decreased the slow closed-state time constant. In neither case was there a significant change in the fast closed-state time constant nor the open-state time constant. 5. The non-hydrolysable ATP analogue adenylylimidodiphosphate (AMP(PNP)) and 'Mg2(+)-free' ATP produced little change in the Po of the ATP-activated K+ channel when applied to the intracellular surface of excised patches. These results suggest that activation of this channel is via an enzymic mechanism. 6. ADP, GTP and GDP also activated the channel in a Mg(2+)-dependent manner. ADP and ATP activated the channel in an additive manner and neither GTP nor GDP inhibited channel activity induced by ATP. 7. It is concluded that the ATP-activated K+ channel observed in isolated inside-out patches from hypothalamic neurones is the same as the channel activated by an increase in the concentration of extracellular glucose in cell-attached recordings from glucose-sensitive neurones.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Boden P. R., Treherne J. M. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990 Jan;415(4):479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Boden P. R., Treherne J. M. Tolbutamide excites rat glucoreceptive ventromedial hypothalamic neurones by indirect inhibition of ATP-K+ channels. Br J Pharmacol. 1990 Nov;101(3):531–540. doi: 10.1111/j.1476-5381.1990.tb14116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J. Pharmacological approaches to appetite suppression. Trends Pharmacol Sci. 1991 Apr;12(4):147–157. doi: 10.1016/0165-6147(91)90532-w. [DOI] [PubMed] [Google Scholar]
- Boden P., Hill R. G. Effects of cholecystokinin and related peptides on neuronal activity in the ventromedial nucleus of the rat hypothalamus. Br J Pharmacol. 1988 May;94(1):246–252. doi: 10.1111/j.1476-5381.1988.tb11521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. K., Reinhart P. H., Martin B. L., Brautigan D., Levitan I. B. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991 Aug 2;253(5019):560–562. doi: 10.1126/science.1857986. [DOI] [PubMed] [Google Scholar]
- Frohman L. A. CNS peptides and glucoregulation. Annu Rev Physiol. 1983;45:95–107. doi: 10.1146/annurev.ph.45.030183.000523. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heidbüchel H., Callewaert G., Vereecke J., Carmeliet E. ATP-dependent activation of atrial muscarinic K+ channels in the absence of agonist and G-nucleotides. Pflugers Arch. 1990 Apr;416(1-2):213–215. doi: 10.1007/BF00370246. [DOI] [PubMed] [Google Scholar]
- Heidbüchel H., Callewaert G., Vereecke J., Carmeliet E. Acetylcholine-mediated K+ channel activity in guinea-pig atrial cells is supported by nucleoside diphosphate kinase. Pflugers Arch. 1993 Jan;422(4):316–324. doi: 10.1007/BF00374286. [DOI] [PubMed] [Google Scholar]
- Jiang C., Haddad G. G. Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. J Neurophysiol. 1991 Jul;66(1):103–111. doi: 10.1152/jn.1991.66.1.103. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Ashford M. L. ATP-sensitive K(+)-channel run-down is Mg2+ dependent. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1298):397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Luiten P. G., ter Horst G. J., Steffens A. B. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol. 1987;28(1):1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- Minami T., Oomura Y., Sugimori M. Electrophysiological properties and glucose responsiveness of guinea-pig ventromedial hypothalamic neurones in vitro. J Physiol. 1986 Nov;380:127–143. doi: 10.1113/jphysiol.1986.sp016276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y., Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Brain Res. 1984 Jul 30;307(1-2):109–116. doi: 10.1016/0006-8993(84)90466-9. [DOI] [PubMed] [Google Scholar]
- Morley J. E., Levine A. S. The pharmacology of eating behavior. Annu Rev Pharmacol Toxicol. 1985;25:127–146. doi: 10.1146/annurev.pa.25.040185.001015. [DOI] [PubMed] [Google Scholar]
- Niijima A. Nervous regulation of metabolism. Prog Neurobiol. 1989;33(2):135–147. doi: 10.1016/0301-0082(89)90037-3. [DOI] [PubMed] [Google Scholar]
- Ono T., Nishino H., Fukuda M., Sasaki K., Muramoto K., Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Res. 1982 Jan 28;232(2):494–499. doi: 10.1016/0006-8993(82)90295-5. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Nakamura T., Sugimori M., Yamada Y. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol Behav. 1975 Apr;14(04):483–486. doi: 10.1016/0031-9384(75)90015-3. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Ono T., Ooyama H., Wayner M. J. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969 Apr 19;222(5190):282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Ooyama H., Sugimori M., Nakamura T., Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974 Feb 1;247(5439):284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Chung S., Martin B. L., Brautigan D. L., Levitan I. B. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci. 1991 Jun;11(6):1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Oomura Y. Calcitonin-induced anorexia in rats: evidence for its inhibitory action on lateral hypothalamic chemosensitive neurons. Brain Res. 1986 Mar 5;367(1-2):128–140. doi: 10.1016/0006-8993(86)91586-6. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]


